Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
33results about How to "Long survival" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
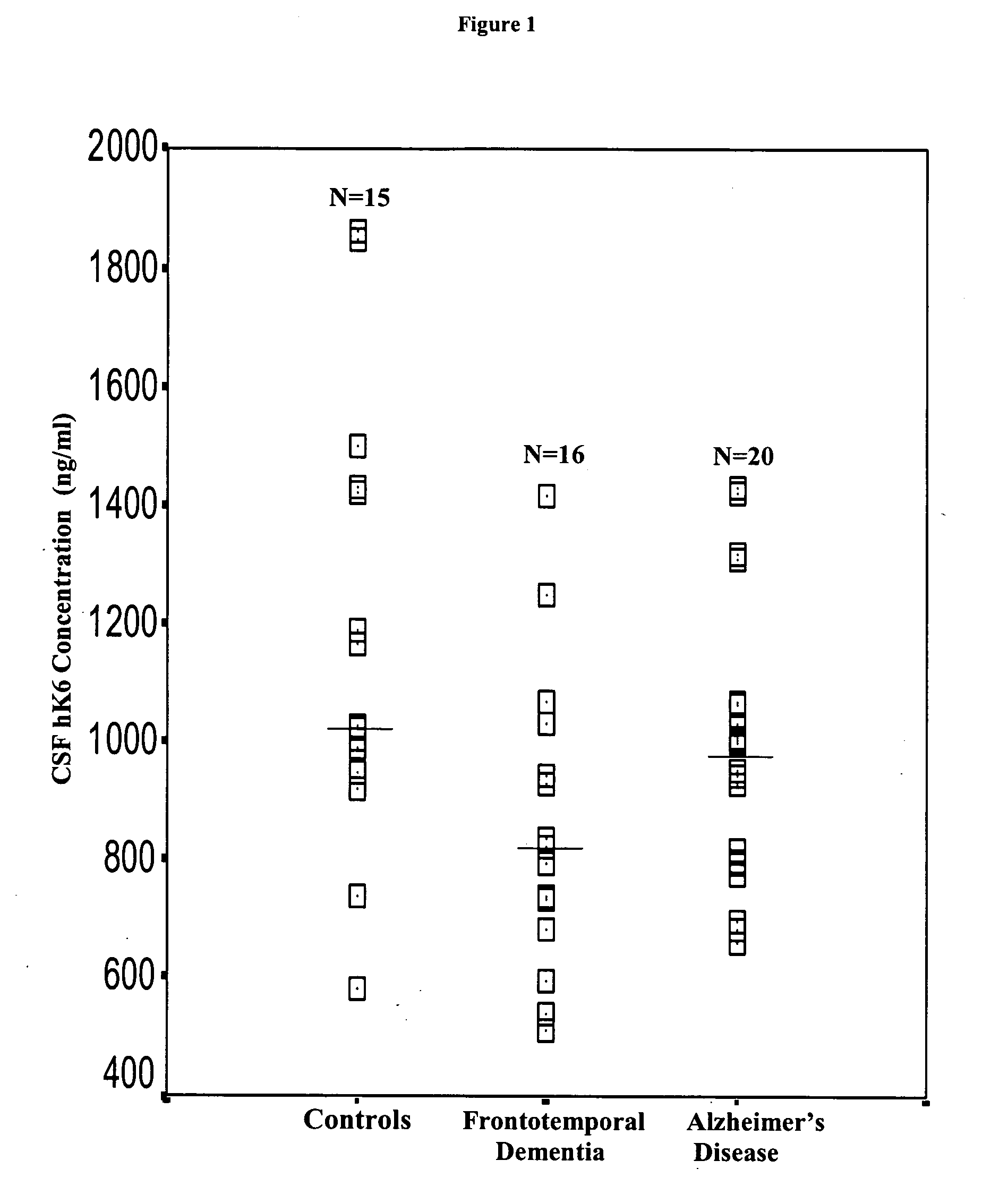
Detection of neurodegenerative diseases
InactiveUS20050106586A1Long survivalHigh expressionNervous disorderMicrobiological testing/measurementKallikreinPlasma kallikrein inhibitor
The invention relates to compositions, kits, and methods for detecting, characterizing, preventing, and treating neurodegenerative diseases. In particular, the invention utilizes kallikrein 7 and kallikrein 10 and nucleic acids encoding same, to detect, characterize, prevent and treat neurodegenerative diseases.
Owner:MOUNT SINAI HOSPITAL
Nanoparticles loaded with chemotherapeutic antitumoral drug
InactiveUS20140024610A1Long survivalStrong signalBiocideNanostructure manufactureSide effectHepatocellular carcinoma
The invention relates to new therapeutic approaches for treating cancer, in particular hepatocellular carcinoma, with Nanoparticules loaded with a chemotherapeutic antitumoral agent. In particular, it relates to the treatment of cancer by administration of said Nanoparticules by intravenous infusion for at least 2 hours in order to prevent toxicological side effects and increase the benefit / risk ratio of the treatment.
Owner:ONXEO SA
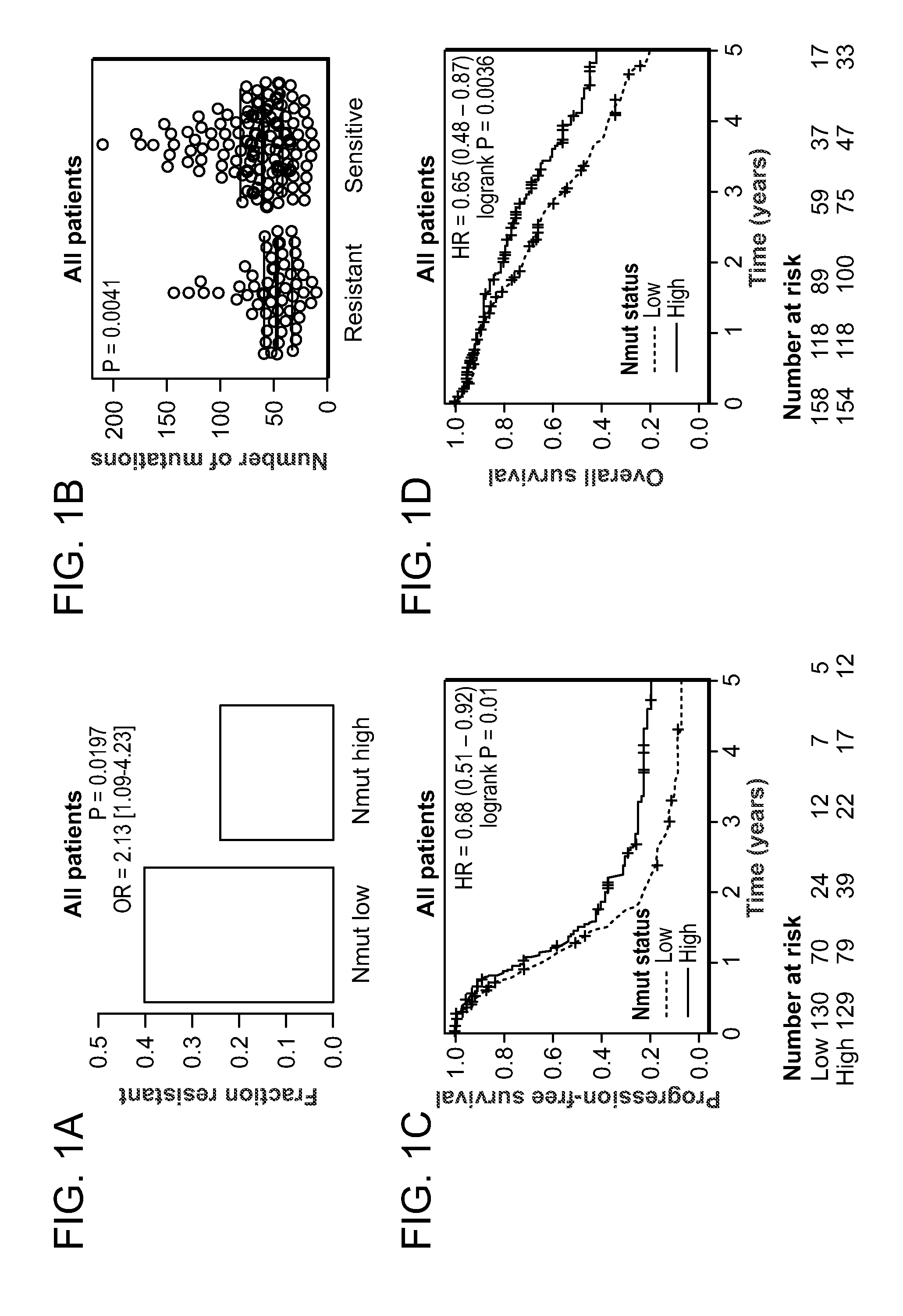
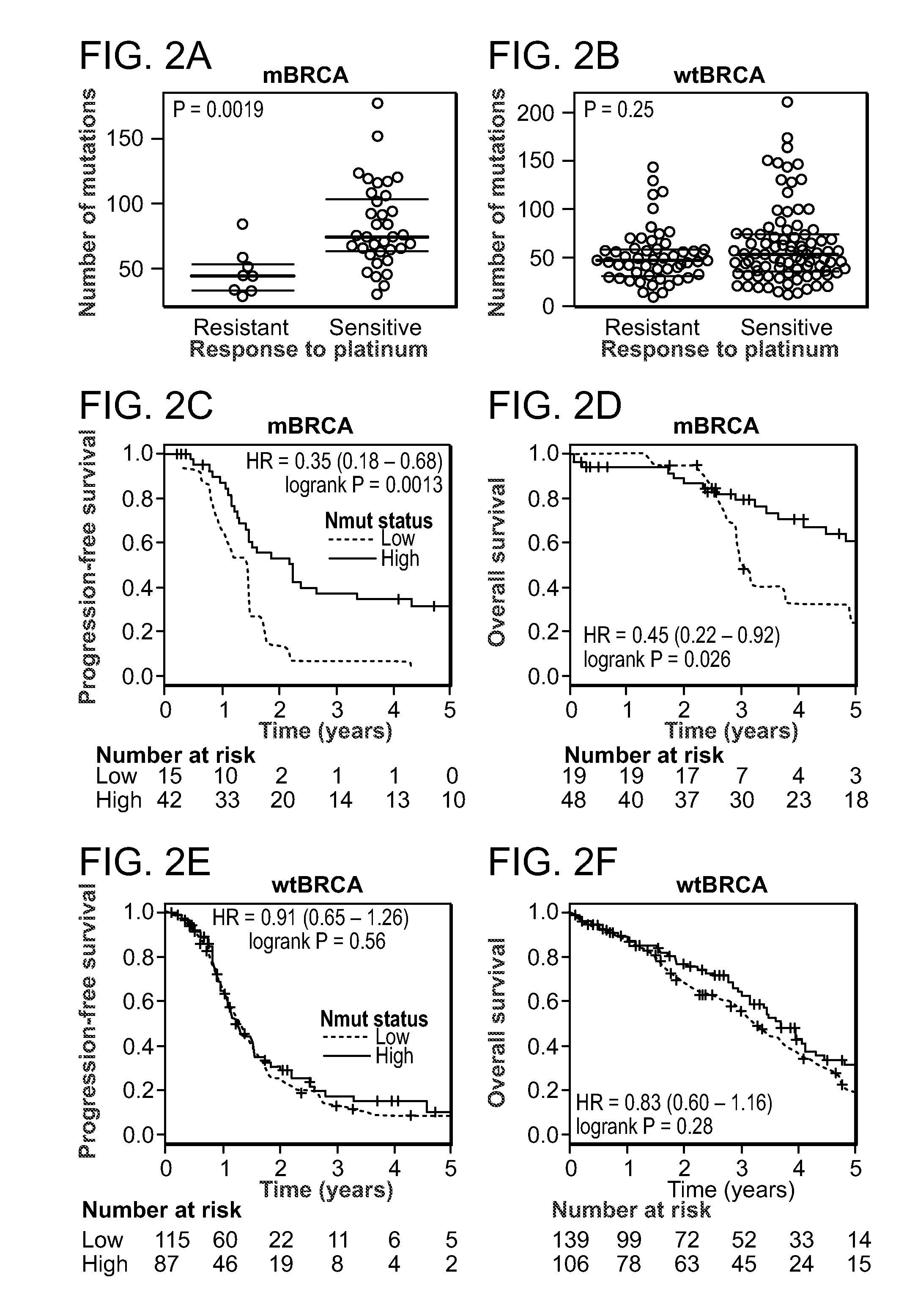
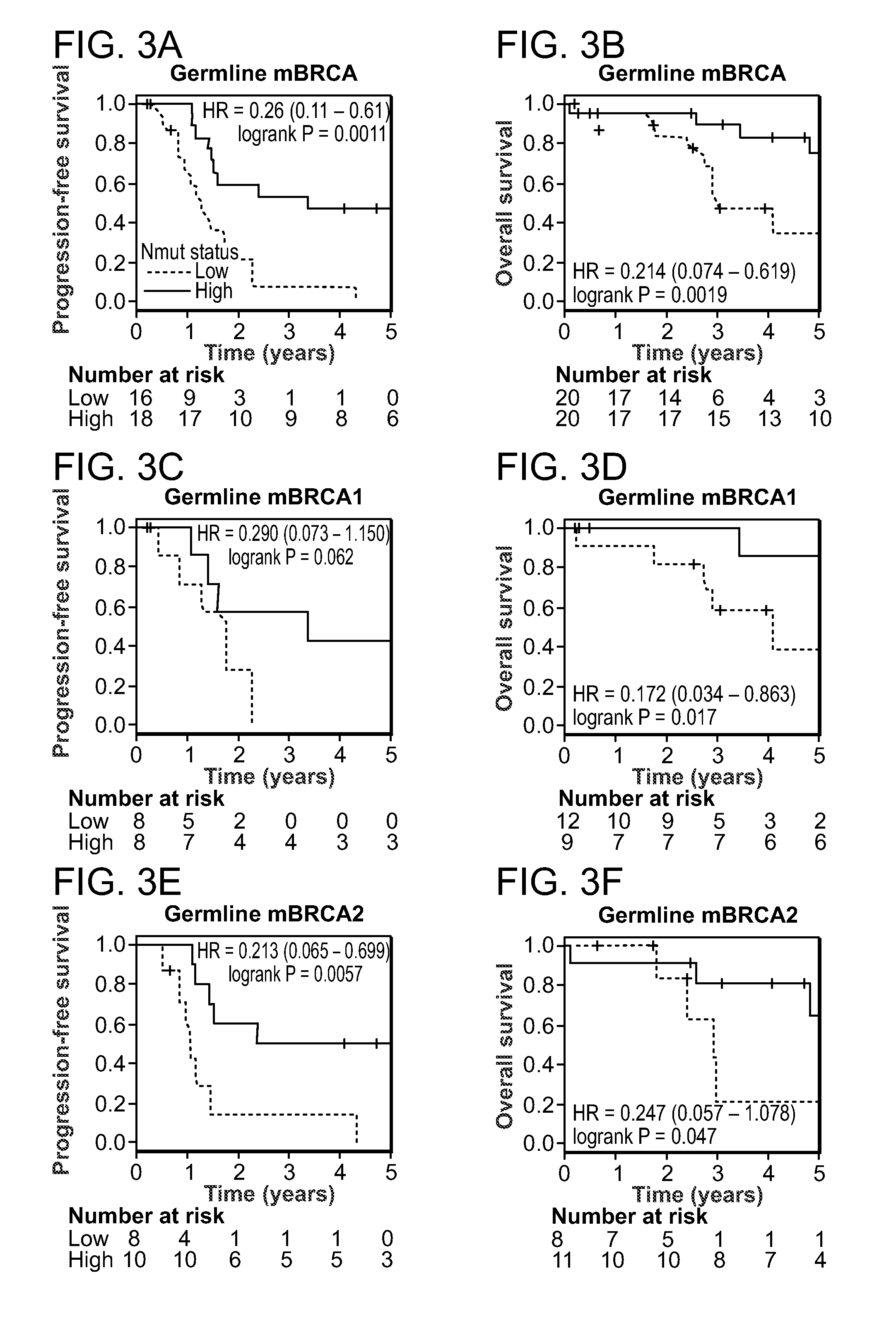
Method of determining cancer prognosis
InactiveUS20150292033A1Increase the burdenLong progression-free survivalMicrobiological testing/measurementLibrary screeningMutationSomatic cell
Provided is a method of predicting the prognosis of a patient with ovarian cancer by determining the total number of somatic exome mutations per genome (Nmut) and status of the BRCA1 and / or BRCA2 in the subject.
Owner:THE BRIGHAM & WOMENS HOSPITAL INC +3
Novel biomarkers for a prediction of the outcome of an immunotherapy against cancer
ActiveUS20120128702A1Long survivalLong progression-free survivalSnake antigen ingredientsLibrary screeningBiologic markerImmunotherapy
The present invention relates to methods for predicting the effect of an immunotherapy against cancer in a patient based on new biomarkers. The present invention furthermore relates to a prognosis regarding the outcome based on said biomarkers. The present invention furthermore relates to panels of biomarkers for use in the above methods.
Owner:IMMATICS BIOTECHNOLOGIES GMBH
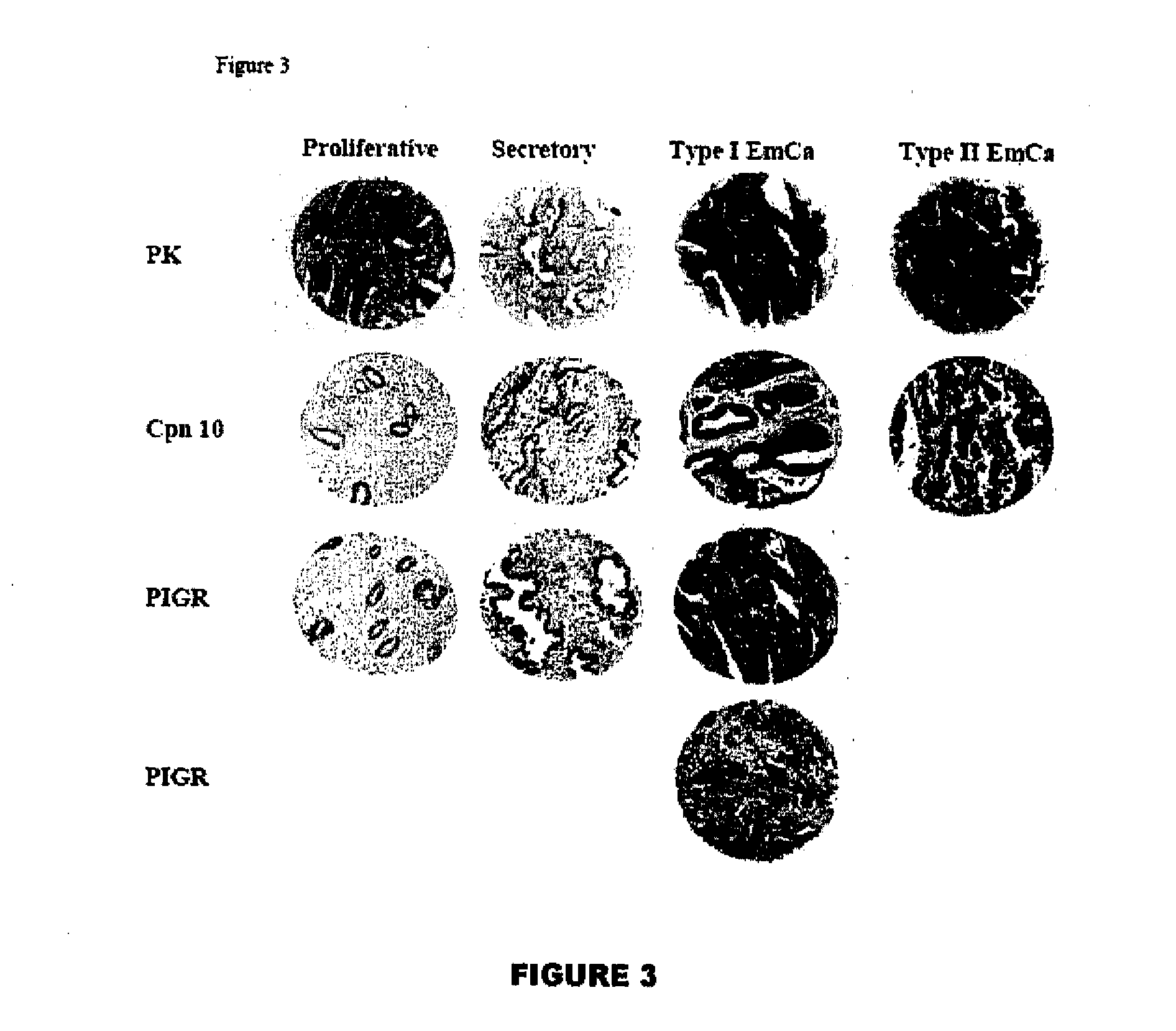
Endometrial biomarkers
Methods for detecting endometrial diseases or an endometrium phase in a subject are described comprising measuring endometrial markers or polynucleotides encoding the markers in a sample from the subject. The invention also provides localization or imaging methods for endometrial diseases, and kits for carrying out the methods of the invention. The invention also contemplates therapeutic applications for endometrial diseases employing endometrial markers, polynucleotides encoding the markers, and / or binding agents for the markers.
Owner:WALFISH PAUL +1
Markers of the Male Urogenital Tract
ActiveUS20130210666A1Positive prognosisLong survivalPeptide librariesNucleotide librariesMale genderMale genitourinary tract
Owner:SINAI HEALTH SYST
Method and Kit for the Prognosis of Mantle Cell Lymphoma
InactiveUS20120225432A1Remarkable differential expression patternImprove accuracyMicrobiological testing/measurementBiological testingMantle lymphomaGene
The method and the kit are useful as tools for classifying a patient diagnosed with mantle cell lymphoma into the category of: indolent or conventional. The method comprises: a) providing a sample from a patient suffering from mantle cell lymphoma; b) determining the level of expression of at least one gene selected from the group consisting of: RNGTT, HDGFRP3, FARP1, HMGB3, LGALS3BP, PON2, CDK2AP1, DBN1, CNR1, CNN3, SOX11, SETMAR and CSNK1E in said sample; and c) comparing the level of expression of each of the measured genes with respect to the level of expression of the same genes in a control sample; wherein the absence of expression or the underexpression of said genes with respect to the same genes in said control sample is indicative of the indolent clinical course of the MCL.
Owner:HOSPITAL CLINIC DE BARCELONA +3
Methods of using biomarkers for predicting the outcome of an immunotherapy against cancer
ActiveUS8669063B2Long survivalLong progression-free survivalSnake antigen ingredientsLibrary screeningBiomarker (petroleum)Immunotherapy
The present invention relates to methods for predicting the effect of an immunotherapy against cancer in a patient based on new biomarkers. The present invention furthermore relates to a prognosis regarding the outcome based on said biomarkers. The present invention furthermore relates to panels of biomarkers for use in the above methods.
Owner:IMMATICS BIOTECHNOLOGIES GMBH
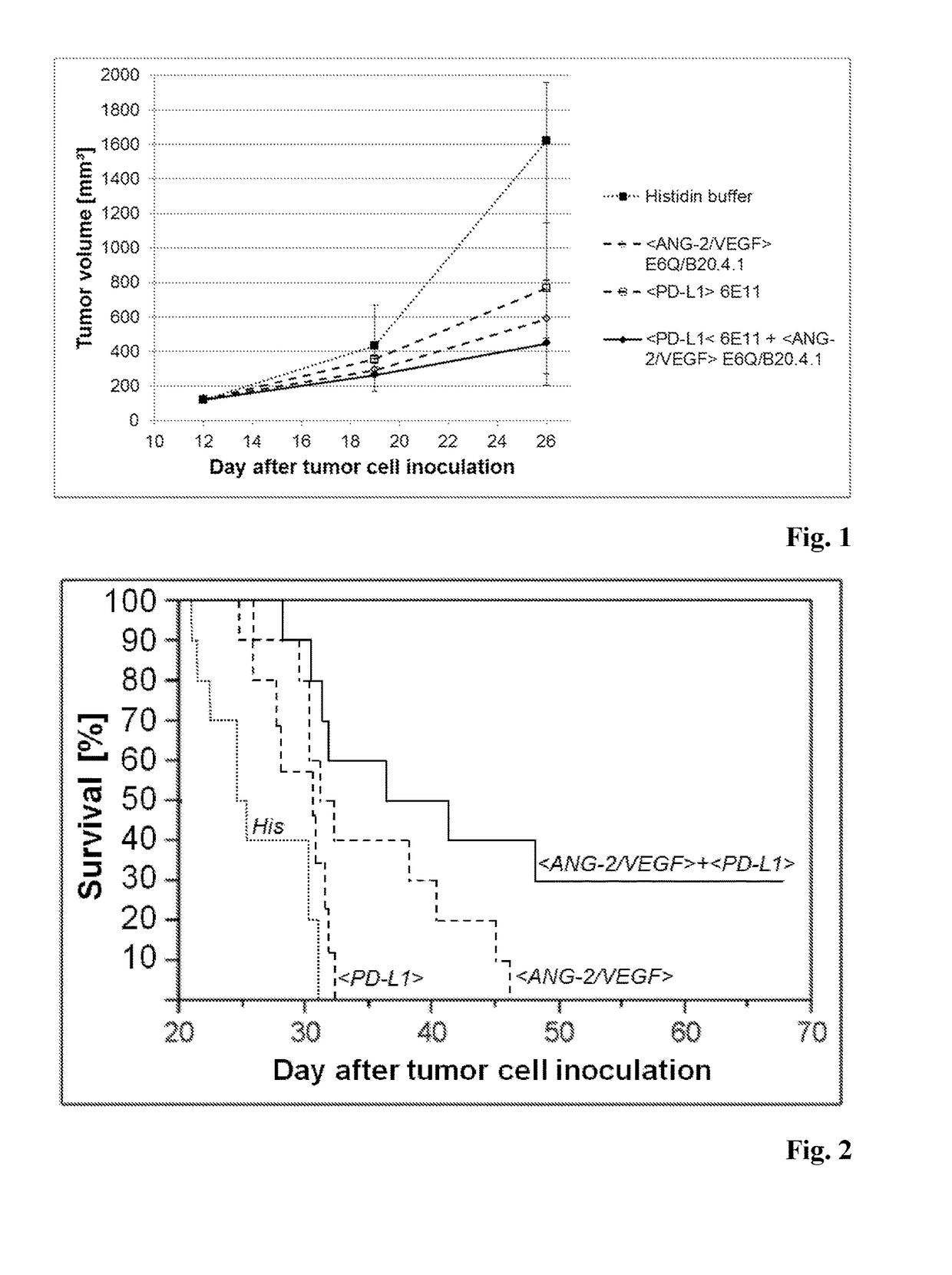
Combination therapy of antibody binding to angiopoietin 2 with antibody binding to programmed death ligand 1
InactiveUS20180155431A1Good curative effectDelay is slowImmunoglobulins against growth factorsImmunoglobulins against cell receptors/antigens/surface-determinantsProgrammed deathPD-L1
The present invention relates to a combination therapy of an antibody specifically binding to Angiopoietin 2 (ANG-2), and an antibody specifically binding to VEGF with an antibody specifically binding to programmed death ligand 1 (PD-L1).
Owner:F HOFFMANN LA ROCHE & CO AG
Method for producing preparations of mature and immature pancreatic endocrine cells, the cell preparation and its use for treatment of diabetes mellitus
InactiveUS20020177228A1Predictable insulin biosynthetic capacityMass productionMetabolism disorderPancreatic cellsDiabetes mellitusMammal
A method for preparing a preparation of mammalian pancreatic endocrine cells comprising the steps: dissociating intact pancreatic tissue into a cell suspension comprising single cells and cell aggregates; enriching said cell suspension with regard to the content in endocrine cells by separating single cells and cellular aggregates with size <100 mum; and removing contaminating non-endocrine cells by density centrifugation.
Owner:BETA CELL
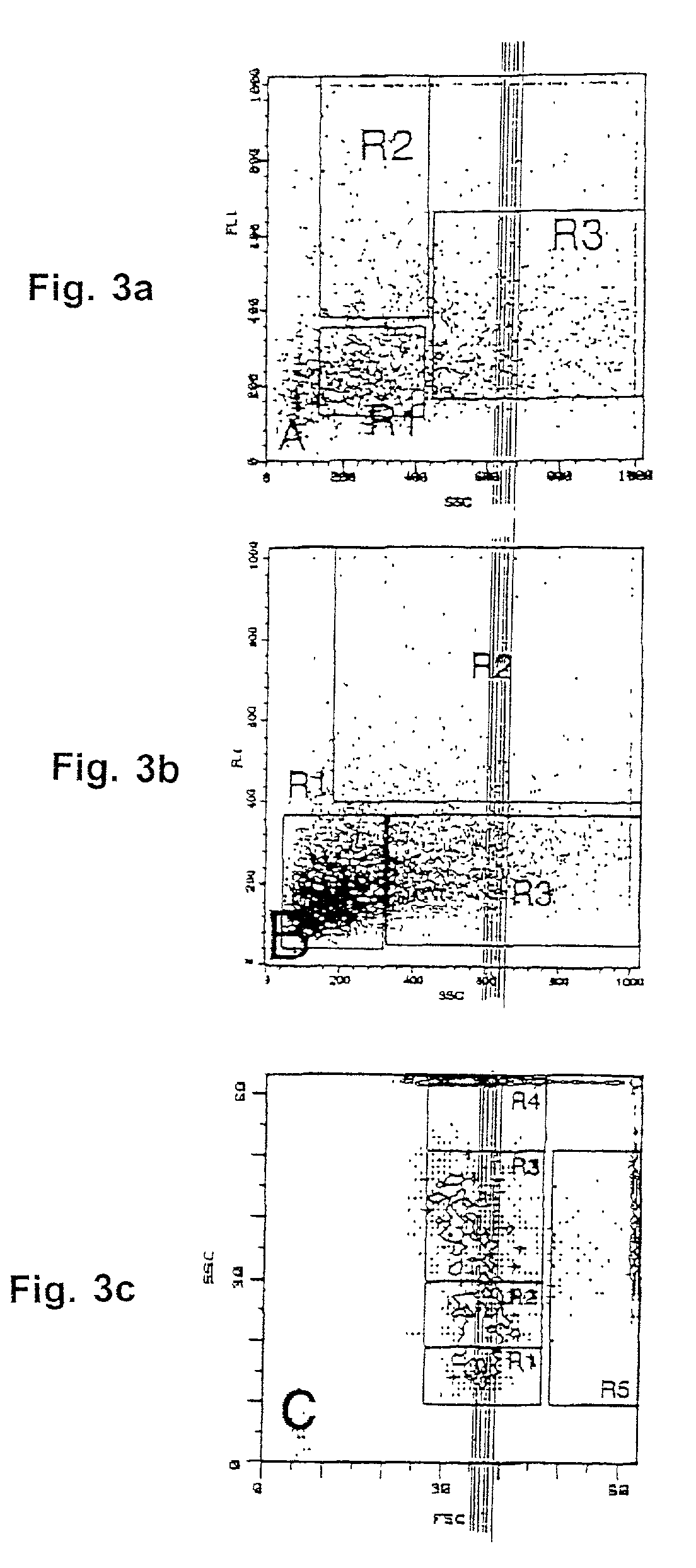
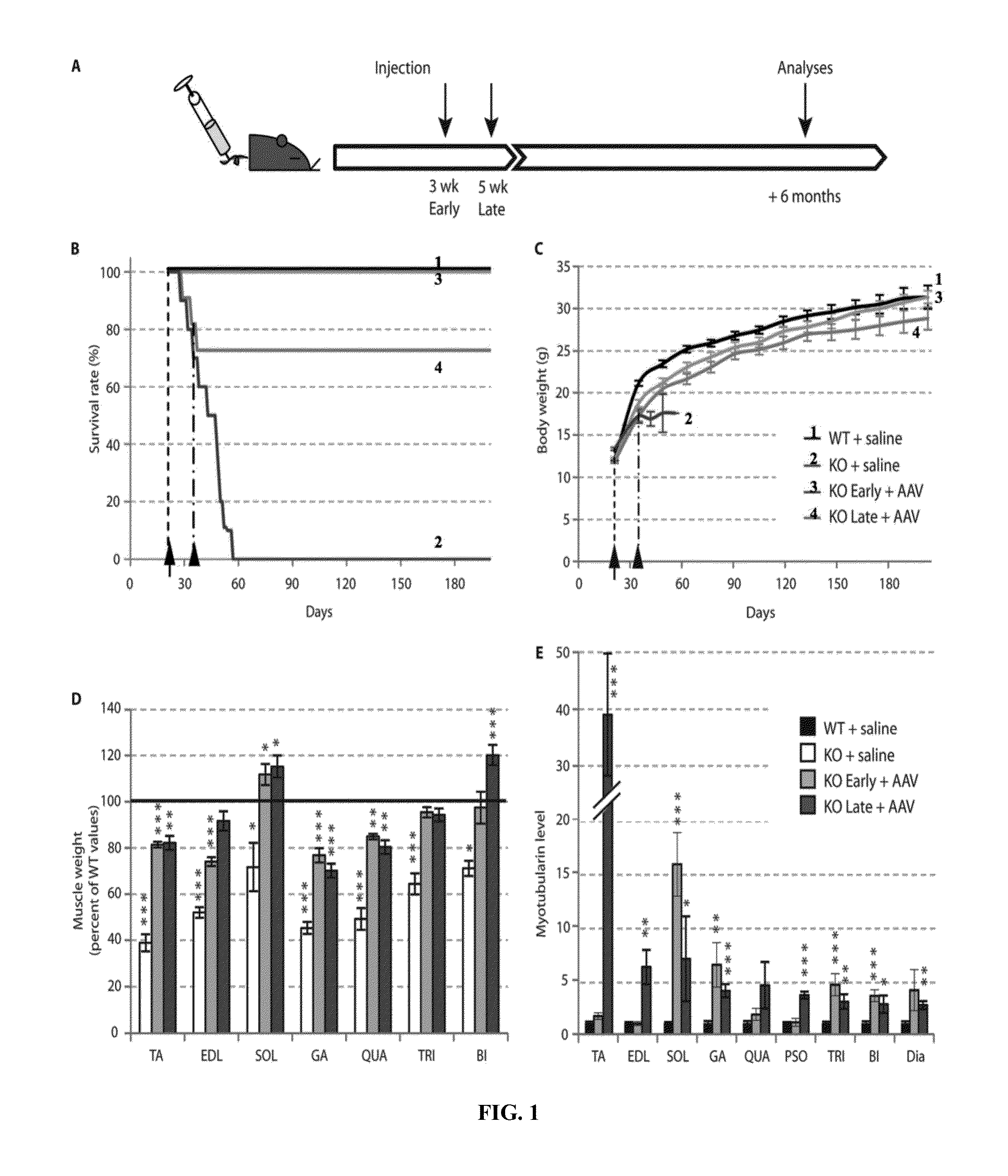
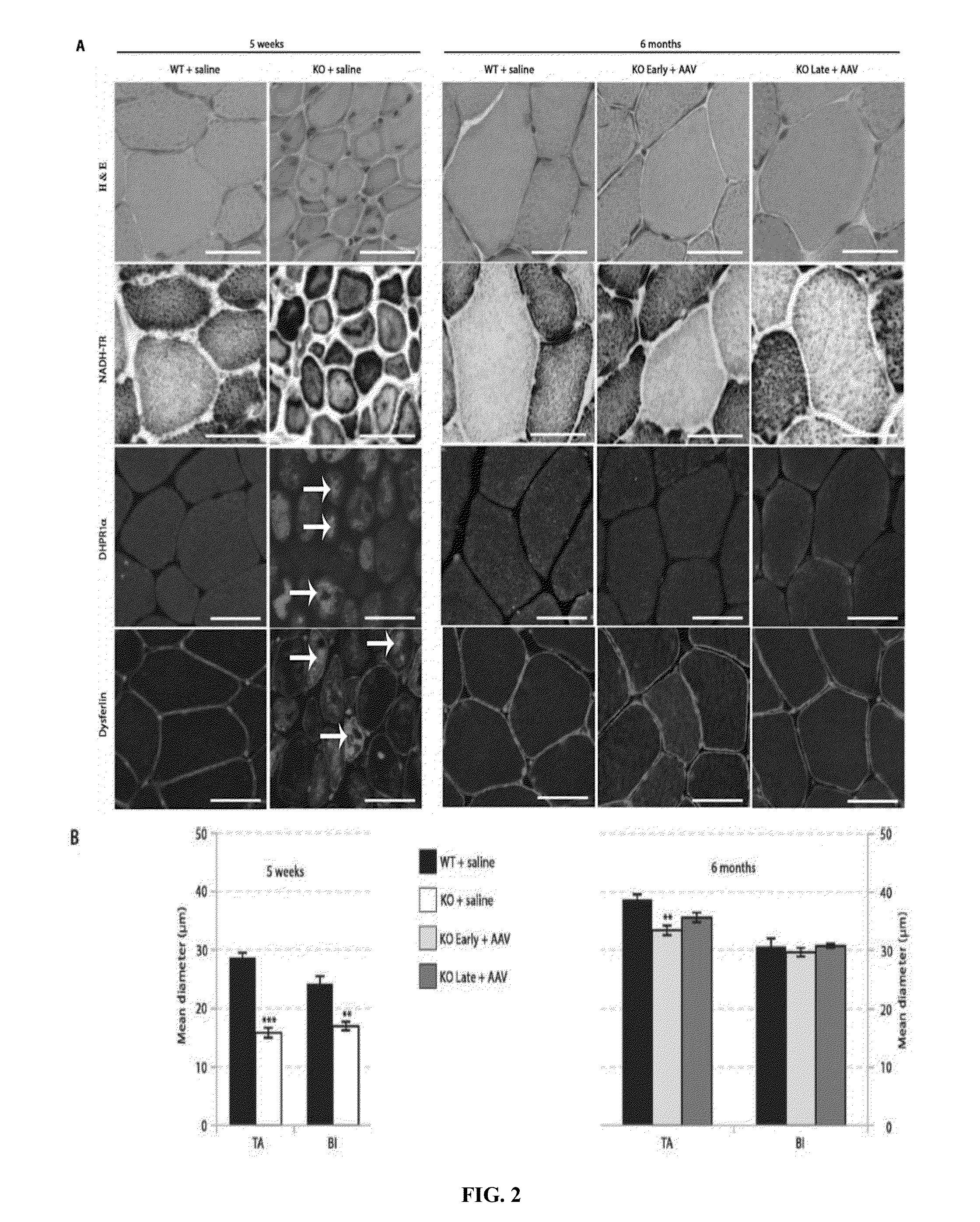
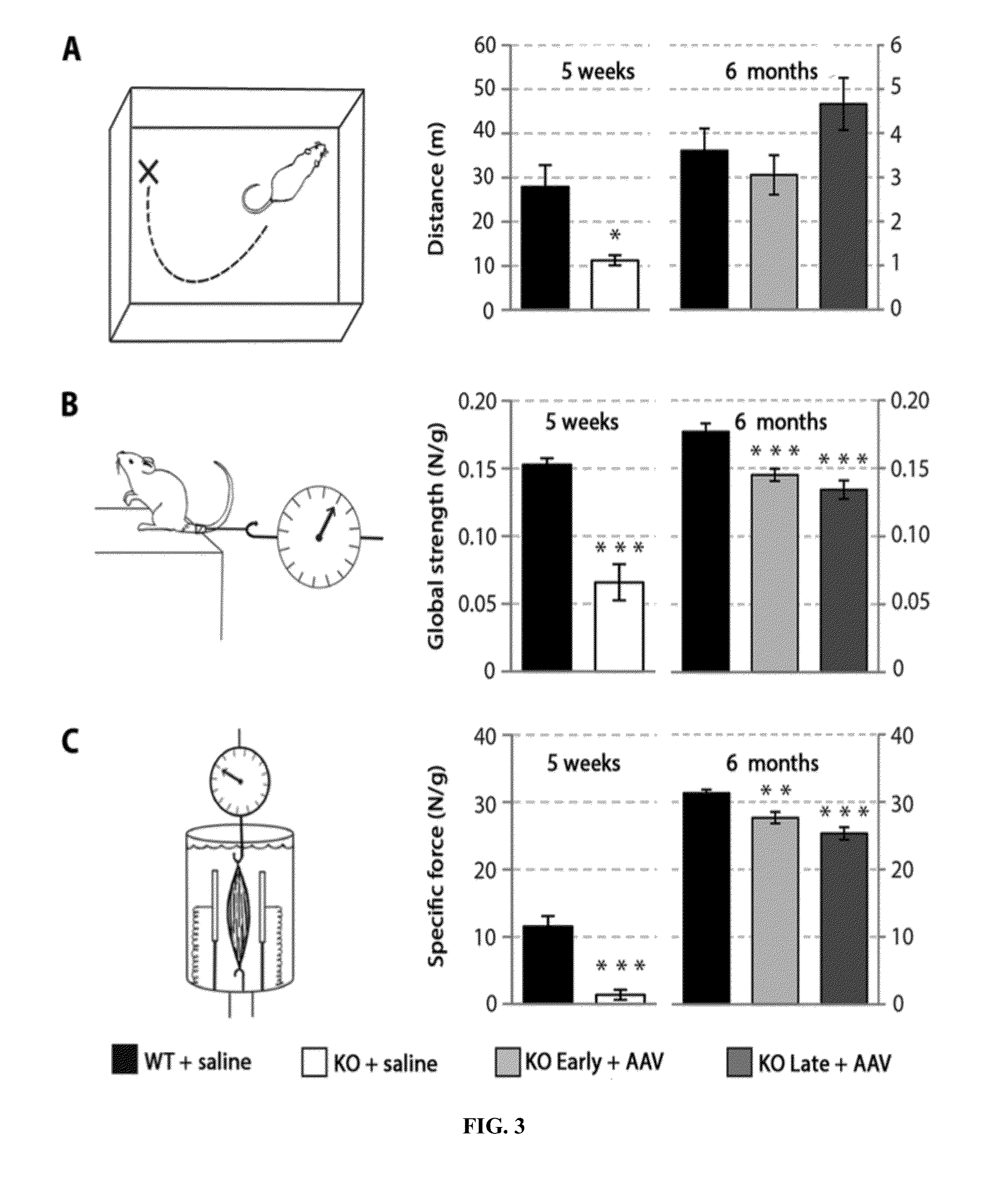
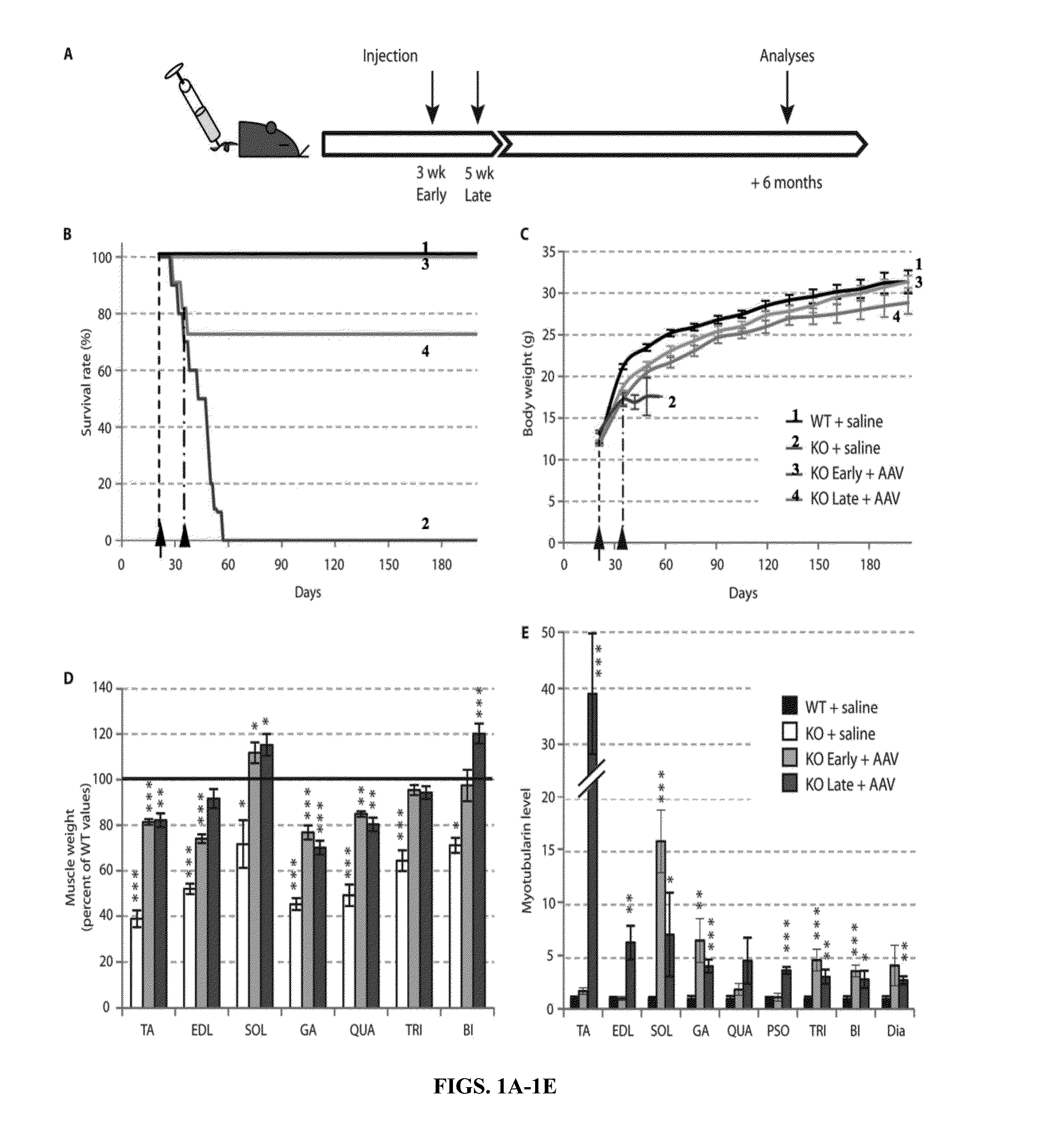
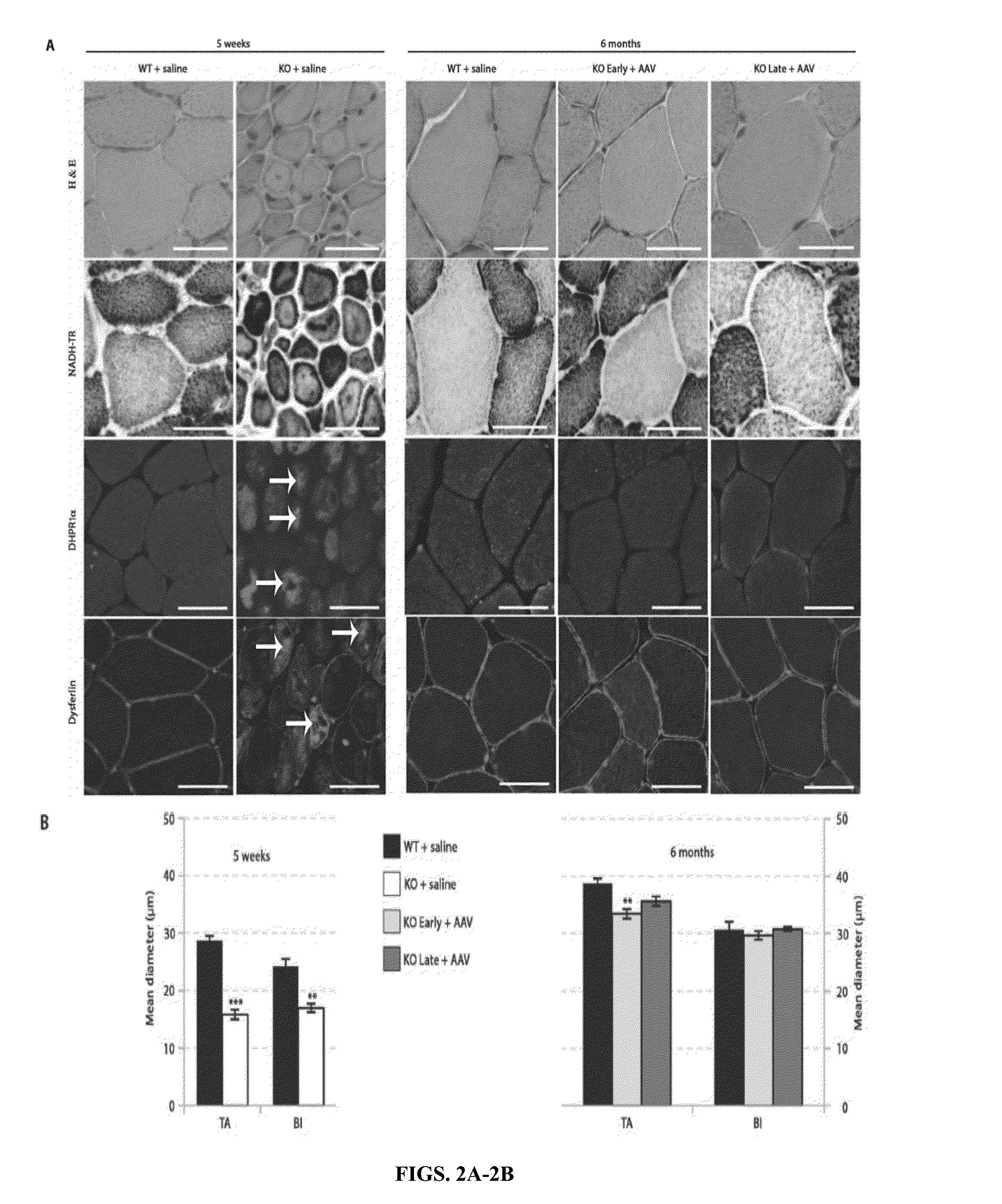
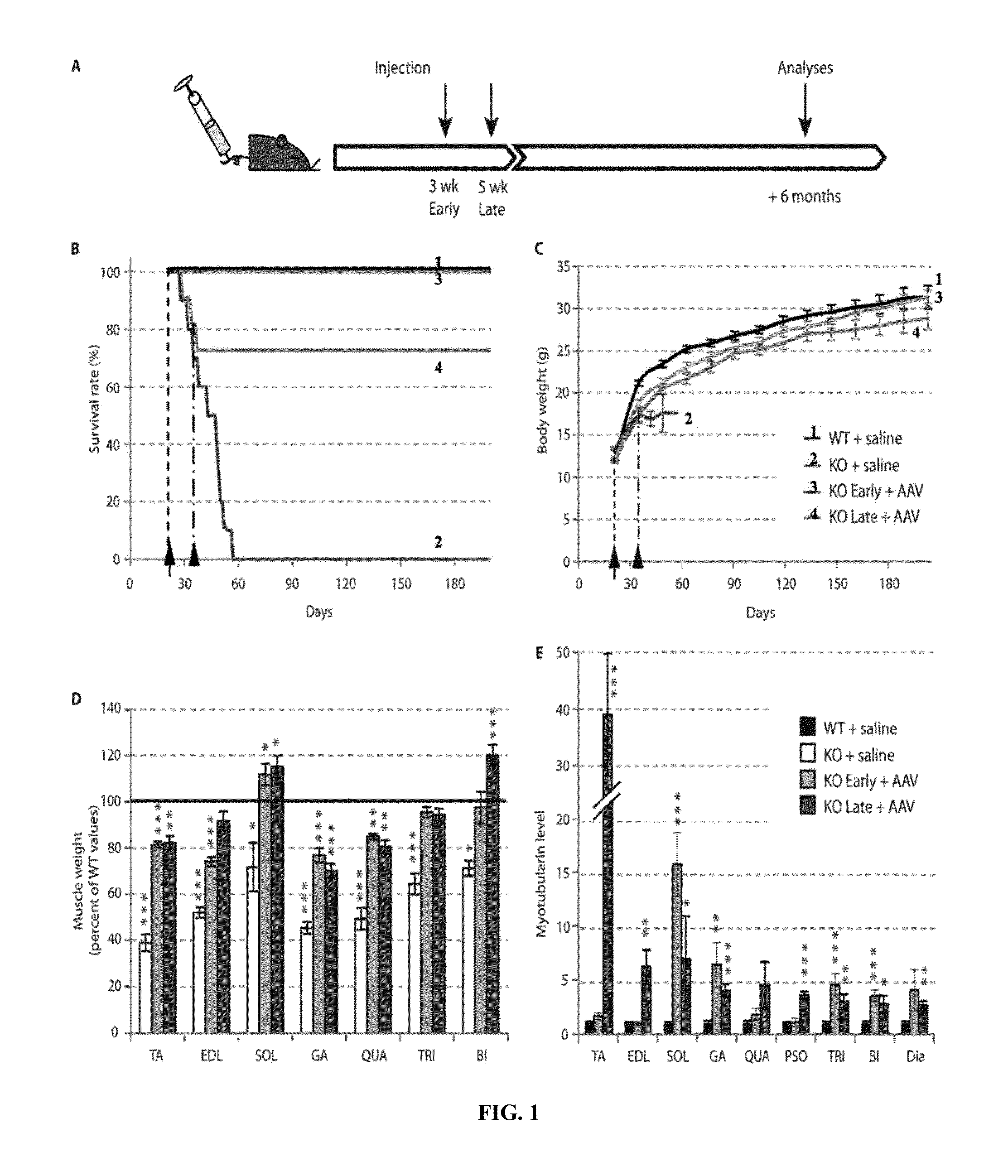
Systemic gene replacement therapy for treatment of X-linked myotubular myopathy (XLMTM)
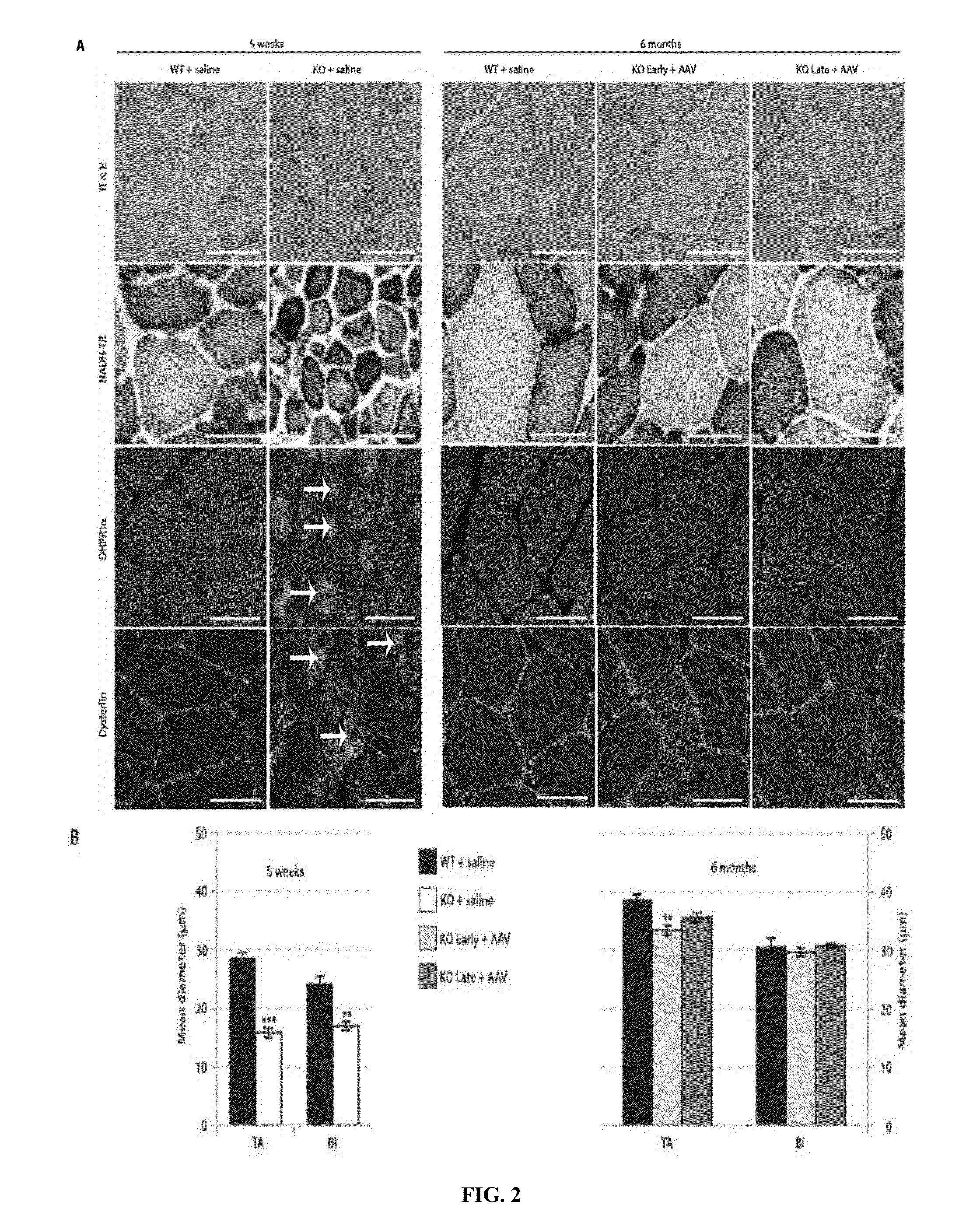
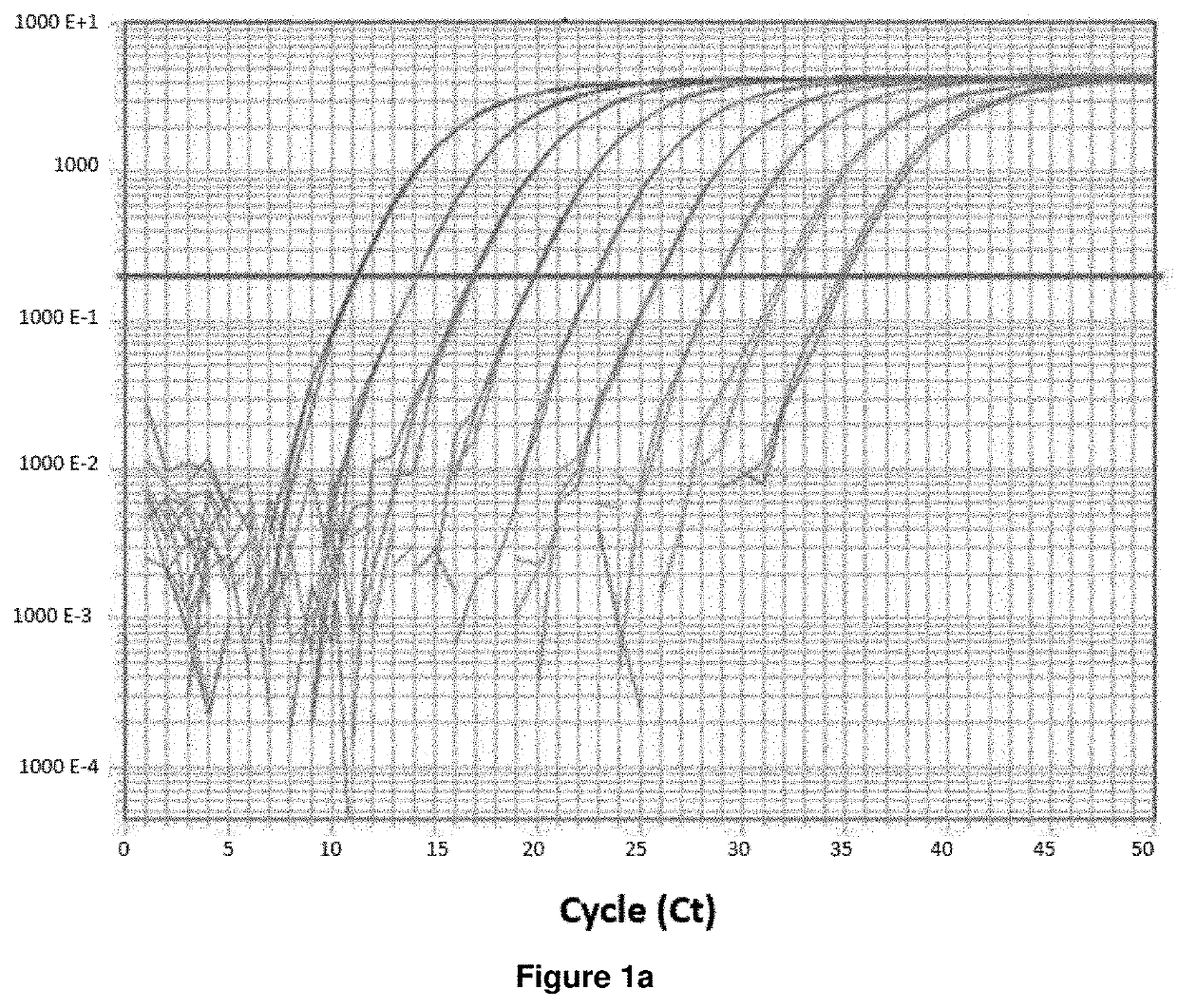
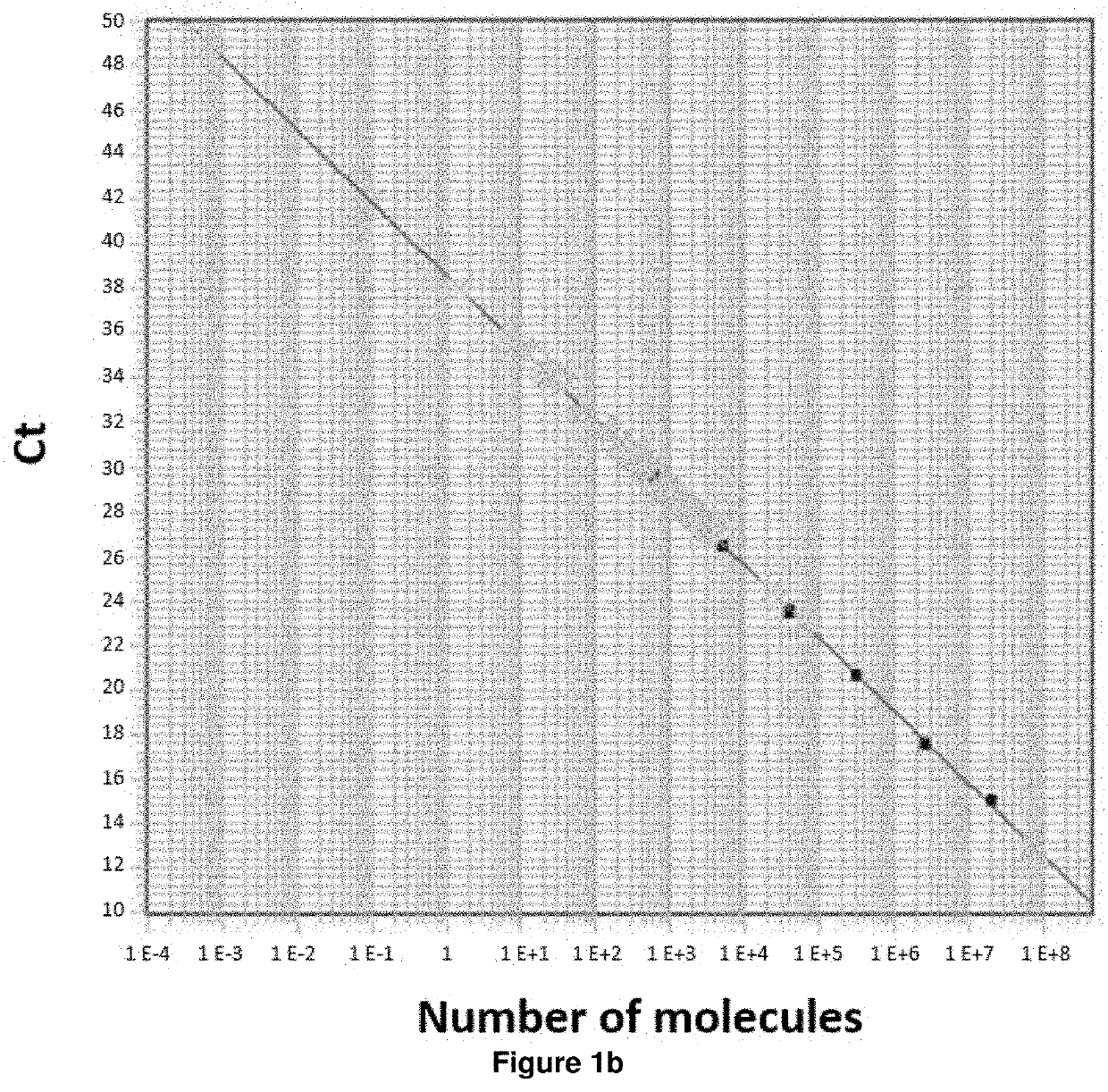
ActiveUS8957044B2Increase in myotubularin expressionSustained increase in strengthSugar derivativesHydrolasesMyopathyWhole body
The present invention provides compositions and methods for treating a myopathy. In certain embodiments, the invention provides compositions and methods for treating, improving muscle function, and prolonging survival in a subject with X-linked myotubular myopathy (XLMTM). The present invention provides a method comprising systemic administration of a composition that induces the increased expression of myotubularin in the muscle of a subject. The invention provides sustained regional and global increases in muscle function.
Owner:GENETHON +2
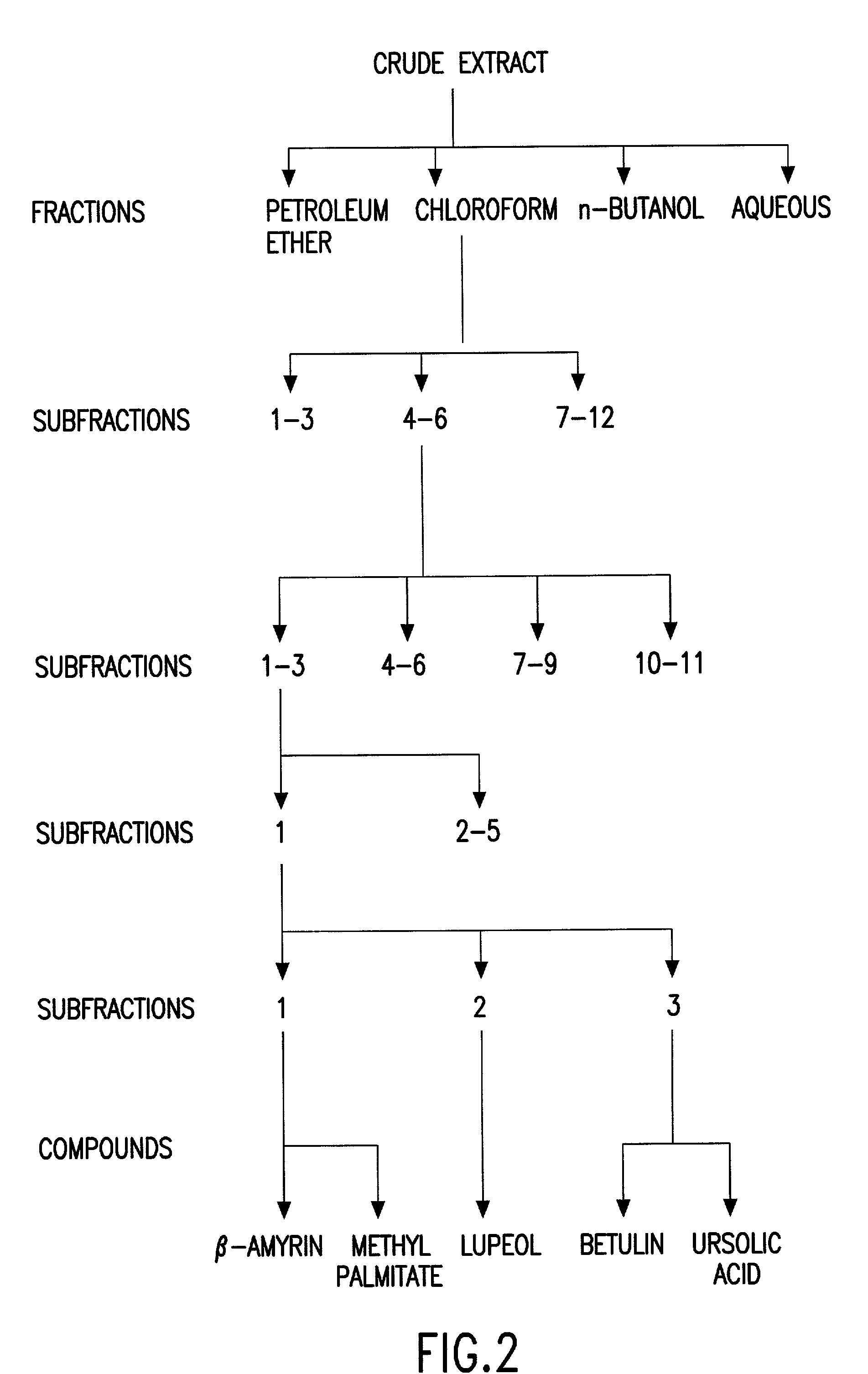
Biologically active chloroform fraction of an extract obtained from a mangroone plant Salvadora persica L
The invention discloses a process of extracting, fractionating and purifying bioactive molecules from an associated mangrove plant, methods of screening for pharmacological activities of crude extract, its fractions and purified compounds and use of the chloroform fraction of the crude extract as anti-spasmodic, anti-arrhythmic and anti-cholinergic agent.
Owner:COUNCIL OF SCI & IND RES
Method for producing preparations of mature and immature pancreatic endocrine cells, the cell preparation and its use for treatment of diabetes mellitus
InactiveUS6686197B2Predictable insulin biosynthetic capacityMass productionMetabolism disorderPancreatic cellsDiabetes mellitusMammal
Owner:BETA CELL
Genotype and Expression Analysis for Use in Predicting Outcome and Therapy Selection
InactiveUS20110178110A1Less aggressive cancer treatmentShort overall survivalOrganic active ingredientsBiocideGastrointestinal cancerGenotype
The invention provides compositions and methods for determining the likelihood of successful treatment with a various treatment regimens available to gastrointestinal cancer patients. After determining if a patient is likely to be successfully treated, the invention also provides methods for treating these patients.
Owner:UNIV OF SOUTHERN CALIFORNIA
Method for treatment of mesothelioma
InactiveUS20140294993A1Survival time is longProlong survival timeBiocideHeavy metal active ingredientsGrowth retardantTreatment choices
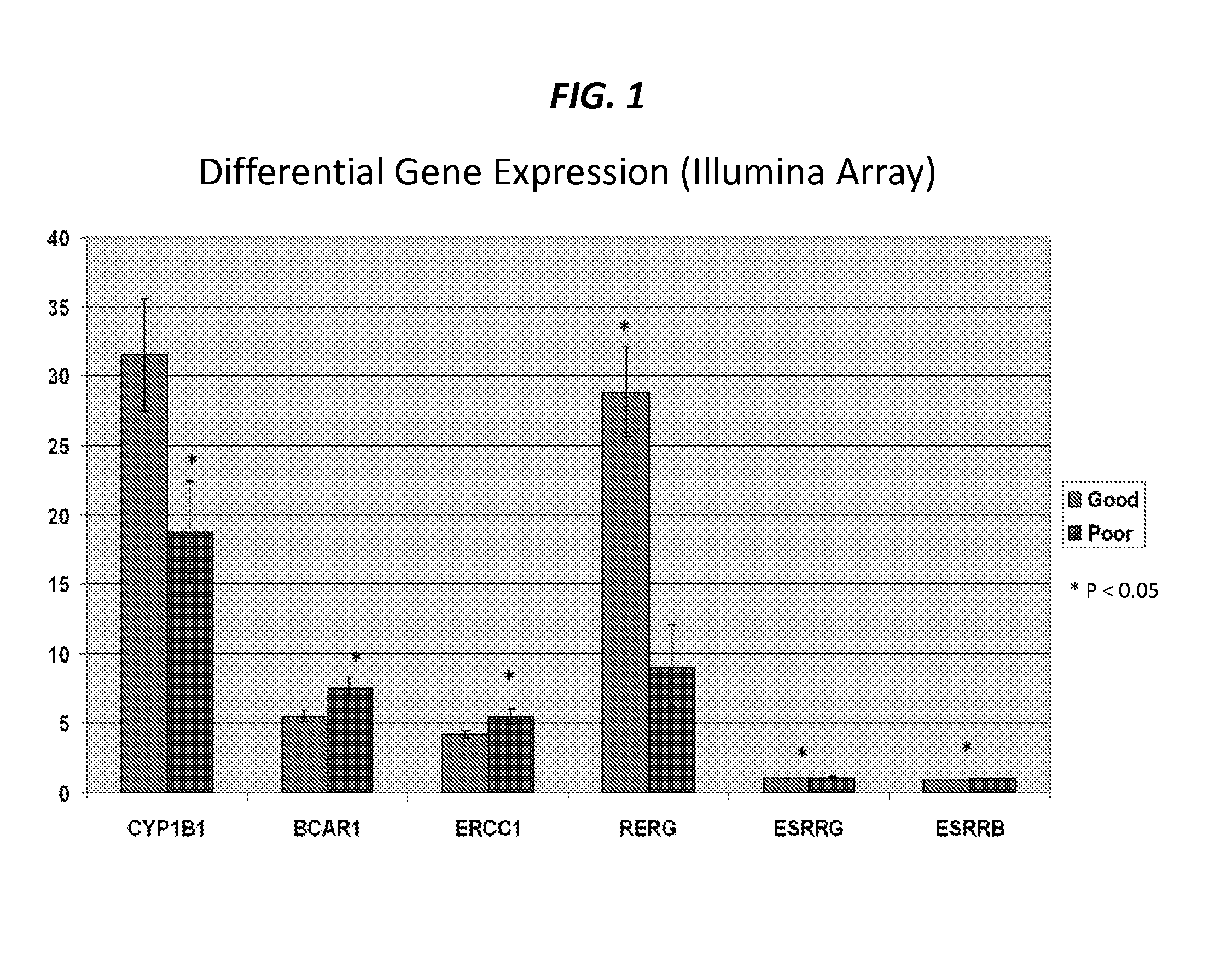
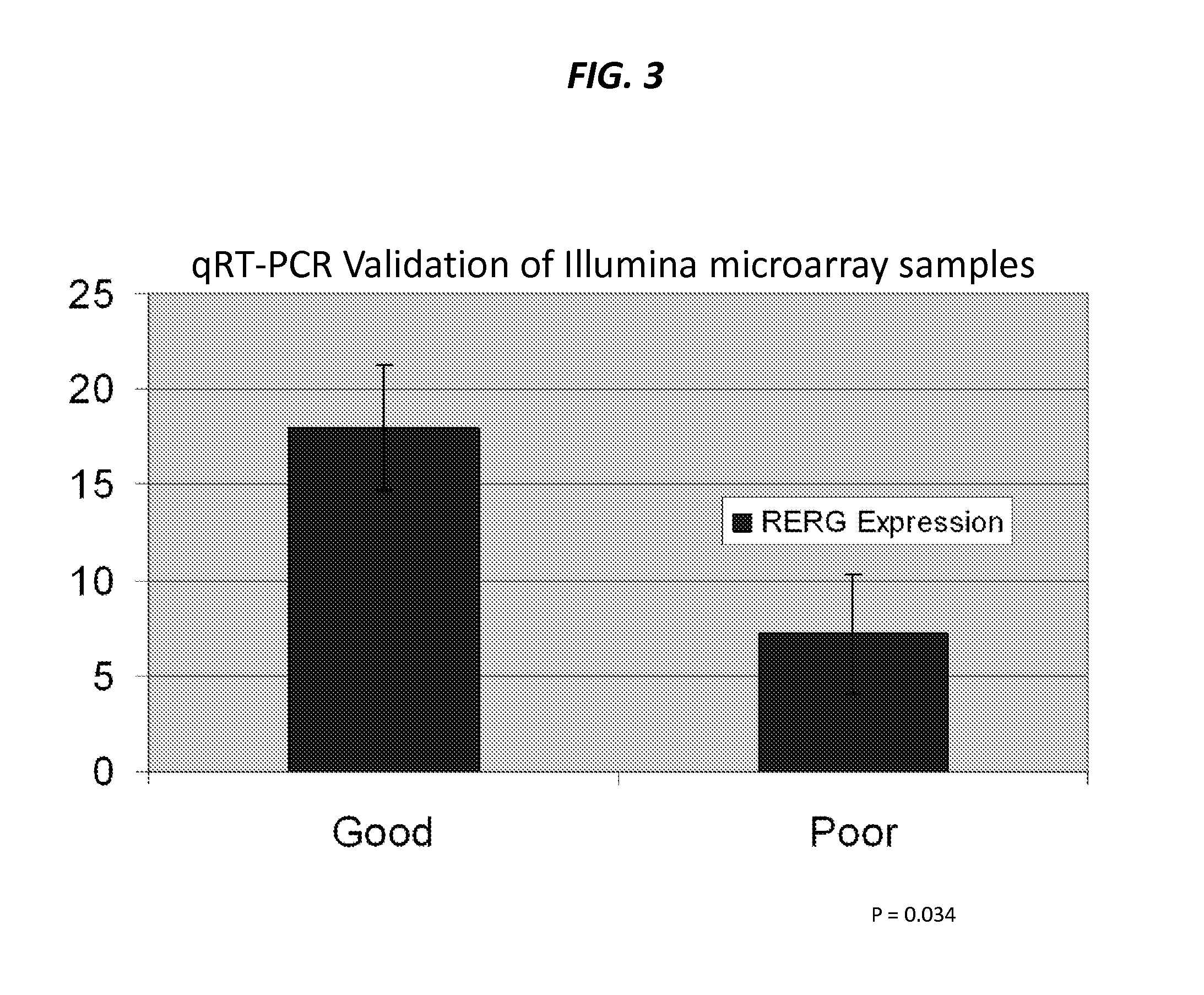
The present invention provides that Ras-like, estrogen-regulated, growth inhibitor (RERG) is a malignant mesothelioma biomarker of clinical course and treatment sensitivity and, itself a target for mesothelioma treatment. A low RERG level in a mesothelioma subject indicates poor prognosis. Analyzing RERG expression level along can help mesothelioma patients make treatment choices. Furthermore, mesothelioma can be treated by modulating RERG activity, for example, with treatment with estrogen or estrogen-like agents.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC
Systemic Gene Replacement Therapy for Treatment of X-Linked MyoTubular Myopathy (XLMTM)
ActiveUS20150258215A1Increase in myotubularin expressionSustained increase in strengthPeptide/protein ingredientsHydrolasesMyopathyWhole body
Owner:WAKE FOREST UNIV HEALTH SCI INC +2
Cancer prognostic diagnostic and treatment methods
InactiveUS20090324595A1Increased EphB expressionProlong survival timeMicrobiological testing/measurementAntibody ingredientsOncologyPolynucleotide
The invention disclosed herein provides methods comprising detection of EphB2 polypeptide and / or polynucleotide in a biological sample from a subject, wherein the detection of EphB2 is predictive or indicative of cancer prognosis for the subject. The invention also provides methods for selecting cancer treatment, methods comprising detection of EphB2 polypeptide and / or polynucleotide expression in colon adenomas, and methods for treating a colon adenoma disorder. Kits, compositions, and articles of manufacture are also provided.
Owner:GENENTECH INC
Novel Protein With Anti-Inflammatory Properties
ActiveUS20190382457A1Drop in level of CRPLong survivalPeptide/protein ingredientsSkeletal disorderDiseaseAmino acid
The present invention provides an isolated or recombinant protein consisting of the amino acid sequence according to SEQ ID NO: 3 or SEQ ID: NO: 4 and its use in the prevention or treatment of an inflammatory condition.
Owner:REVOLO BIOTHERAPEUTICS LTD
Mycobacterium mutants for vaccines with improved protective efficacy
InactiveUS8685415B2Improved vaccine efficiencyGood curative effectAntibacterial agentsBiocideAbnormal macrophageMannosyltransferase
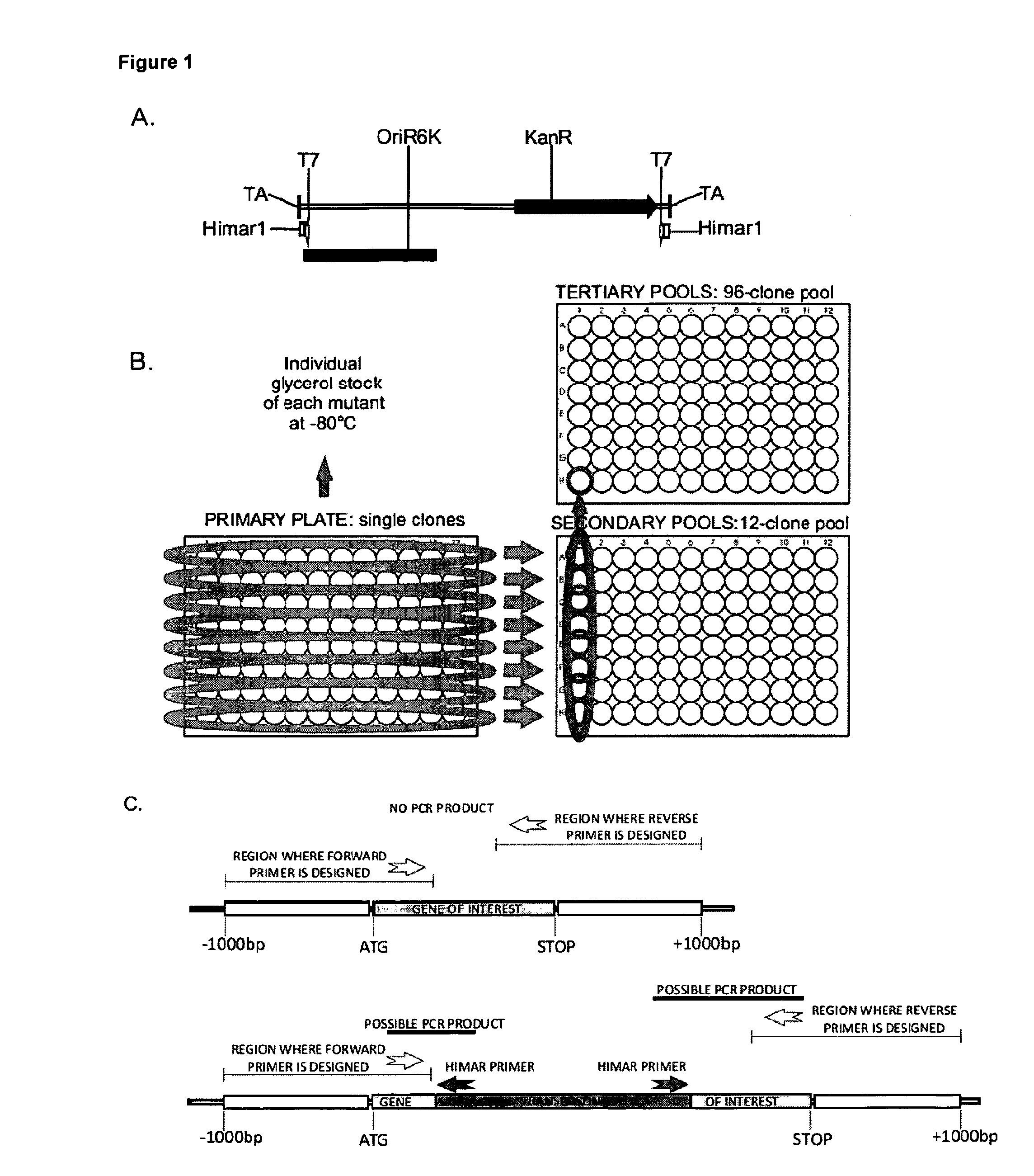
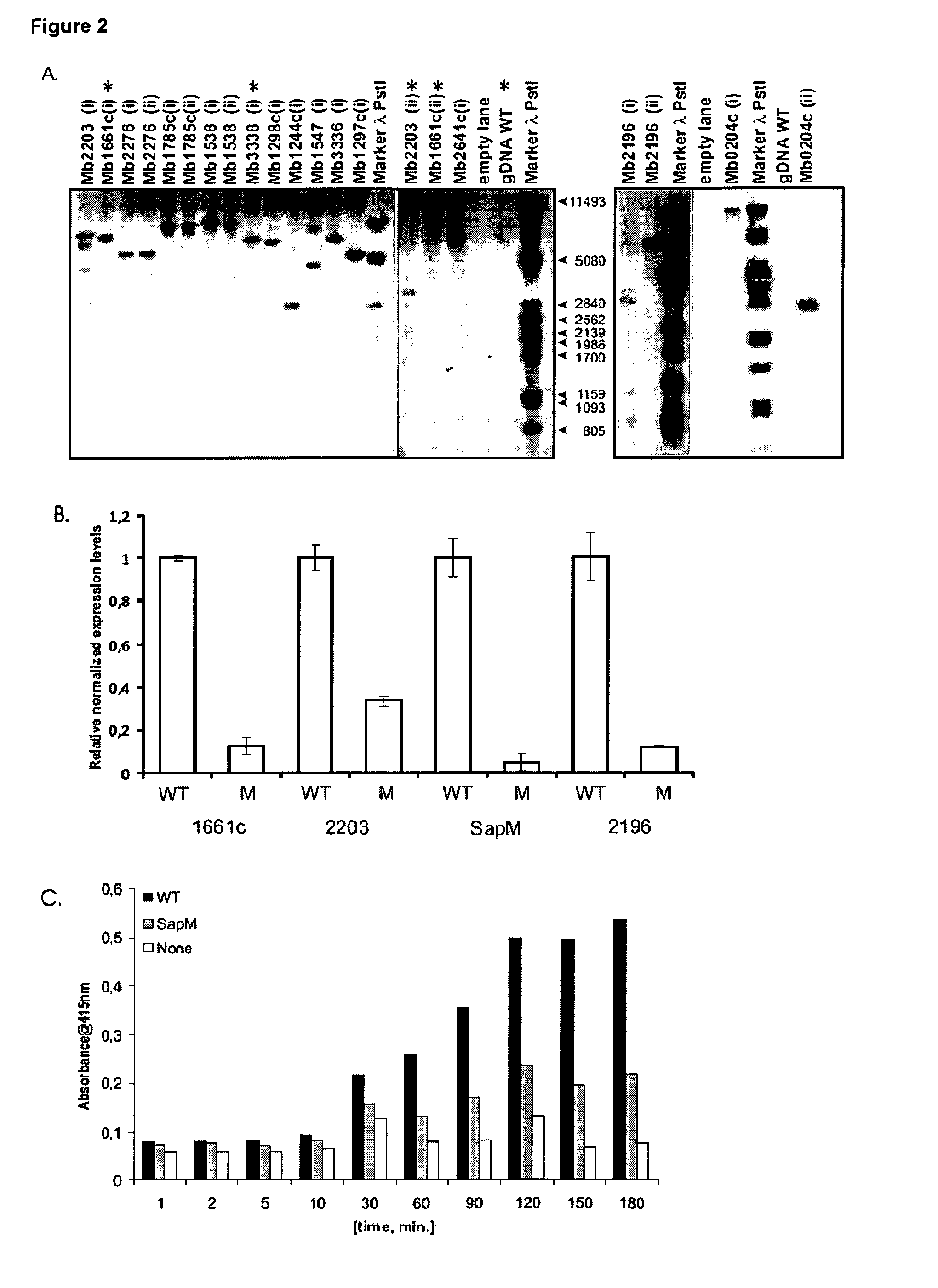
Tuberculosis (TB) is a major health problem and currently, the only licensed TB vaccine is Mycobacterium bovis Bacille Calmette-Guerin (M. bovis BCG). In the present invention, mutation of mycobacterial components reportedly involved in phagosome maturation inhibition was evaluated for vaccine purposes, as such mutations should result in better vaccine antigen processing and presentation. Thus, BCG mutants in genes coding for ManLAM capping α-1,2-mannosyltransferases and the PI3P phosphatase SapM were evaluated as TB vaccines in a stringent mouse model. Vaccination with both ManLAM capping mutants and the SapM mutant resulted in significantly longer survival as compared to non-vaccinated mice, whereas vaccination with the parental BCG did not. Moreover, mice vaccinated with the SapM mutant survived significantly longer than mice vaccinated with the parental BCG. The mutant BCG strains showed unaltered phagocytosis, replication, lysosome colocalization and oxidant activity in macrophages and similarly induced autophagy in the latter. Additionally, replication and granuloma formation in mice was unaffected, indicating BCG-equivalent safety of these vaccines.
Owner:VLAAMS INTERUNIVERSITAIR INST VOOR BIOTECHNOLOGIE VZW +2
Biologically active chloroform fraction of an extract obtained from a mangrove plant Salvadora persica L
InactiveUS20020172731A1Reduce after tasteHigh sweetnessBiocideUnknown materialsSalvadora persicaAnticholinergic agents
The invention discloses a process of extracting, fractionating and purifying bioactive molecules from an associated mangrove plant, methods of screening for pharmacological activities of crude extract, its fractions and purified compounds and use of the chloroform fraction of the crude extract as anti-spasmodic, anti-arrhythmic and anti-cholinergic agent.
Owner:COUNCIL OF SCI & IND RES
Systemic Gene Replacement Therapy for Treatment of X-Linked MyoTubular Myopathy (XLMTM)
ActiveUS20140249211A1High expressionIncrease in myotubularin expressionPeptide/protein ingredientsHydrolasesMyopathyWhole body
The present invention provides compositions and methods for treating a myopathy. In certain embodiments, the invention provides compositions and methods for treating, improving muscle function, and prolonging survival in a subject with X-linked myotubular myopathy (XLMTM). The present invention provides a method comprising systemic administration of a composition that induces the increased expression of myotubularin in the muscle of a subject. The invention provides sustained regional and global increases in muscle function.
Owner:GENETHON +2
Method of determining prognosis in patients with follicular lymphoma
PendingUS20210388448A1Shorten survival timeEasy to getMicrobiological testing/measurementGenetic engineeringFollicular lymphoma grade IIOncology
A method is disclosed for determining prognosis for a patient suffering from follicular lymphoma, that determines the amount of miR-31 in a biological sample taken from the body of the patient, and assigns the patient to a prognostic group based on the determined amount of miR-31, wherein the prognostic groups and the threshold values for assignment to the prognostic groups are obtained by analyzing the amount of miR-31 in biological samples of patients with known prognosis. In the biological sample taken from the patient, the miR-31 expression may be determined along with the expression of an endogenous control, which is a small nuclear RNA or stably expressed miRNA, and, in case of absolute quantification, the expression of synthetic standards of these miRNAs and endogenous controls of known number of molecules are determined. The method allows the assignment of patients to prognostic groups by determining miR-31 expression.
Owner:MASARYK UNIVERSITY +1
Mutations in pancreatic neoplasms
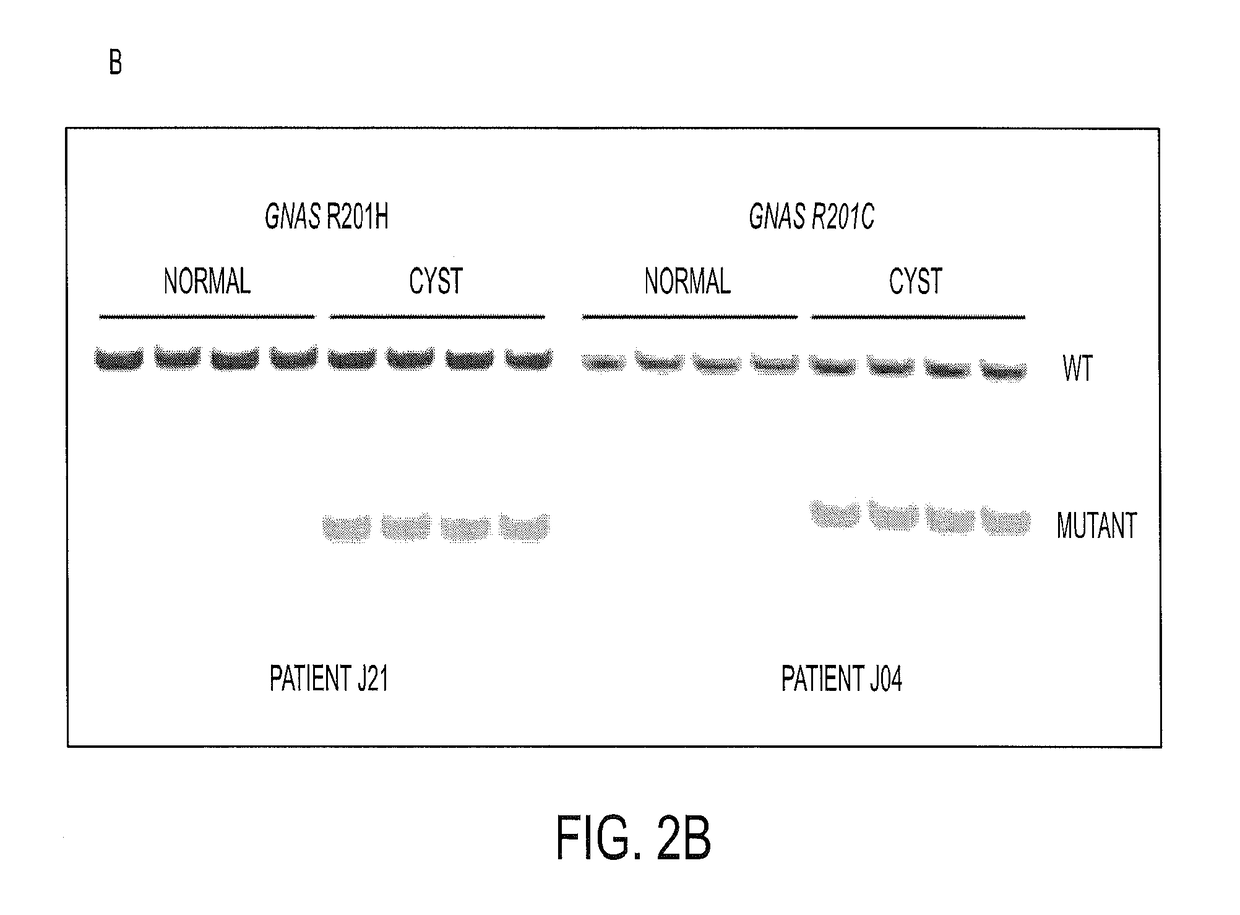
To help reveal the pathogenesis of these lesions, we purified the DNA from Intraductal Papillary Mucinous Neoplasm (IPMN) cyst fluids from 19 patients and searched for mutations in 169 genes commonly altered in human cancers. We identified recurrent mutations at codon 201 of GNAS. We found that GNAS mutations were present in 66% of IPMNs and that either KRAS or GNAS mutations could be identified in 96%. In eight cases, we could investigate invasive adenocarcinomas that developed in association with IPMNs containing GNAS mutations. In seven of these eight cases, the GNAS mutations present in the IPMNs were also found in the invasive lesion. GNAS mutations were not found in other types of cystic neoplasms of the pancreas or in invasive adenocarcinomas not associated with IPMNs. These data suggest that GNAS mutations can inform the diagnosis and management of patients with cystic pancreatic lesions.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Profiling and treatment of myc-associated cancers
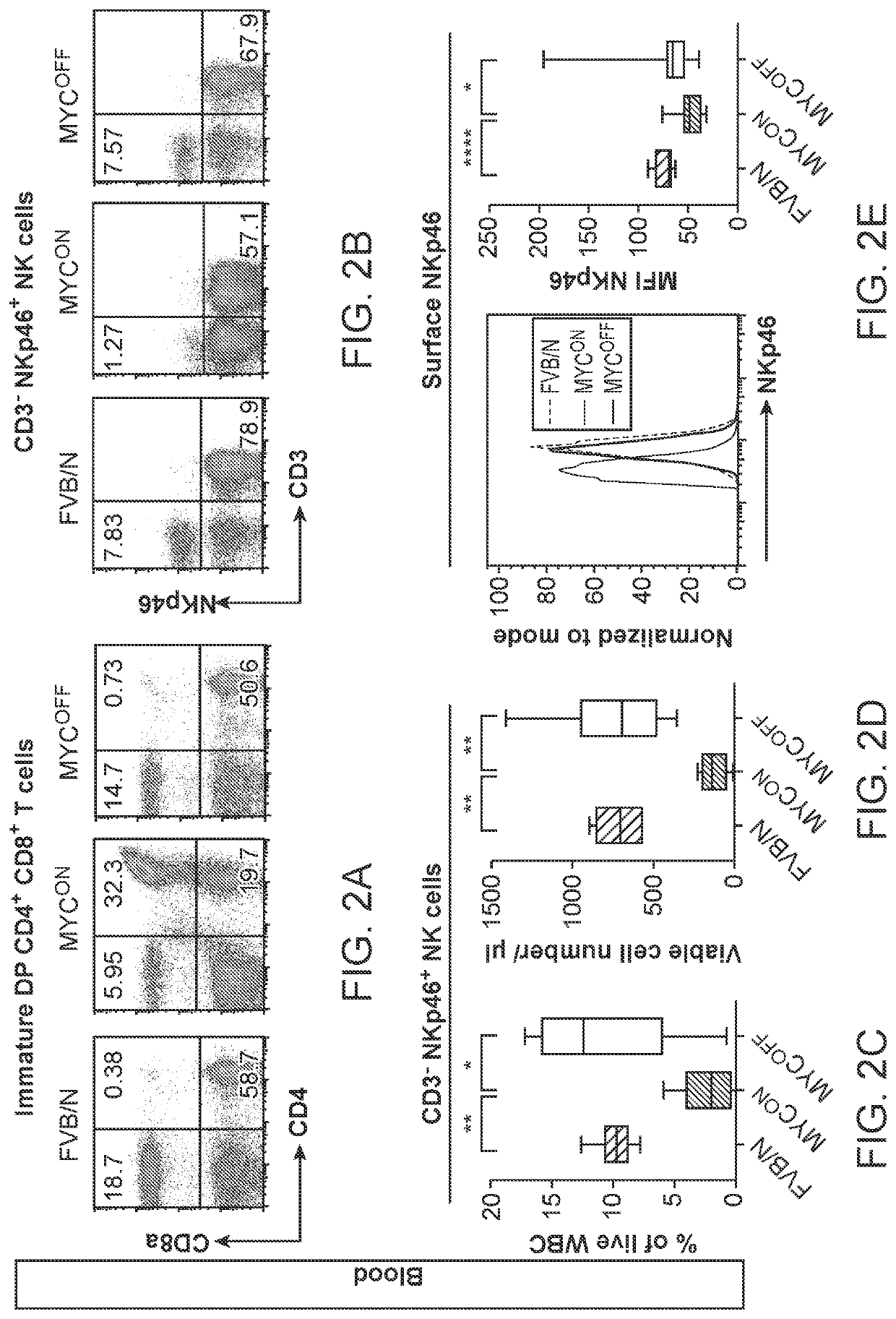
ActiveUS20200353000A1Long progression-free survivalLong survivalPeptide/protein ingredientsMammal material medical ingredientsOncologyChromatosome
Compositions and methods are provided for classification and treatment of MYC-driven cancers, i.e. causally dependent on MYC as a result of, over-expression of MYC, constitutive expression of MYC, chromosomal translocation resulting in overactive MYC, and the like. Specifically, the methods comprising determining the MYC status of the cancer, and in a cancer that is determined to be driven by MYC activation, administering a composition of an effective dose of one or both of activated natural killer (NK) cells and a type 1 interferon.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Neoadjuvant treatment of breast cancer
InactiveUS20060160755A1Effective procedureLong survivalBiocideCarbohydrate active ingredientsTaxaneOncology
Owner:MANITOBA UNIV OF THE
Protein with anti-inflammatory properties
ActiveUS10858409B2Long survivalDiminishment of extentPeptide/protein ingredientsSkeletal disorderAmino acidProtein
The present invention provides an isolated or recombinant protein consisting of the amino acid sequence according to SEQ ID NO: 3 or SEQ ID: NO: 4 and its use in the prevention or treatment of an inflammatory condition.
Owner:REVOLO BIOTHERAPEUTICS LTD
Method for assessing the response to PD-1/PDL-1 targeting drugs
ActiveUS11293066B2Good for healthMinimizing spread and worseningMicrobiological testing/measurementPharmaceutical drugBiochemistry
Owner:INSTITUT GUSTAVE ROUSSY
Endometrial Phase or Endometrial Cancer Biomarkers
Methods for detecting endometrial diseases or an endometrium phase in a subject are described comprising measuring endometrial markers or polynucleotides encoding the markers in a sample from the subject. The invention also provides localization or imaging methods for endometrial diseases, and kits for carrying out the methods of the invention. The invention also contemplates therapeutic applications for endometrial diseases employing endometrial markers, polynucleotides encoding the markers, and / or binding agents for the markers.
Owner:WALFISH PAUL +1
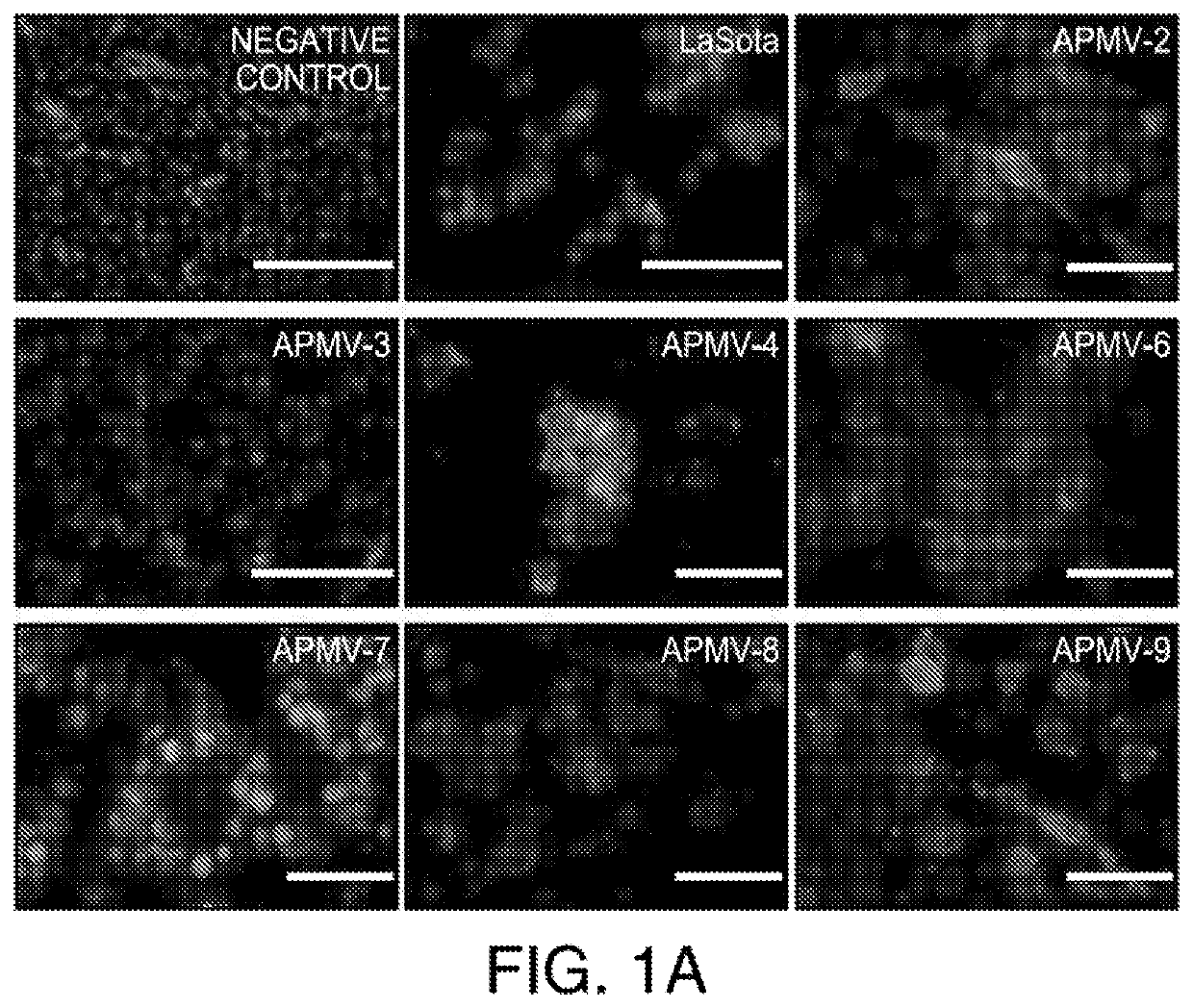
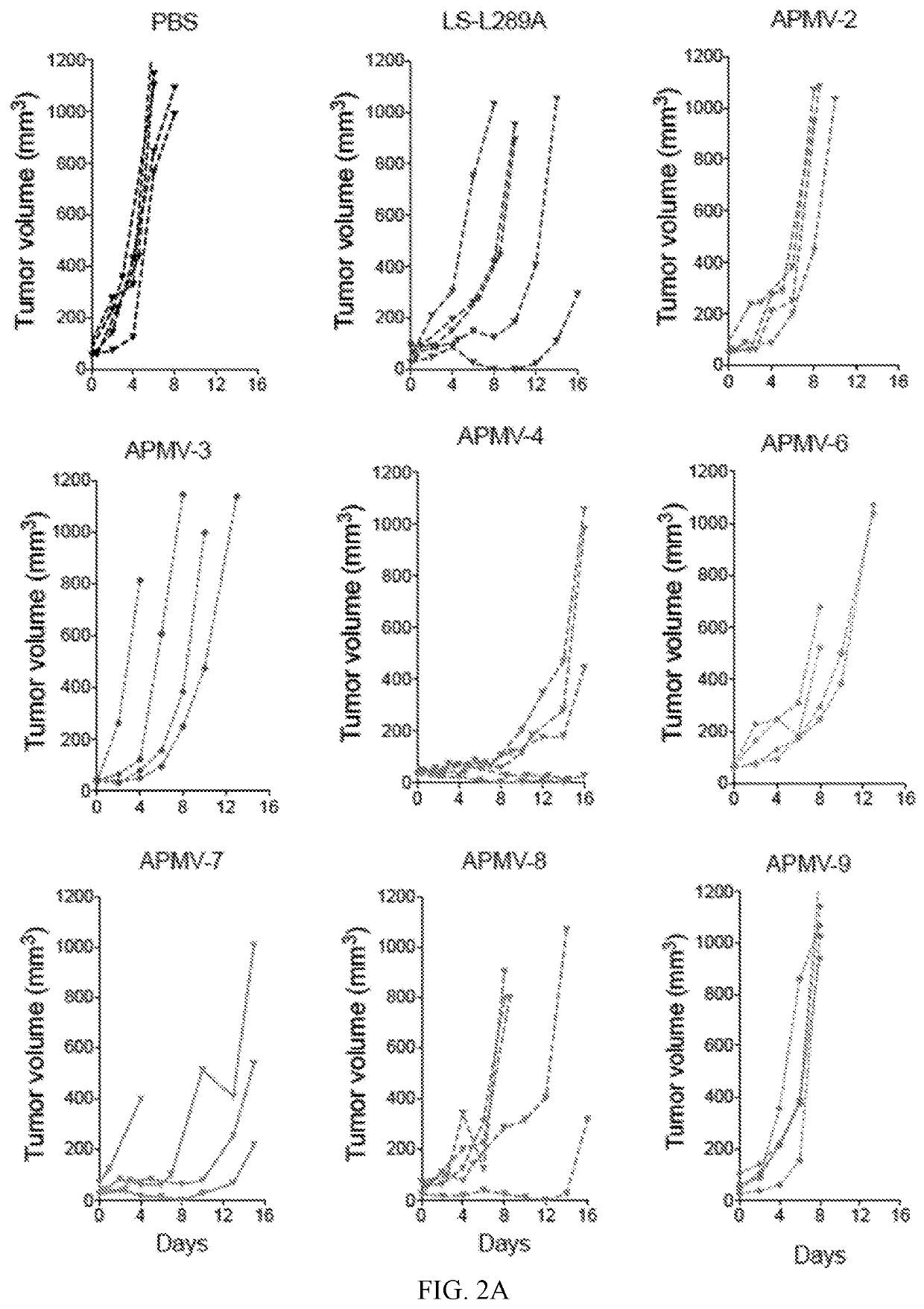
Apmv and uses thereof for the treatment of cancer
InactiveUS20200297787A1Slow tumor growthImprove survivalSsRNA viruses negative-sensePeptide/protein ingredientsAvian paramyxovirusSerotype
In one aspect, provided herein are naturally occurring and recombinantly produced avian paramyxovirus (APMV) (e.g., an APMV-2, APMV-3, APMV-4, APMV-6, APMV-7, APMV-8, and APMV-9 strain) and uses of such APMV for the treatment of cancer. In particular, provided herein are methods for treating cancer comprising administering a naturally occurring or recombinantly produced APMV-4 strain to a subject in need thereof. In another aspect, provided herein are recombinant APMV comprising a packaged genome, wherein the packaged genome comprises a transgene. In particular, described herein are recombinant APMV (e g., APMV-2, APMV-3, APMV-4, APMV-6, APMV-7, APMV-8, and APMV-9). In another aspect, provided herein are methods for treating cancer comprising administering a recombinant APMV (e g., APMV-2, APMV-3, APMV-4, APMV-6, APMV-7, APMV-8, and APMV-9) to a subject in need thereof, wherein the recombinant APMV comprises a packaged genome comprising a transgene. In particular, provided herein are methods for treating cancer comprising administering a recombinant APMV-4 to a subject in need thereof, wherein the recombinant APMV-4 comprises a packaged genome comprising a transgene. In specific aspects, the use of APMV serotypes other than APMV-1 (such as described herein, in particular AMPV-4) to treat cancer is based, in part, on the similar or enhanced in vivo anti-tumor activities when compared to oncolytic NDV La Sota-L289A strain.
Owner:MT SINAI SCHOOL OF MEDICINE
Mutations in pancreatic neoplasms
ActiveUS20140179538A1Well managementLong survivalMicrobiological testing/measurementLibrary screeningNeoplasmHuman cancer
To help reveal the pathogenesis of these lesions, we purified the DNA from Intraductal Papillary Mucinous Neoplasm (IPMN) cyst fluids from 19 patients and searched for mutations in 169 genes commonly altered in human cancers. We identified recurrent mutations at codon 201 of GNAS. We found that GNAS mutations were present in 66% of IPMNs and that either KRAS or GNAS mutations could be identified in 96%. In eight cases, we could investigate invasive adenocarcinomas that developed in association with IPMNs containing GNAS mutations. In seven of these eight cases, the GNAS mutations present in the IPMNs were also found in the invasive lesion. GNAS mutations were not found in other types of cystic neoplasms of the pancreas or in invasive adenocarcinomas not associated with IPMNs. These data suggest that GNAS mutations can inform the diagnosis and management of patients with cystic pancreatic lesions.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com