Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
45 results about "Virus Integration" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Insertion of viral DNA into host-cell DNA. This includes integration of phage DNA into bacterial DNA; (LYSOGENY); to form a PROPHAGE or integration of retroviral DNA into cellular DNA to form a PROVIRUS.
HIV Integrase Inhibitors
The invention encompasses series bicyclic pyrimidinone compounds of Formula I which inhibit HIV integrase and prevent viral integration into human DNA. This action makes the compounds useful for treating HIV infection and AIDS. The invention also encompasses pharmaceutical compositions and methods for treating those infected with HIV.
Owner:BRISTOL MYERS SQUIBB CO
HIV integrase inhibitors: cyclic pyrimidinone compounds
The invention encompasses a series of pyrimidinone compounds which inhibit HIV integrase and thereby prevent viral integration into human DNA. This action makes the compounds useful for treating HIV infection and AIDS. The invention also encompasses intermediates useful for making the pyrimidone compounds. Additionally, pharmaceutical compositions and methods for treating those infected with HIV are encompassed
Owner:BRISTOL MYERS SQUIBB CO
Bicyclic heterocycles as HIV integrase inhibitors
The invention encompasses a series bicyclic pyrimidinone compounds of Formula I which inhibit HIV integrase and prevent viral integration into human DNA. This action makes the compounds useful for treating HIV infection and AIDS. The invention also encompasses pharmaceutical compositions and methods for treating those infected with HIV
Owner:BRISTOL MYERS SQUIBB CO
Bicyclic heterocycles as HIV integrase inhibitors
The invention encompasses a series cyclic bicyclic heterocyclic compounds of Formula I which are inhibitors of HIV integrase and prevent viral integration into human DNA. This action makes the compounds useful for treating HIV infection and AIDS. The invention also encompasses pharmaceutical compositions and methods for treating those infected with HIV
Owner:BRISTOL MYERS SQUIBB CO
HIV Integrase Inhibitors
The disclosure generally relates to the novel compounds of formula I, including their salts, which inhibit HIV integrase and prevent viral integration into human DNA. This action makes the compounds useful for treating HIV infection and AIDS. The invention also encompasses pharmaceutical compositions and methods for treating those infected with HIV.
Owner:BRISTOL MYERS SQUIBB CO
Bicyclic heterocycles as HIV integrase inhibitors
The invention encompasses a series cyclic bicyclic pyrimidinone compounds of Formula I which inhibit HIV integrase and prevent viral integration into human DNA. This action makes the compounds useful for treating HIV infection and AIDS. The invention also encompasses pharmaceutical compositions and methods for treating those infected with HIV.
Owner:BRISTOL MYERS SQUIBB CO
Bicyclic heterocycles as HIV-integrase inhibitors
The invention encompasses a series cyclic bicyclic heterocyclic compounds of Formula I which are inhibitors of HIV integrase and prevent viral integration into human DNA. This action makes the compounds useful for treating HIV infection and AIDS. The invention also encompasses pharmaceutical compositions and methods for treating those infected with HIV.
Owner:BRISTOL MYERS SQUIBB CO
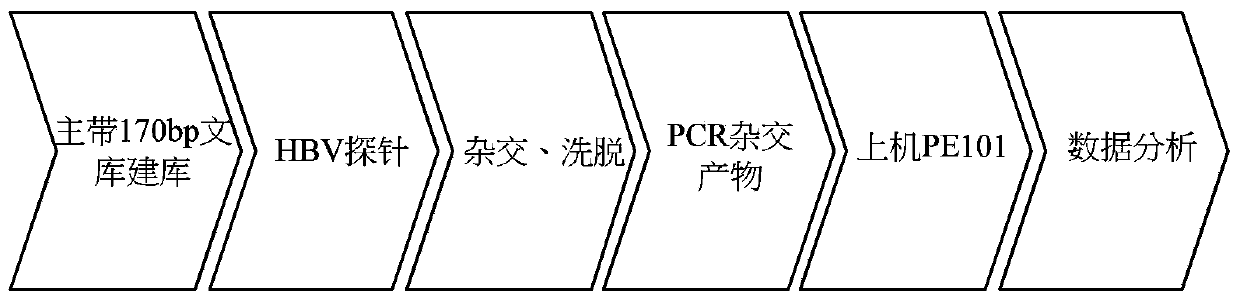
Virus integration site capture sequencing analysis method
ActiveCN103993069AImprove consistencyHigh sensitivityMicrobiological testing/measurementSequence analysisAnalysis method
A virus integration site capture sequencing analysis method includes the steps of: merging a human reference sequence and a virus reference sequence to construct a mixed reference sequence; reading sequencing data, and filtering the unqualified parts to obtain filtered sequencing data; aligning processed sequencing data to the mixed reference sequence by using a piece of alignment software to obtain an alignment result, and then processing the alignment result to obtain an alignment result for the detection of virus integration; according to the alignment result for detection of virus integration, executing the corresponding operations to obtain the virus integration related sequences; integrating alignment information of the above related sequences to obtain the coordinates of a virus integration site in the reference sequence; and integrating coordinate information of the integration sites and outputting the obtained results virus integration result. The method provided by the invention can obtain virus site integration information with high accuracy.
Owner:BGI TECH SOLUTIONS
Bicyclic heterocycles as HIV integrase inhibitors
The invention encompasses a series cyclic bicyclic pyrimidinone compounds of Formula I which inhibit HIV integrase and prevent viral integration into human DNA. This action makes the compounds useful for treating HIV infection and AIDS. The invention also encompasses pharmaceutical compositions and methods for treating those infected with HIV.
Owner:BRISTOL MYERS SQUIBB CO
HIV integrase inhibitors: cyclic pyrimidinone compounds
The invention encompasses a series of pyrimidinone compounds which inhibit HIV integrase and thereby prevent viral integration into human DNA. This action makes the compounds useful for treating HIV infection and AIDS. The invention also encompasses intermediates useful for making the pyrimidone compounds. Additionally, pharmaceutical compositions and methods for treating those infected with HIV are encompassed.
Owner:BRISTOL MYERS SQUIBB CO
HIV integrase inhibitors
The disclosure generally relates to the novel compounds of formula I, including their salts, which inhibit HIV integrase and prevent viral integration into human DNA. This action makes the compounds useful for treating HIV infection and AIDS. The invention also encompasses pharmaceutical compositions and methods for treating those infected with HIV.
Owner:BRISTOL MYERS SQUIBB CO
HIV Integrase Inhibitors
The invention encompasses a series pyrimidinone compounds of Formula I which inhibit HIV integrase and prevent viral integration into human DNA. This action makes the compounds useful for treating HIV infection and AIDS. The invention also encompasses pharmaceutical compositions and methods for treating those infected with HIV.
Owner:BRISTOL MYERS SQUIBB CO
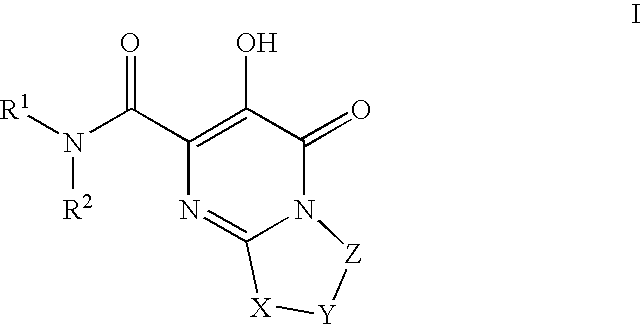
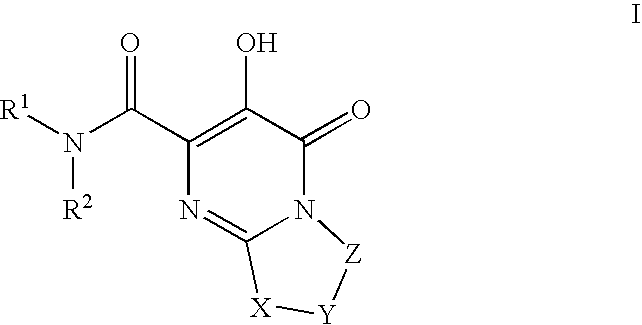
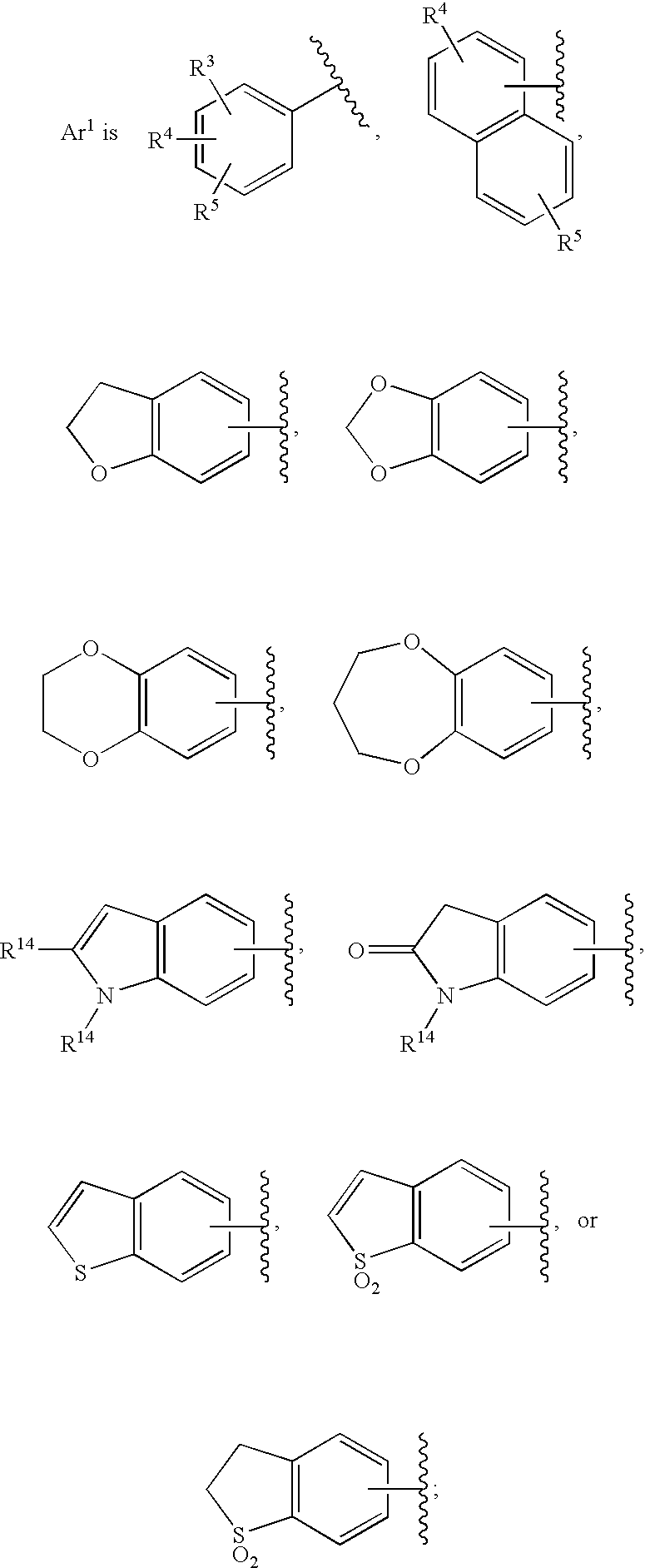
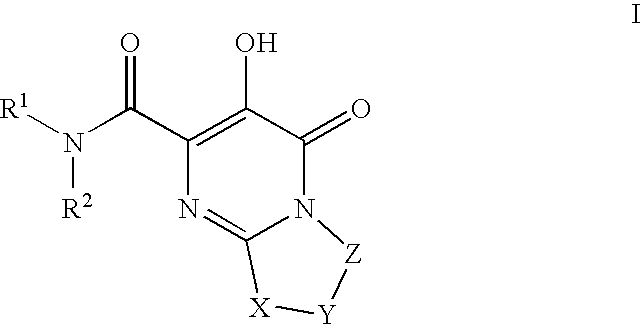
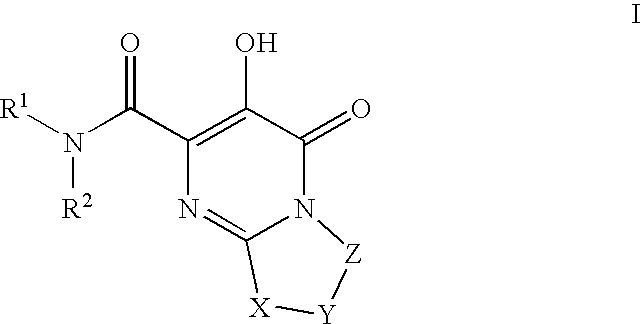
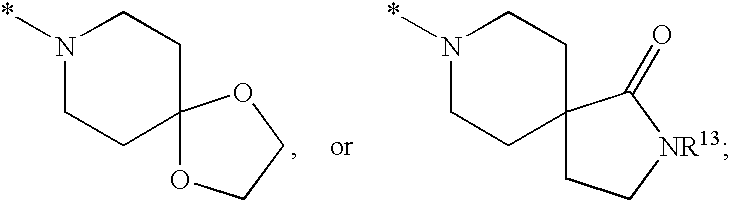
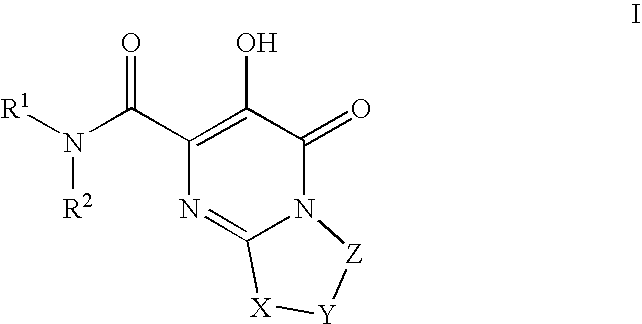
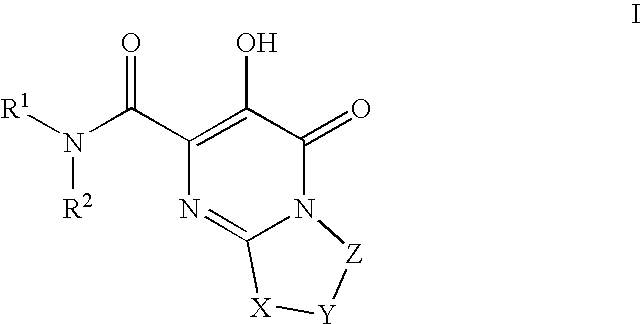
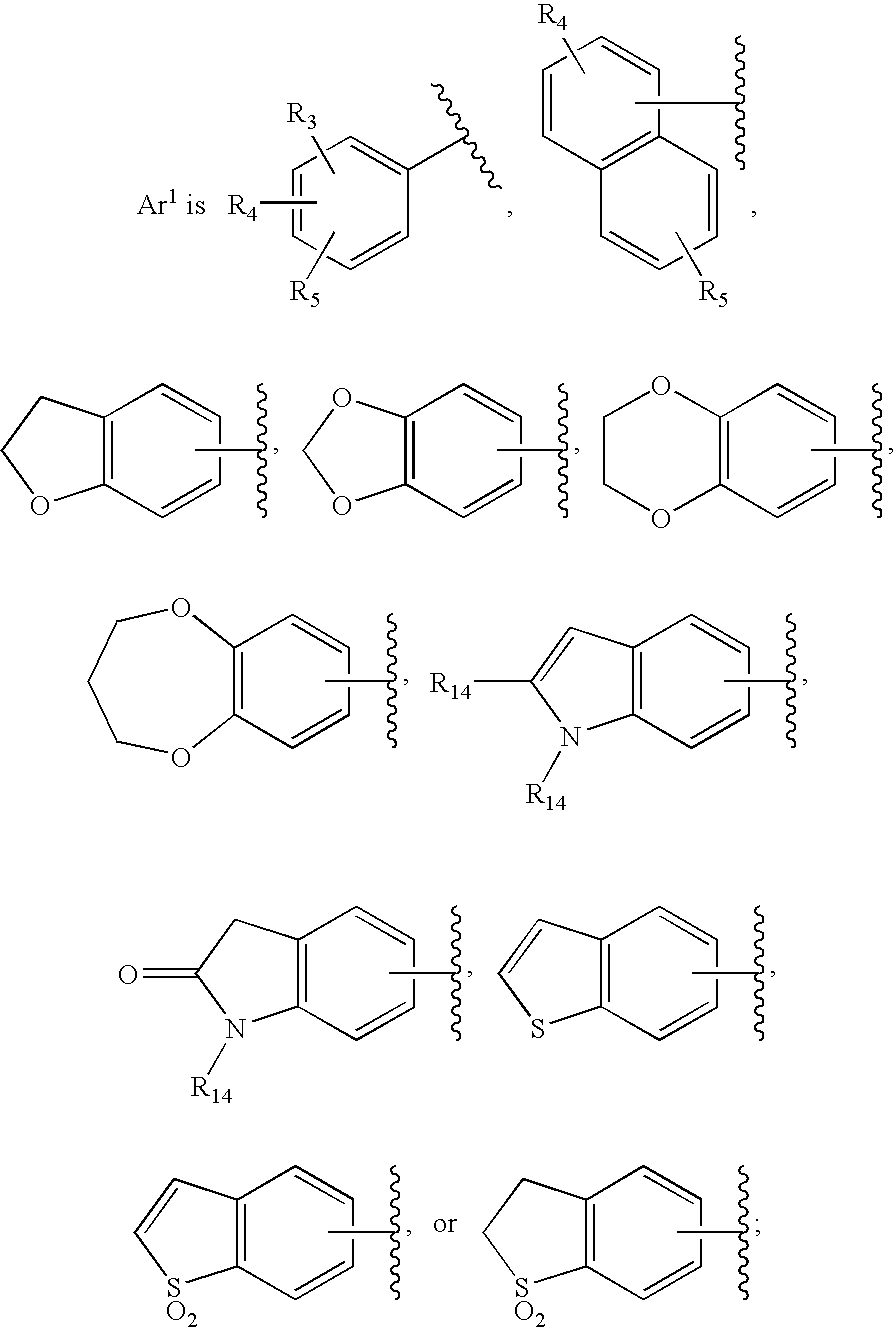
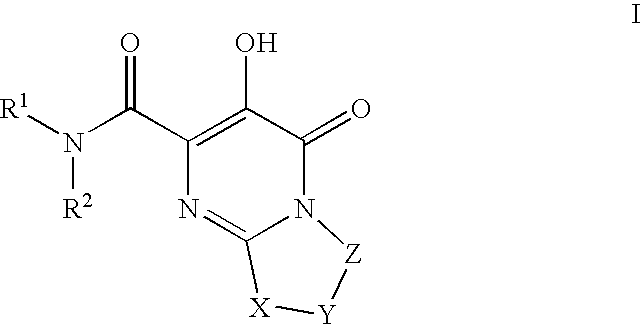
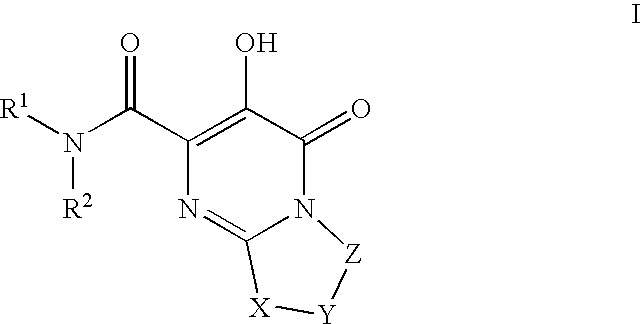
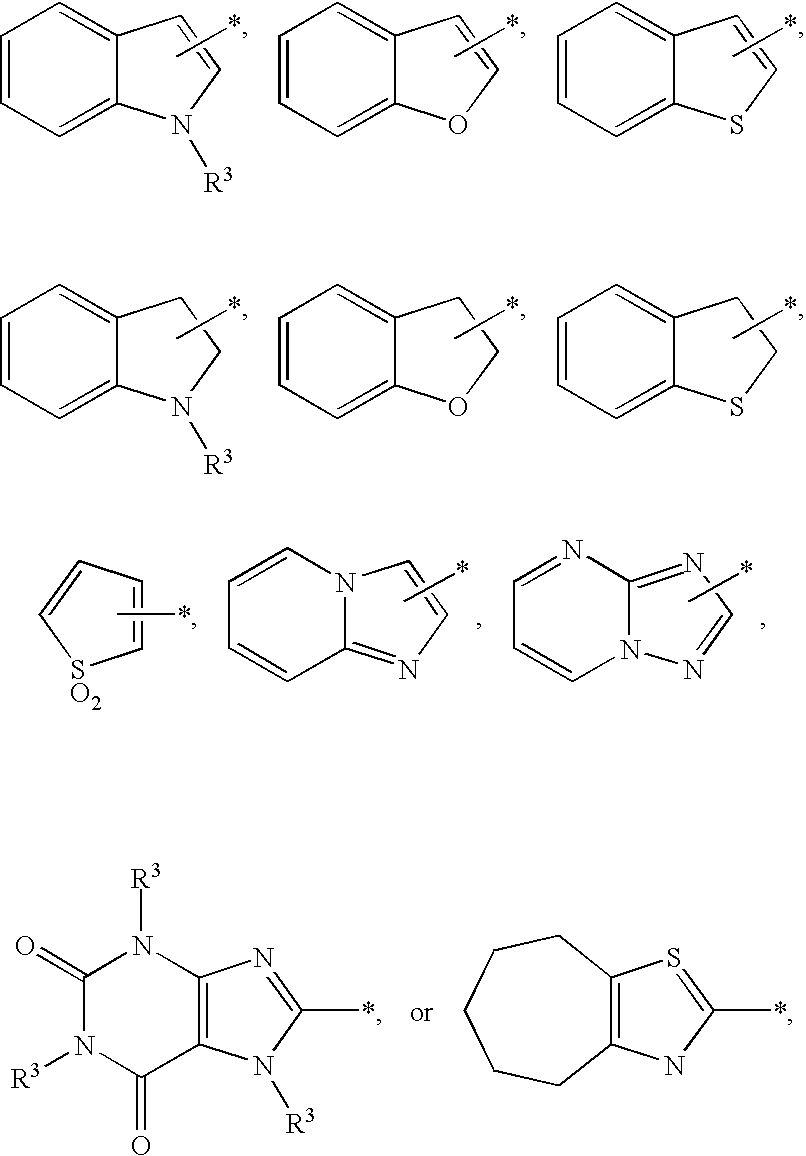
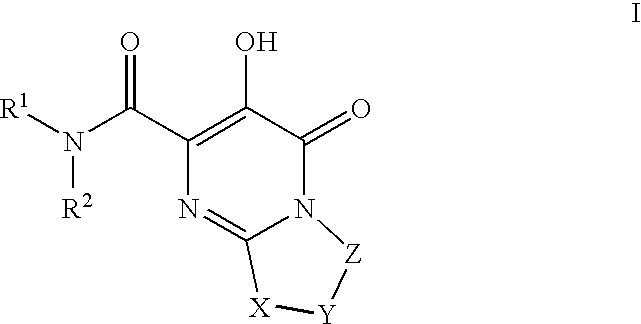
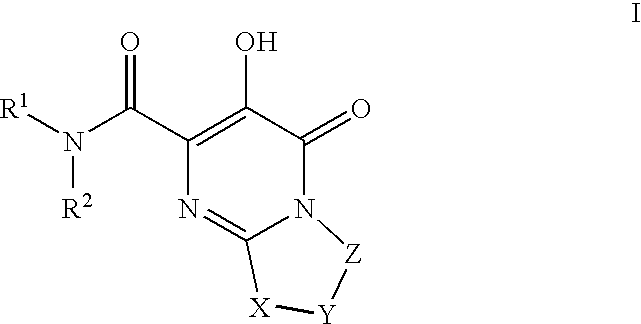
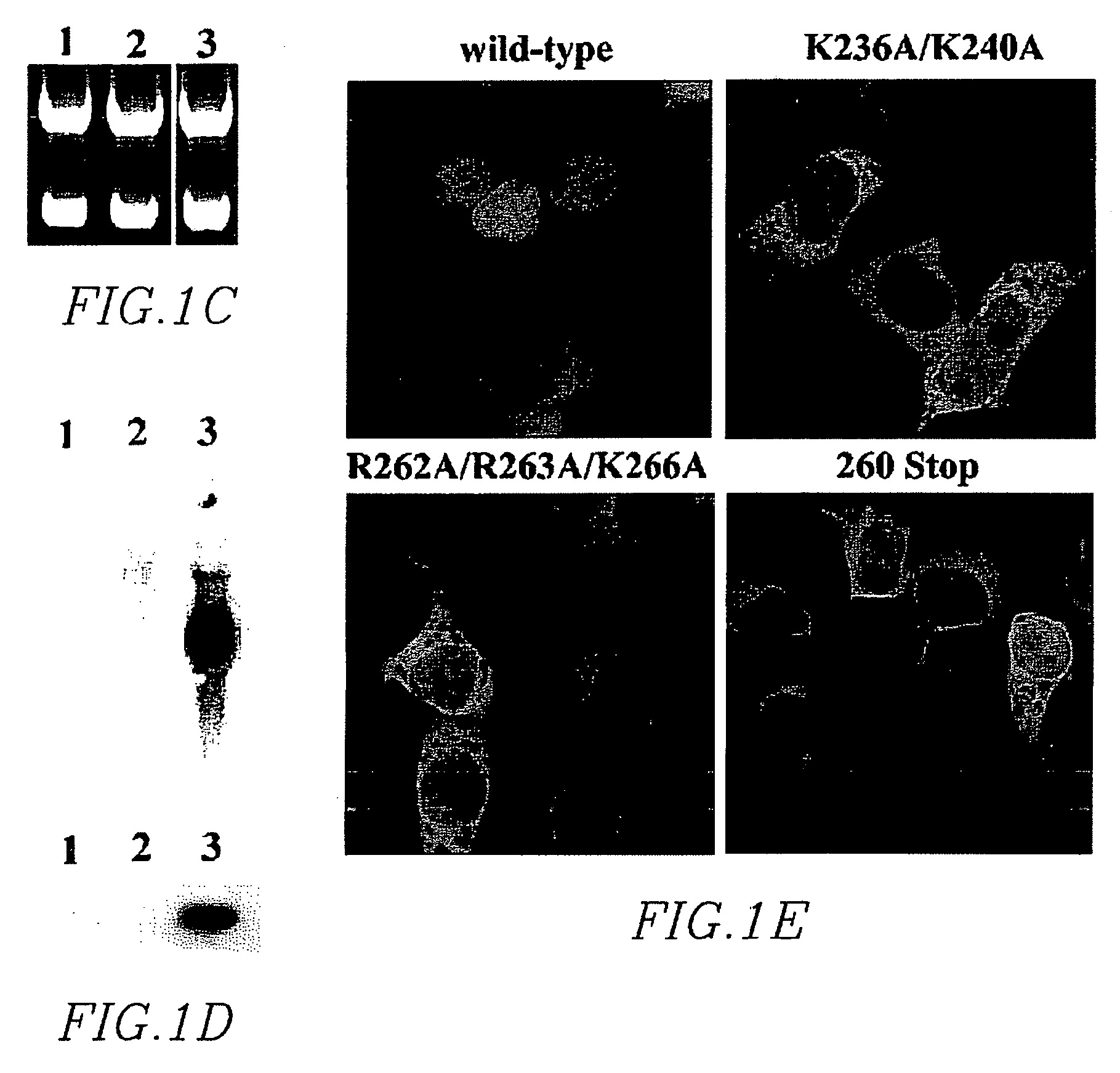
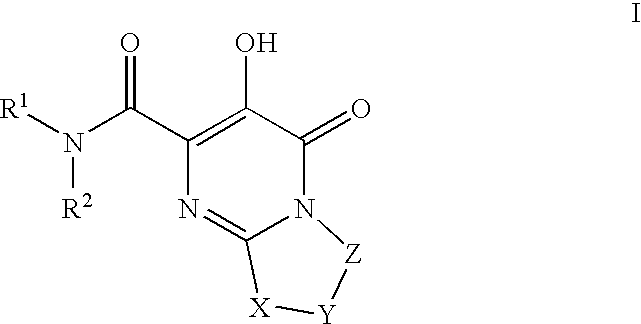
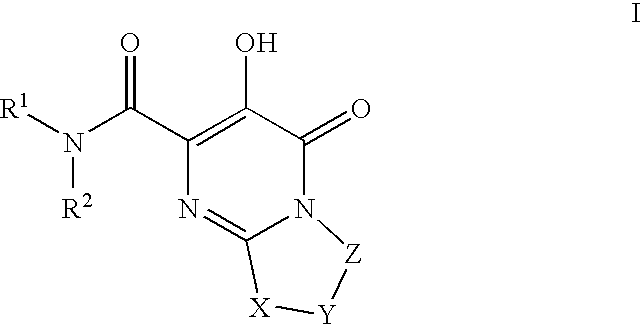
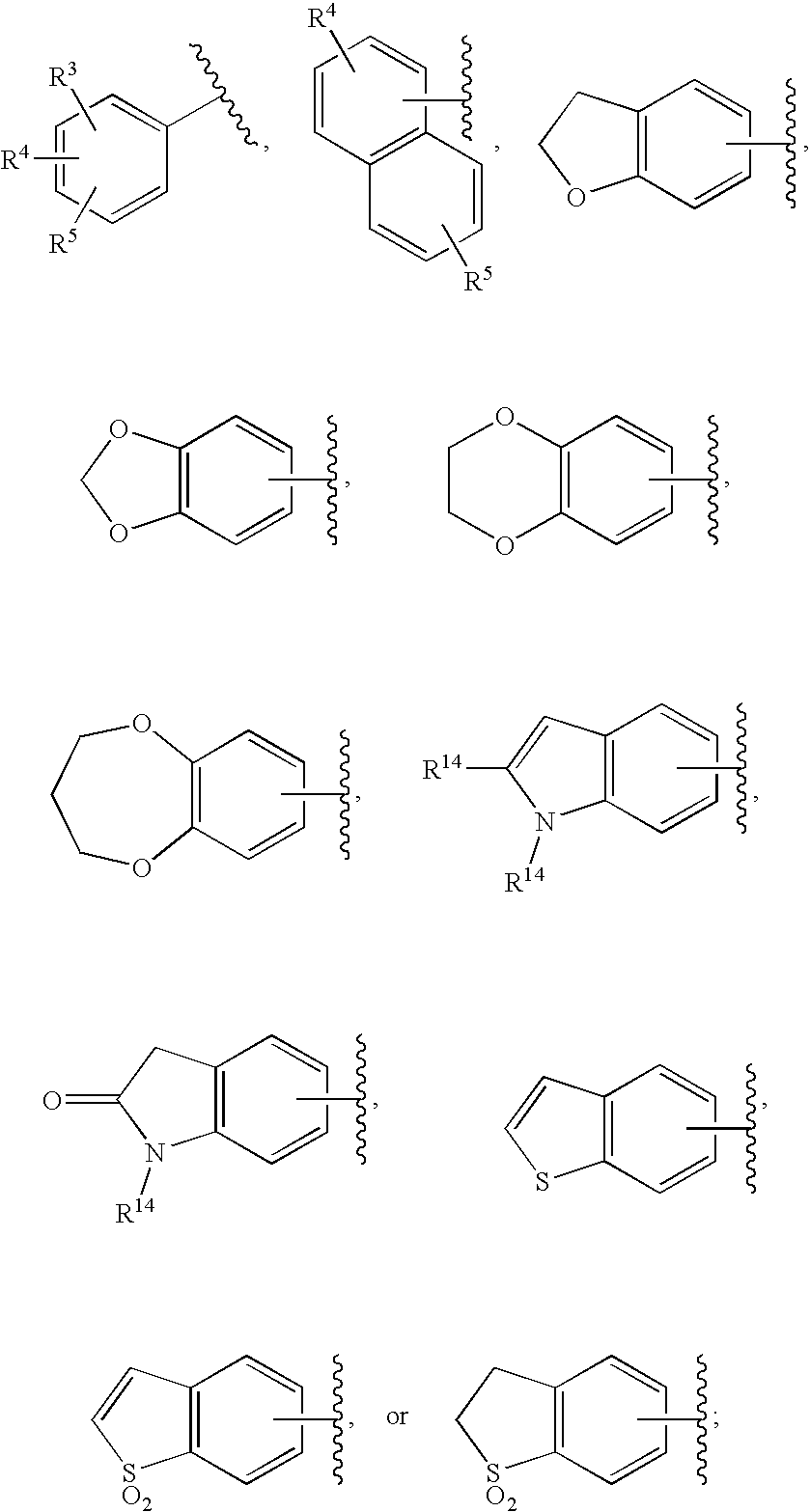
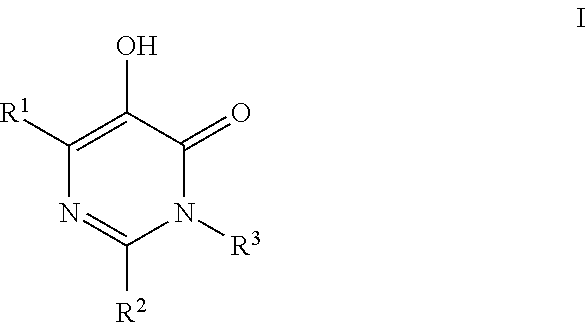
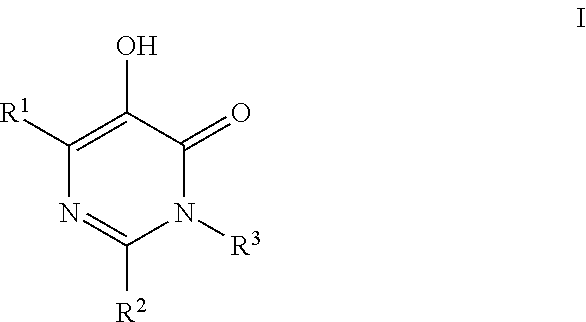
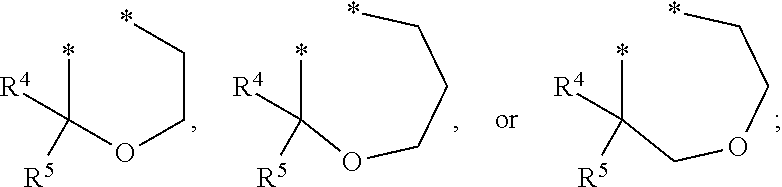
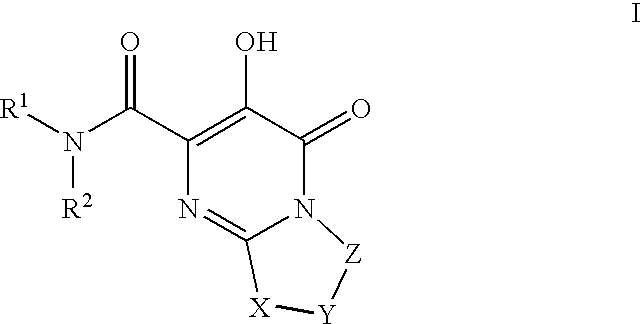
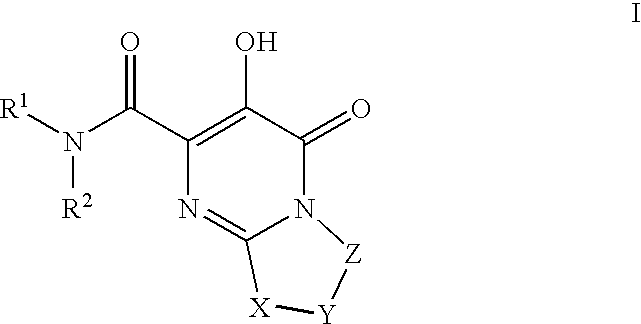
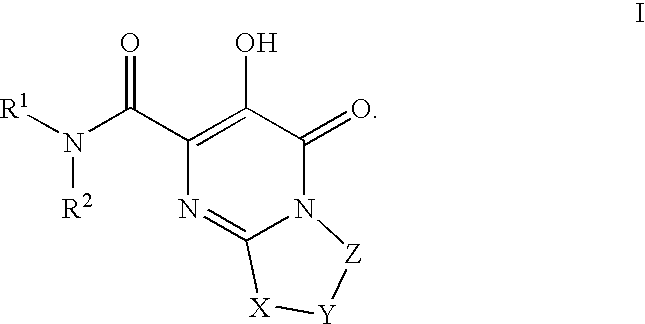
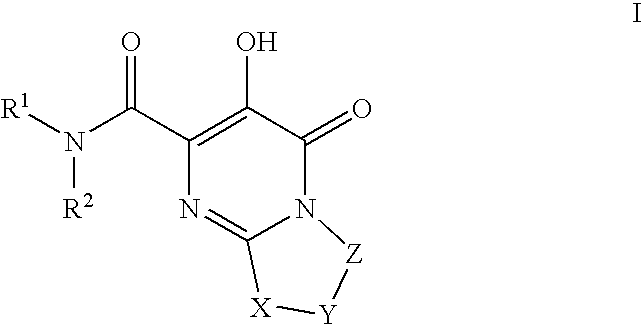
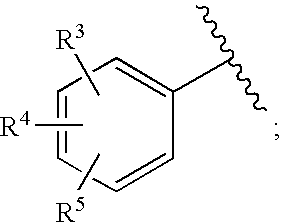
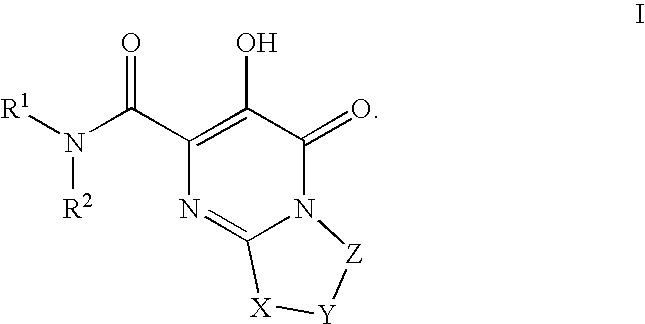
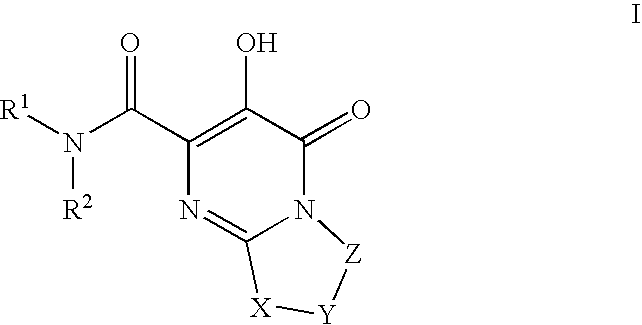
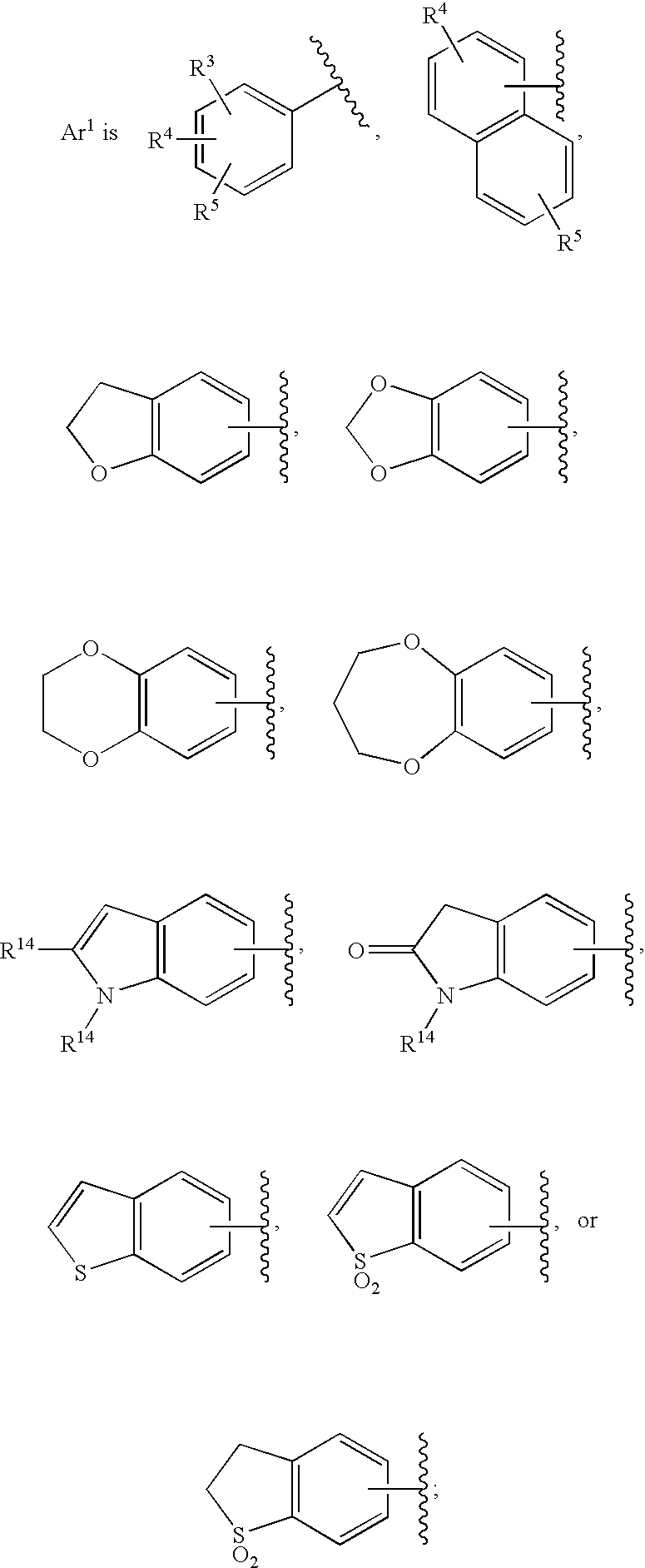
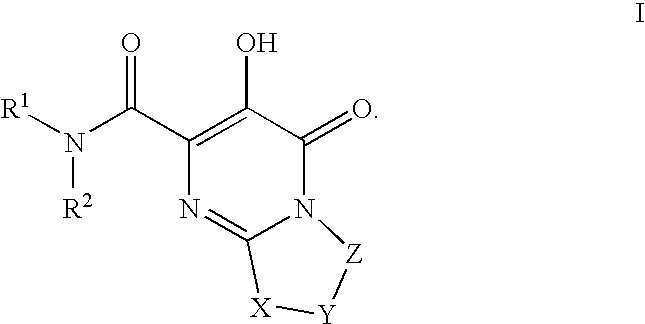
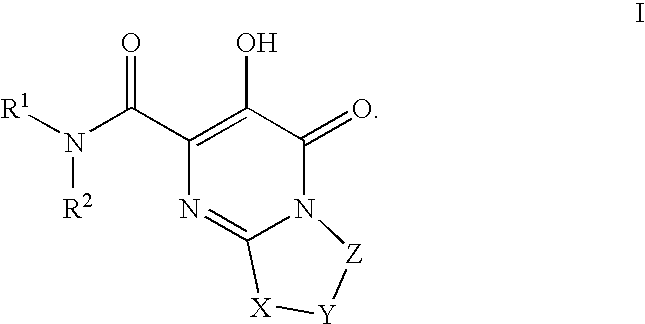
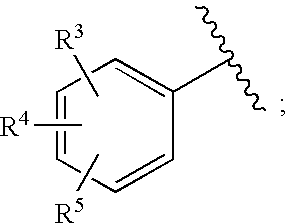
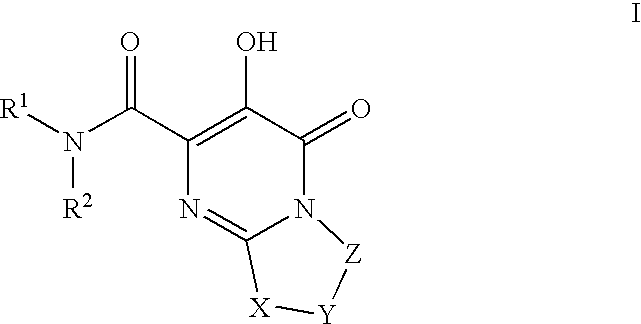
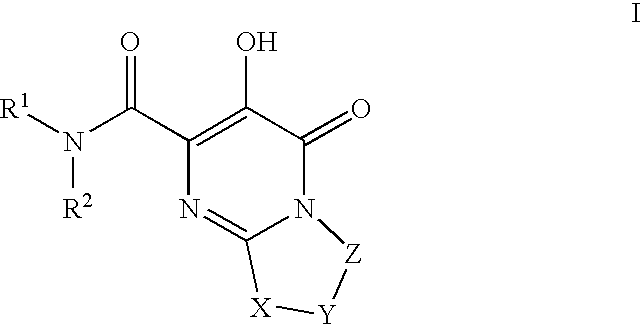
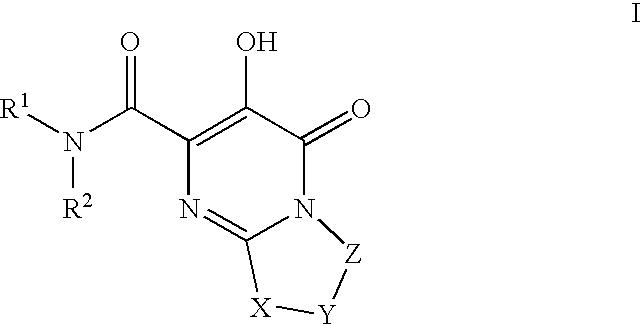
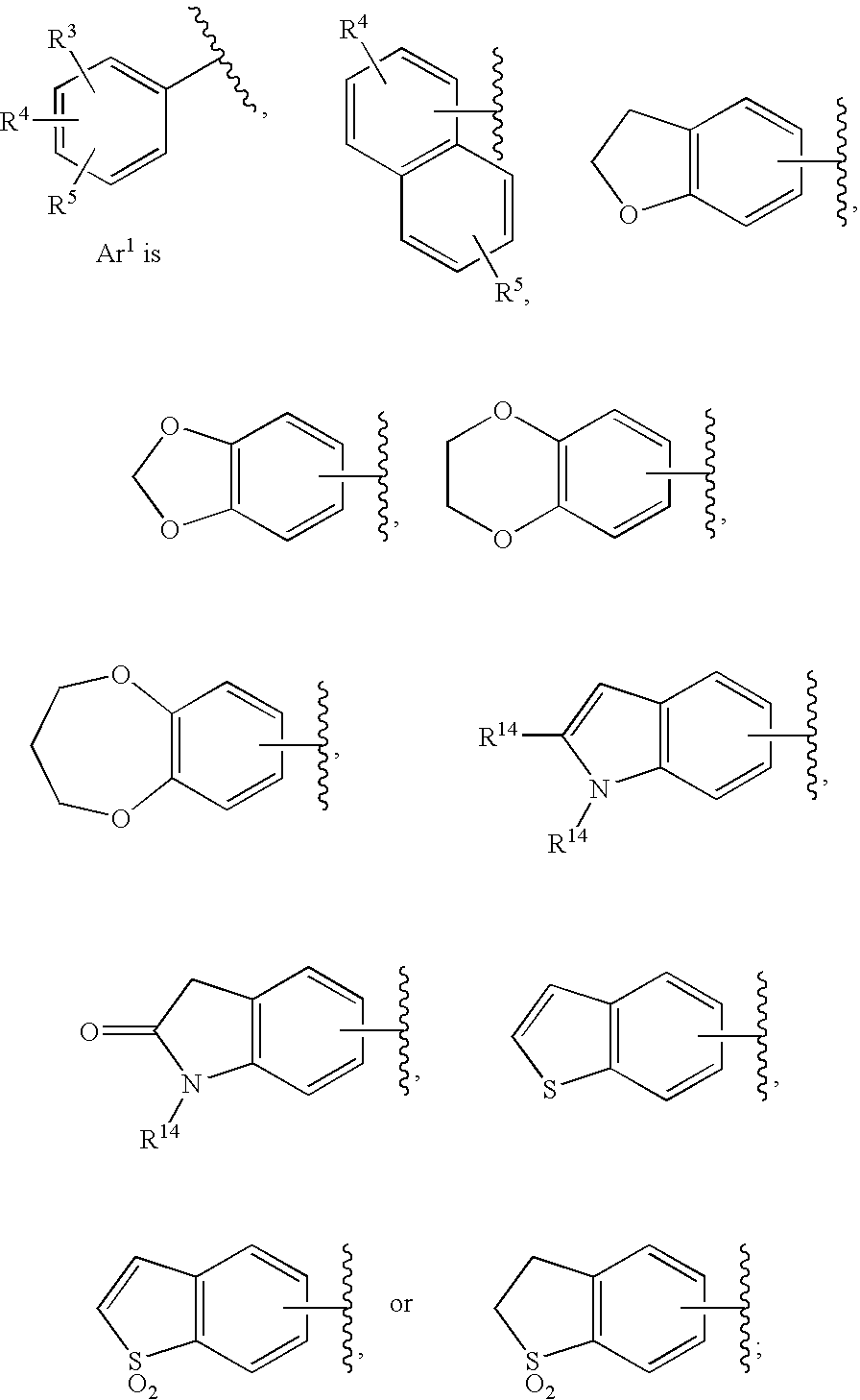
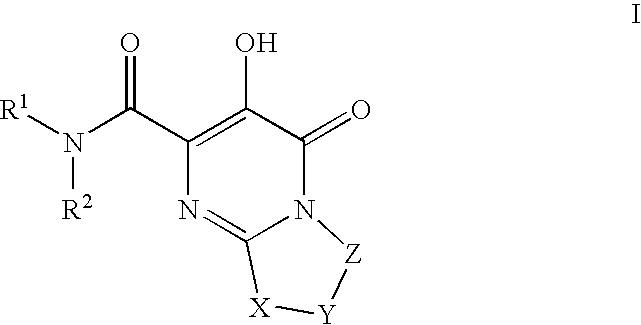
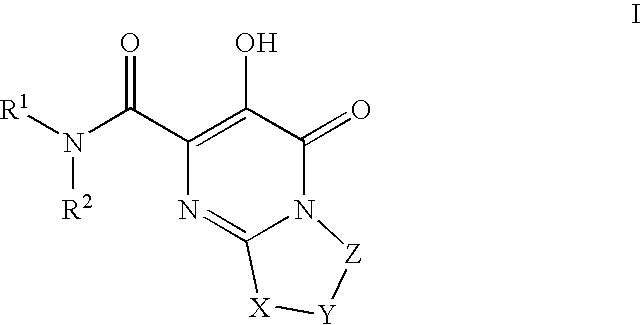
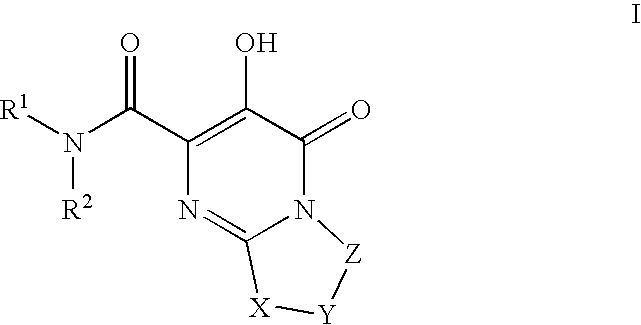
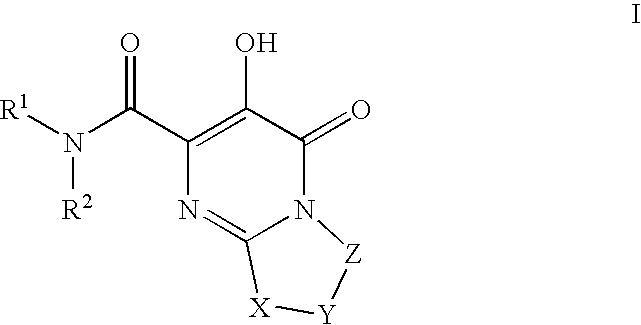
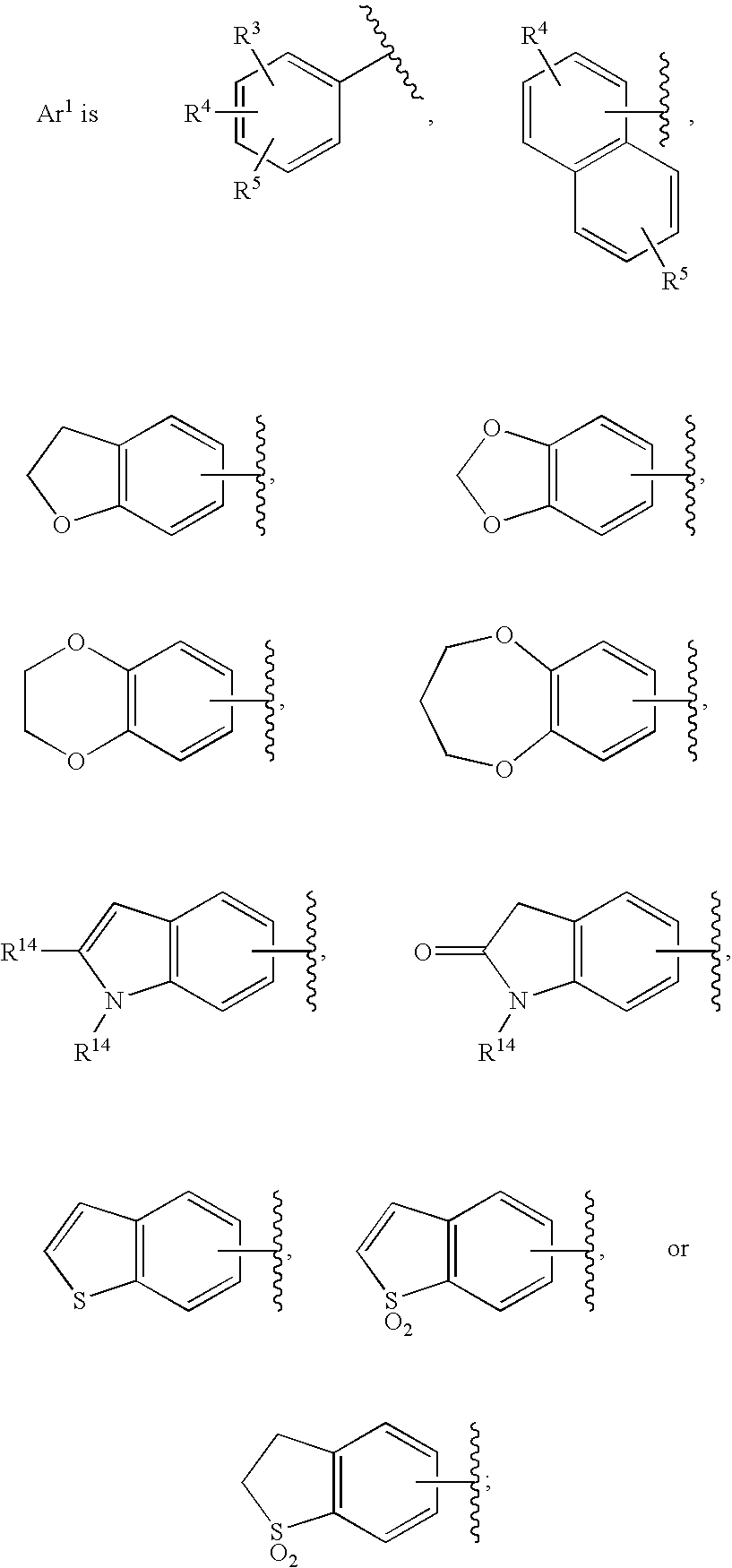
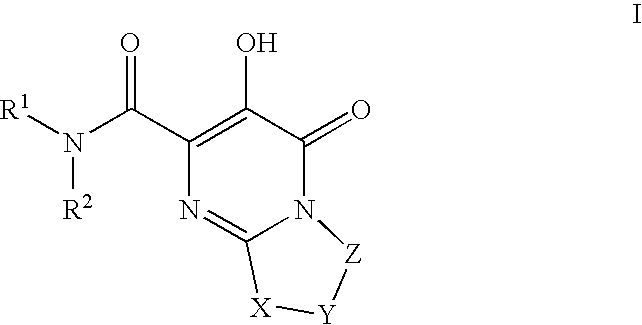
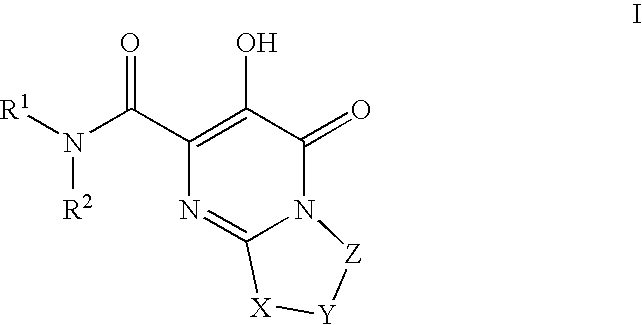
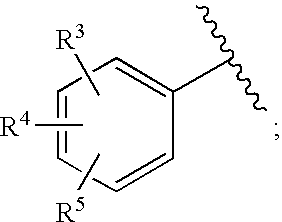
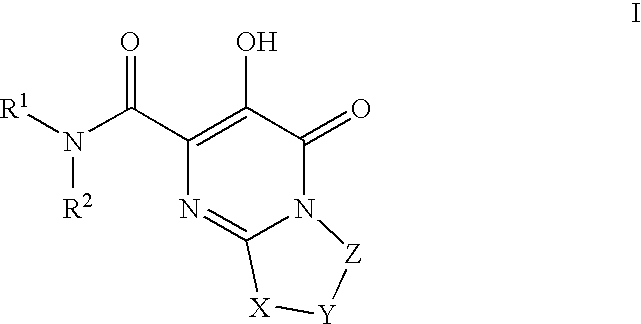
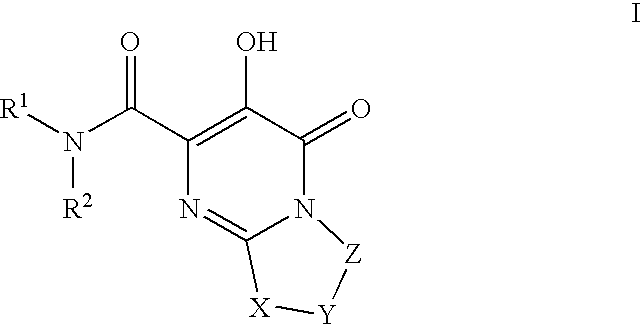
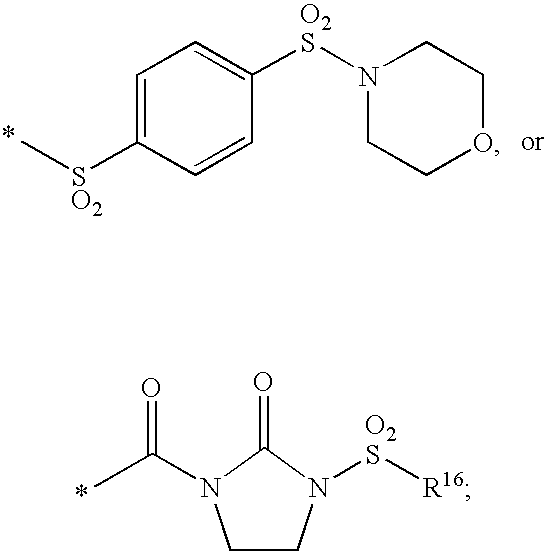
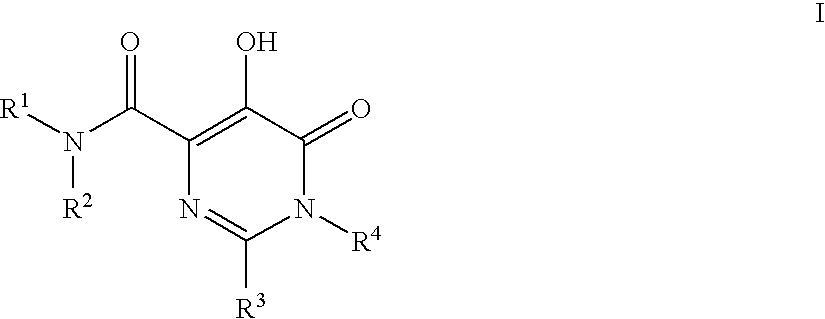
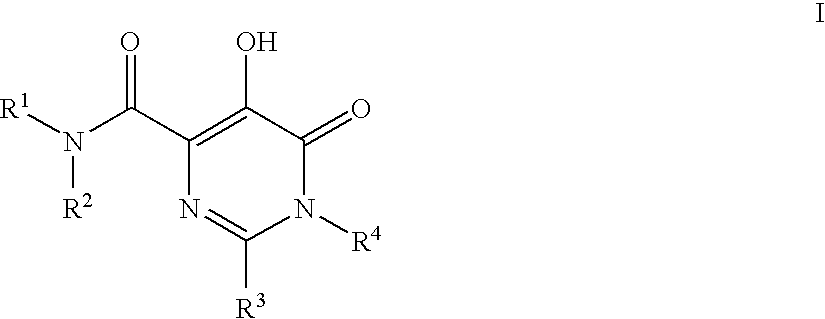
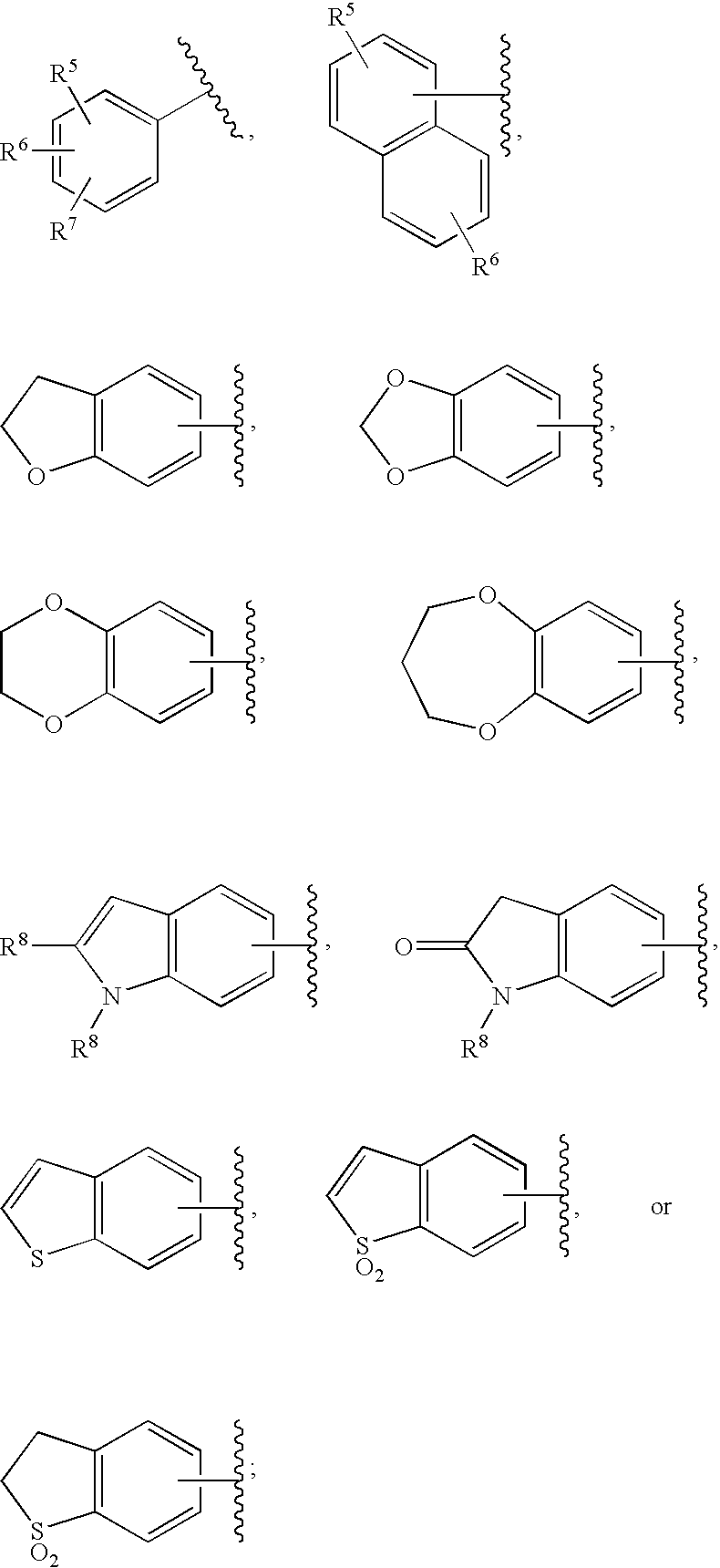
Substituted imidazo[1,2-a]pyrimidines as HIV viral DNA integrase inhibitors
The invention encompasses a series of bicyclic heterocyclic compounds of Formula I which are inhibitors of HIV integrase and prevent viral integration into human DNA. This action makes the compounds useful for treating HIV infection and AIDS. The invention also encompasses pharmaceutical compositions and methods for treating those infected with HIV.
Owner:BRISTOL MYERS SQUIBB CO
Bicyclic heterocycles as HIV integrase inhibitors
The invention encompasses a series cyclic bicyclic pyrimidinone compounds of Formula I which inhibit HIV integrase and prevent viral integration into human DNA. This action makes the compounds useful for treating HIV infection and AIDS. The invention also encompasses pharmaceutical compositions and methods for treating those infected with HIV.
Owner:BRISTOL MYERS SQUIBB CO
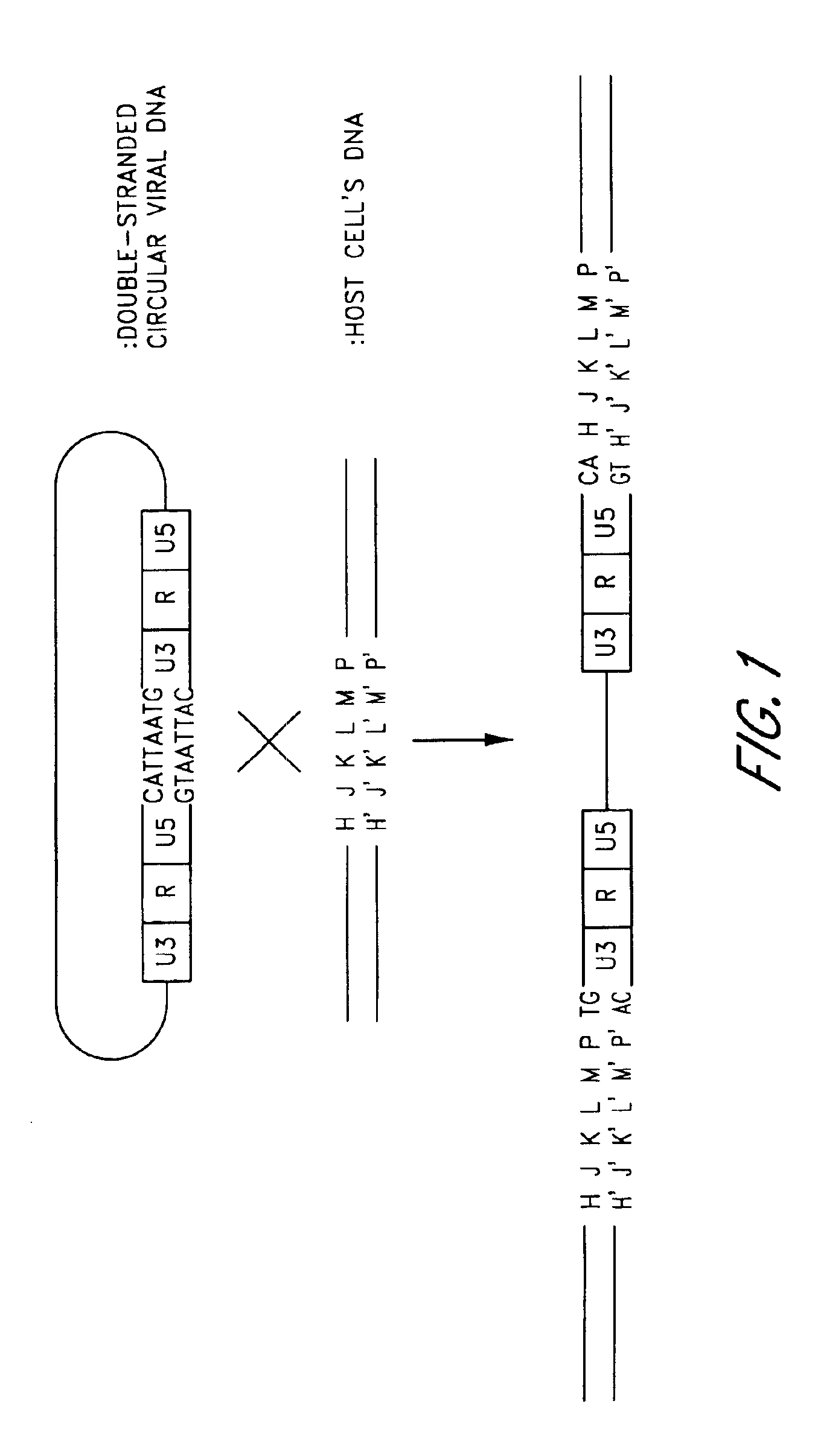
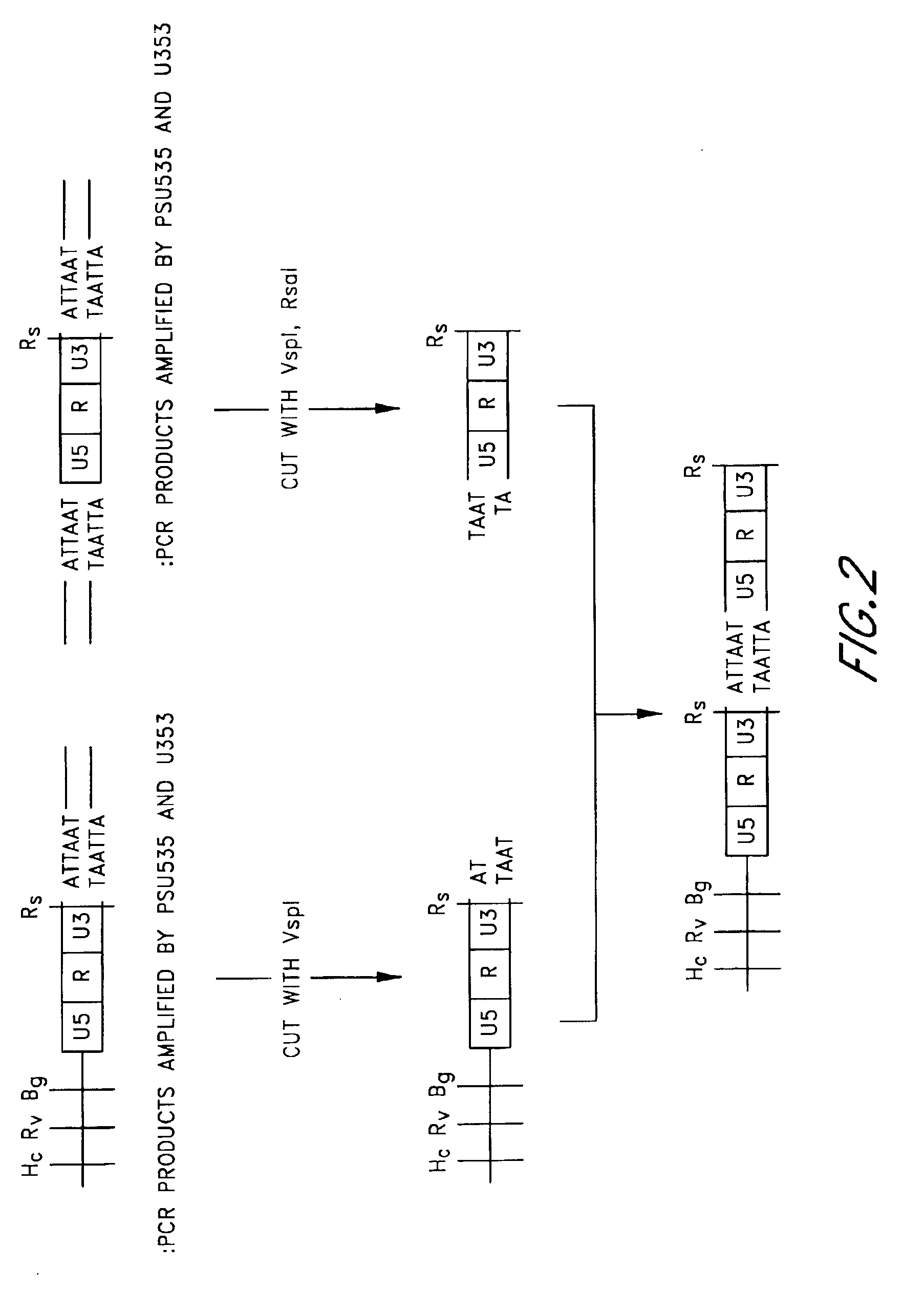
Plasmid vector comprising a retroviral integrase gene and an integrase recognition region
A plasmid vector having components (D1), (D2), and (D3) enables the efficient integration of foreign DNA into host cells. The components are (D1) an integrase gene, (D2) a segment of DNA forming a region for controlling the expression of the integrase gene, and (D3) a segment of DNA serving as an integrase recognition region when integrase catalyzes the integration reaction.
Owner:NIHON SEIBUTSU KAGAKU KENKYUSHO
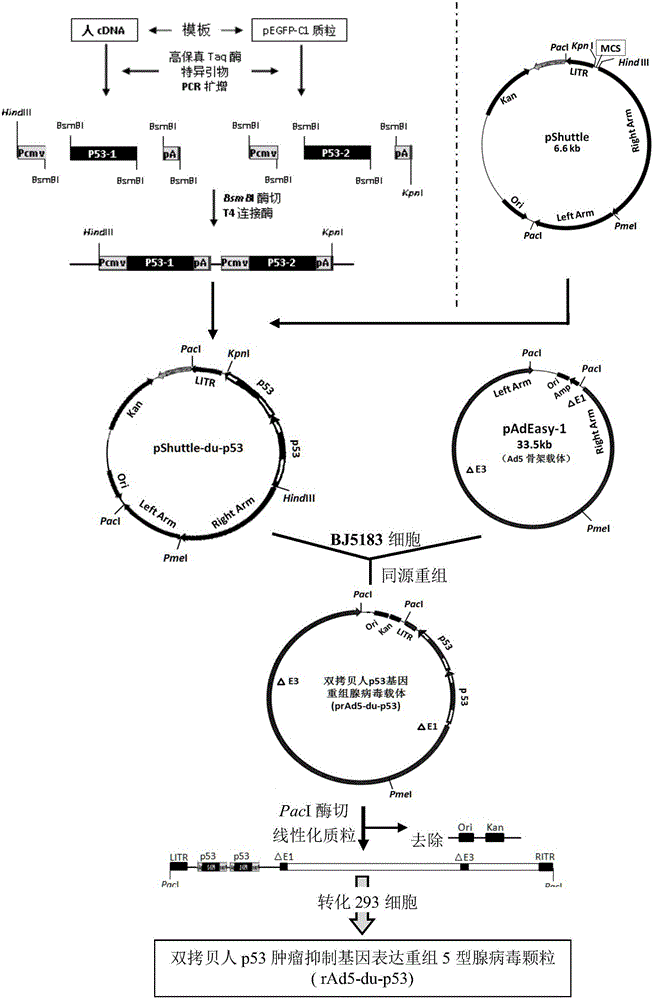
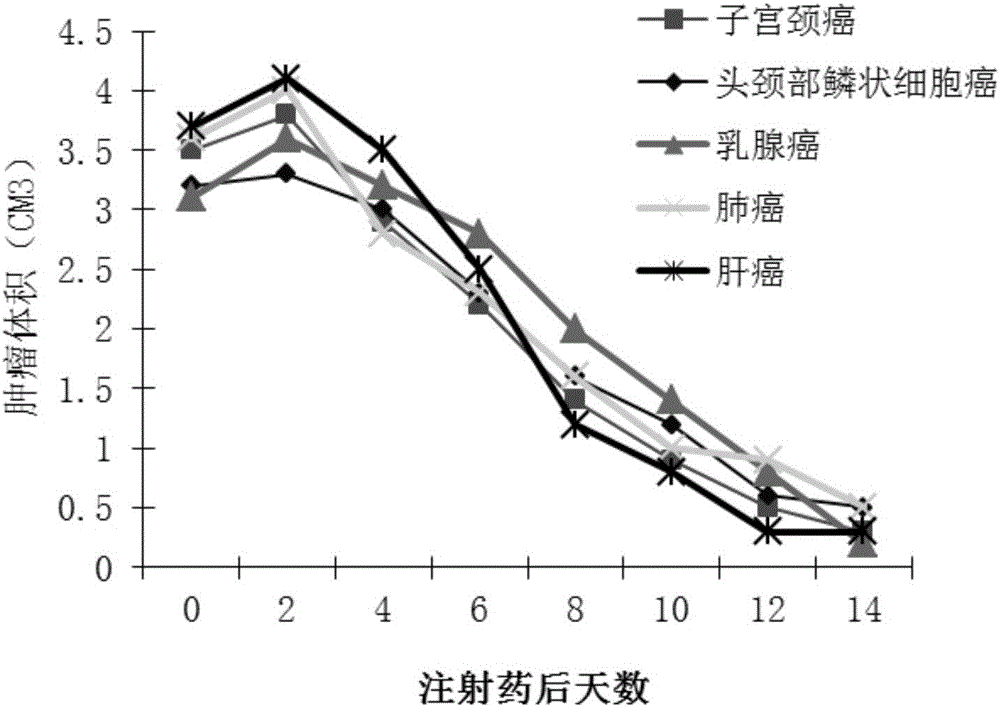
Double-copy human p53 gene recombinant adenovirus and preparation method thereof
ActiveCN105755043AIncreased expression of functional p53 proteinIncreased and accelerated expression of functional p53 proteinPeptide/protein ingredientsFermentationGenes mutationTumour suppressor gene
The invention discloses double-copy human p53 gene recombinant adenovirus and a preparation method thereof. A commercialized 5-type recombinant replication-deficient adenovirus construction system (AdEasy) is inserted into a double-copy human p53 tumor inhibition gene eukaryotic expression box as shown in SEQ ID NO.1 to construct a p53 tumor inhibition gene recombinant adenovirus expression carrier system, and recombinant replication-deficient adenovirus granules for expressing double-copy human p53 tumor inhibition gene are further obtained. Experiment shows that after being injected by tumor cells, the double-copy human p53 gene recombinant adenovirus can efficiently express p53 tumor inhibition genes carried by the virus. As the double-copy human p53 tumor inhibition gene eukaryotic expression box is integrated, the p53 tumor inhibition gene expression amount can be greatly increased, meanwhile the virus amount can be reduced, and a relatively good gene treatment effect can be achieved. The double-copy human p53 gene recombinant adenovirus is good in specificity and wide in spectrum, directly aims at gene mutation of tumor cells, and can be applied to malignant tumor of various tissue types at the early stage, the middle stage and the late stage.
Owner:SINOSHENG SHENZHEN GENE IND DEV CO LTD
HIV integrase inhibitors
Owner:BRISTOL MYERS SQUIBB CO
HIV integrase inhibitors
The invention encompasses a series bicyclic pyrimidinone compounds of Formula I which inhibit HIV integrase and prevent viral integration into human DNA. This action makes the compounds useful for treating HIV infection and AIDS. The invention also encompasses pharmaceutical compositions and methods for treating those infected with HIV.
Owner:BRISTOL MYERS SQUIBB CO
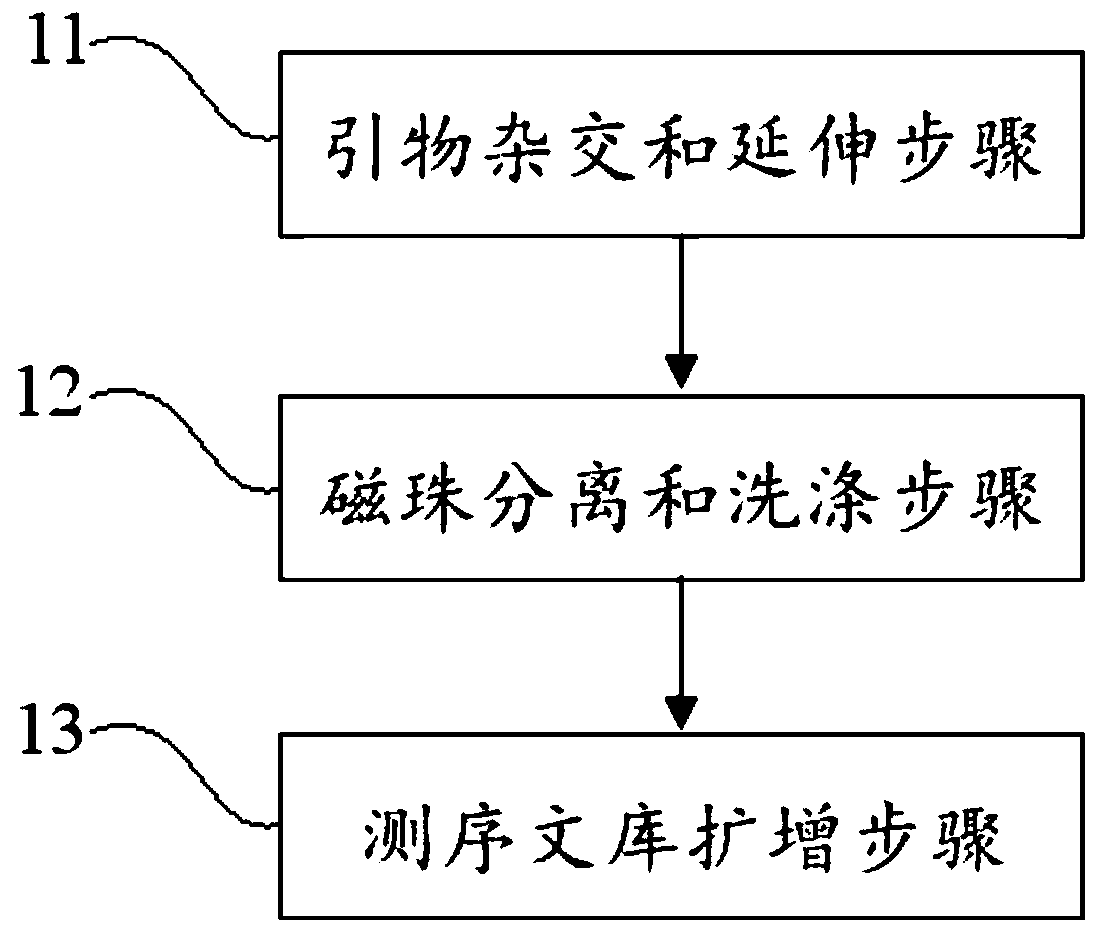
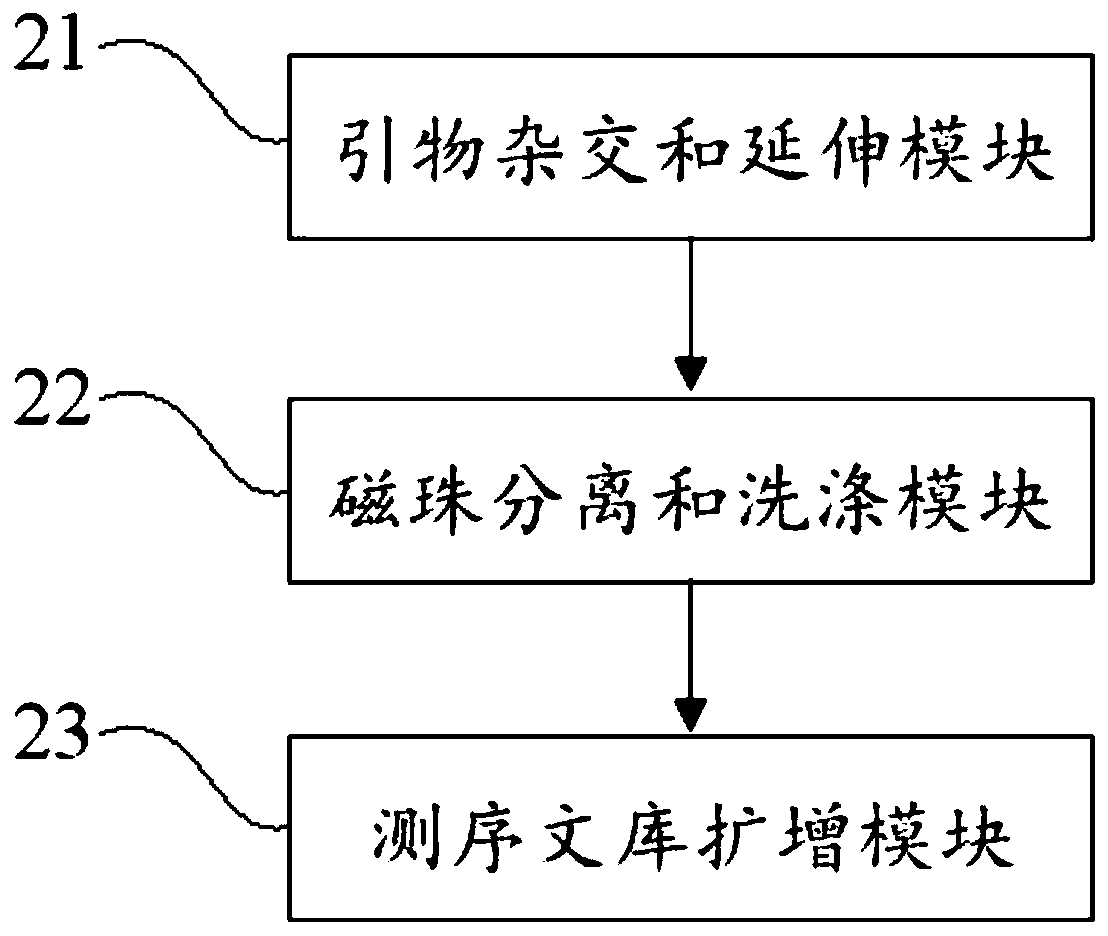
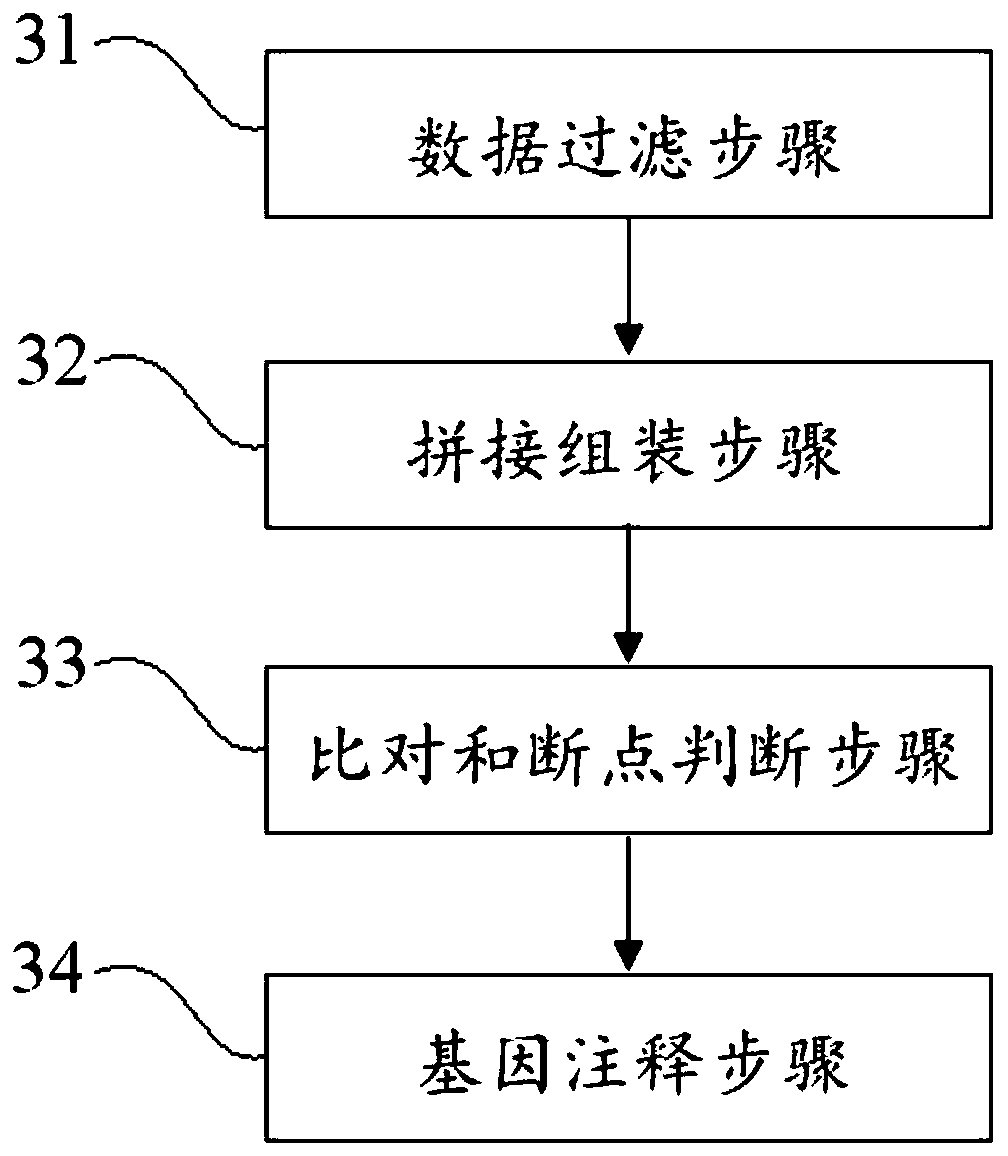
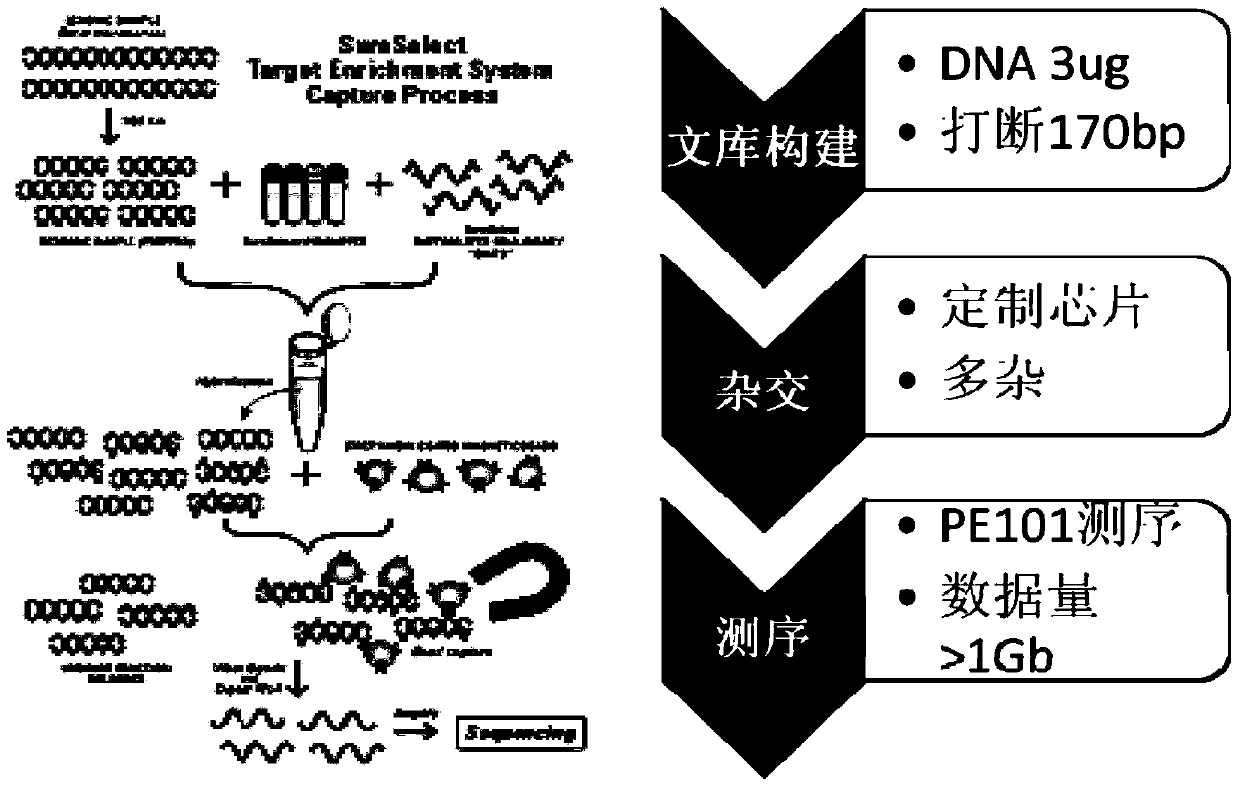
Virus integrated DNA enrichment method, sequencing data analyzing method and device
PendingCN110273028AReduce data volumeIncreased sequencing depthMicrobiological testing/measurementMicroorganism based processesMagnetic beadEnrichment methods
The application discloses a virus integrated DNA enrichment method, a sequencing data analyzing method and a device. The enrichment method of the application comprises the steps of primer hybridizing and extending, magnetic bead separating and washing. The sequencing data analyzing method disclosed by the application comprises the steps of data filtering, splicing and assembling, comparing and breakpoint judging and gene noting. According to the enrichment method disclosed by the application, specific primers are adopted for extending virus genome target genes so as to replace a long probe, so that the cost is low, the efficiency is high, the specificity is high, and the enrichment time is shortened. Compared with a complete genome, the sequencing data analyzing method disclosed by the application can save data amount, can obtain higher sequencing depth, can obtain more virus DNA breakpoint information and integration position information on the host genome, and can obtain clonal integration events supported by more reads, and besides, can detect non-clonal integration supported by single reads, so as to construct a comprehensive prospect integrated on the host genome.
Owner:SHENZHEN HAPLOX BIOTECH
HIV integrase inhibitors
The invention encompasses a series bicyclic pyrimidinone compounds of Formula I which inhibit HIV integrase and prevent viral integration into human DNA. This action makes the compounds useful for treating HIV infection and AIDS. The invention also encompasses pharmaceutical compositions and methods for treating those infected with HIV.
Owner:BRISTOL MYERS SQUIBB CO
HIV integrase inhibitors
The invention encompasses series bicyclic pyrimidinone compounds of Formula I which inhibit HIV integrase and prevent viral integration into human DNA. This action makes the compounds useful for treating HIV infection and AIDS. The invention also encompasses pharmaceutical compositions and methods for treating those infected with HIV.
Owner:BRISTOL MYERS SQUIBB CO
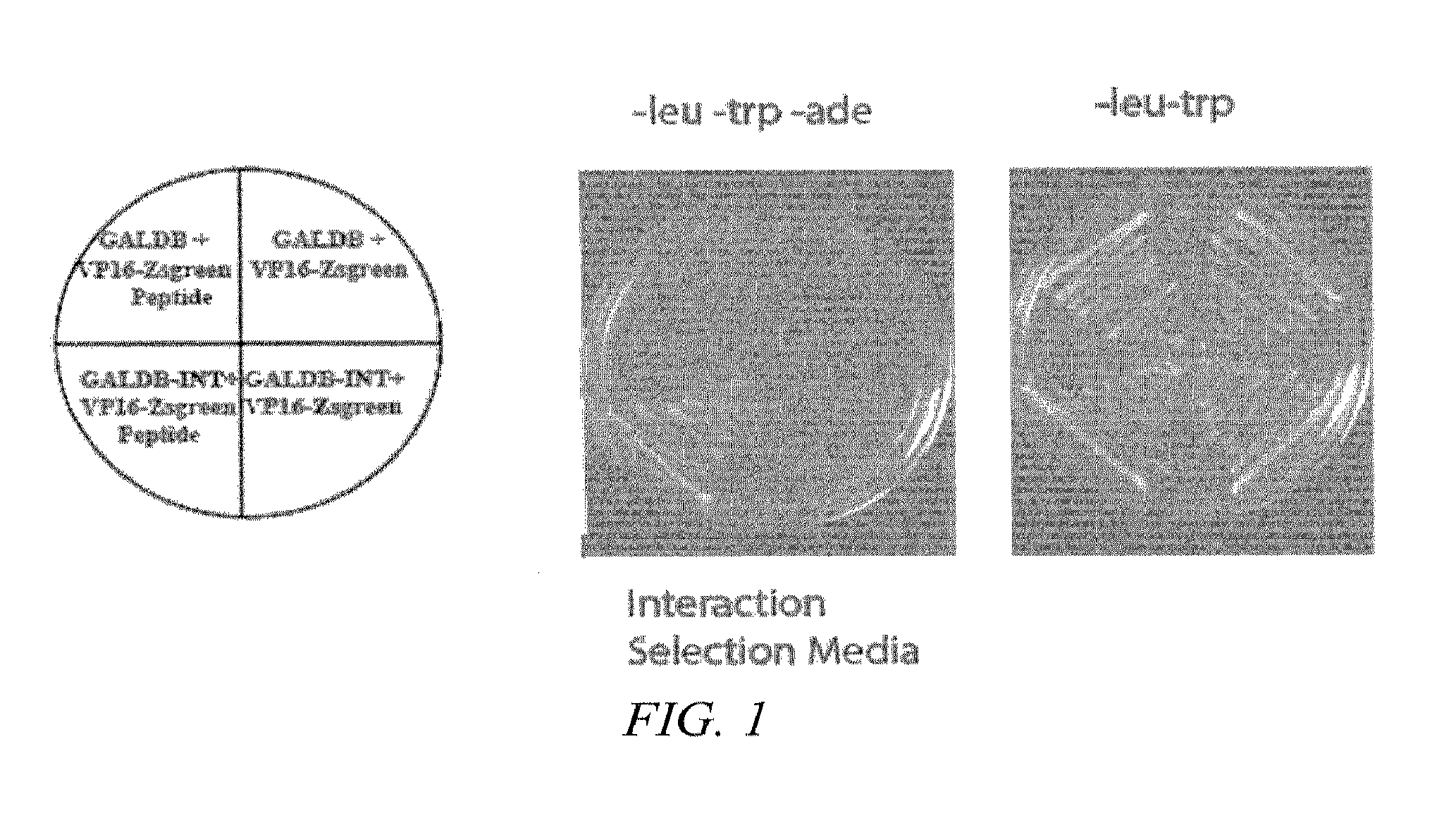
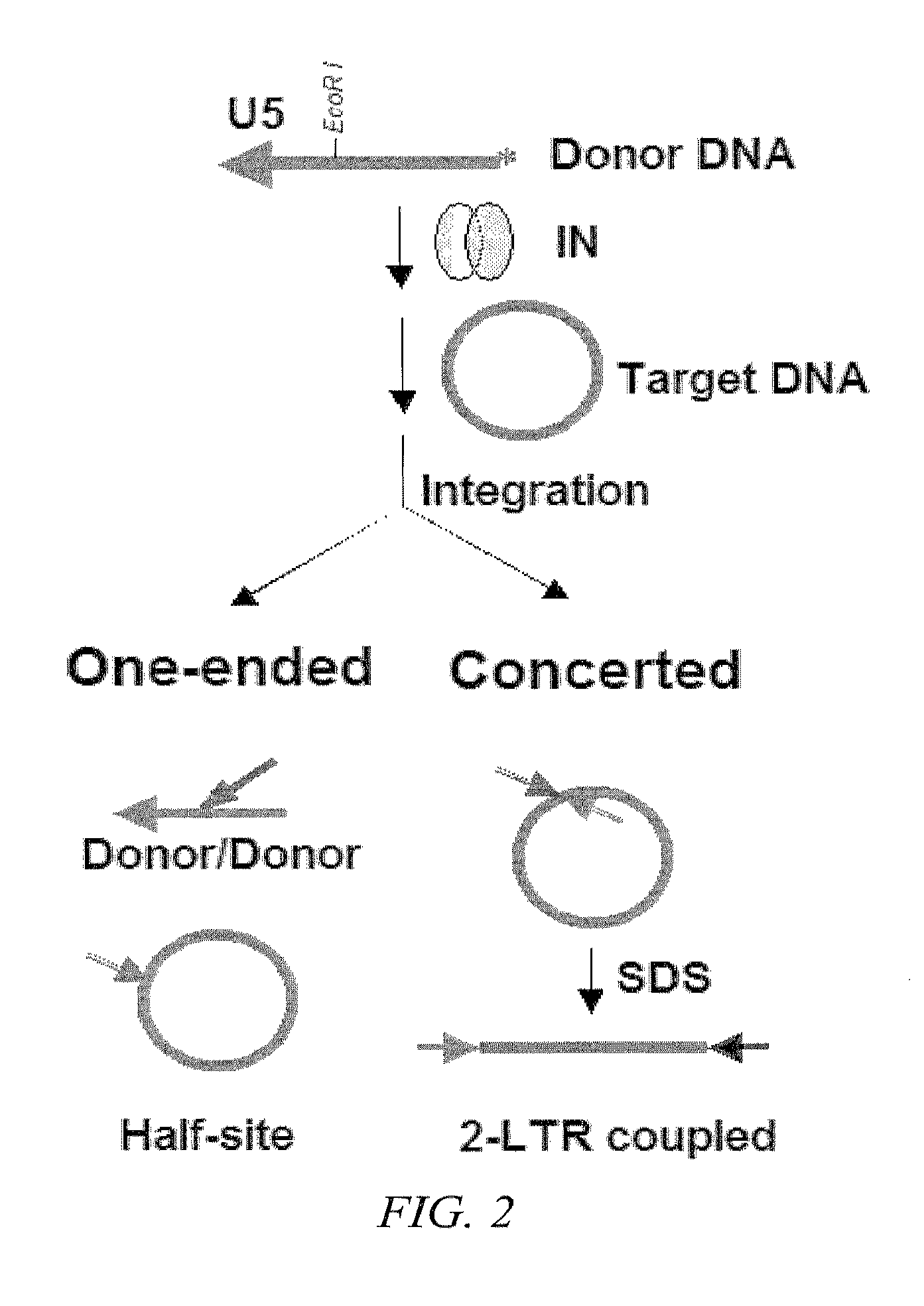
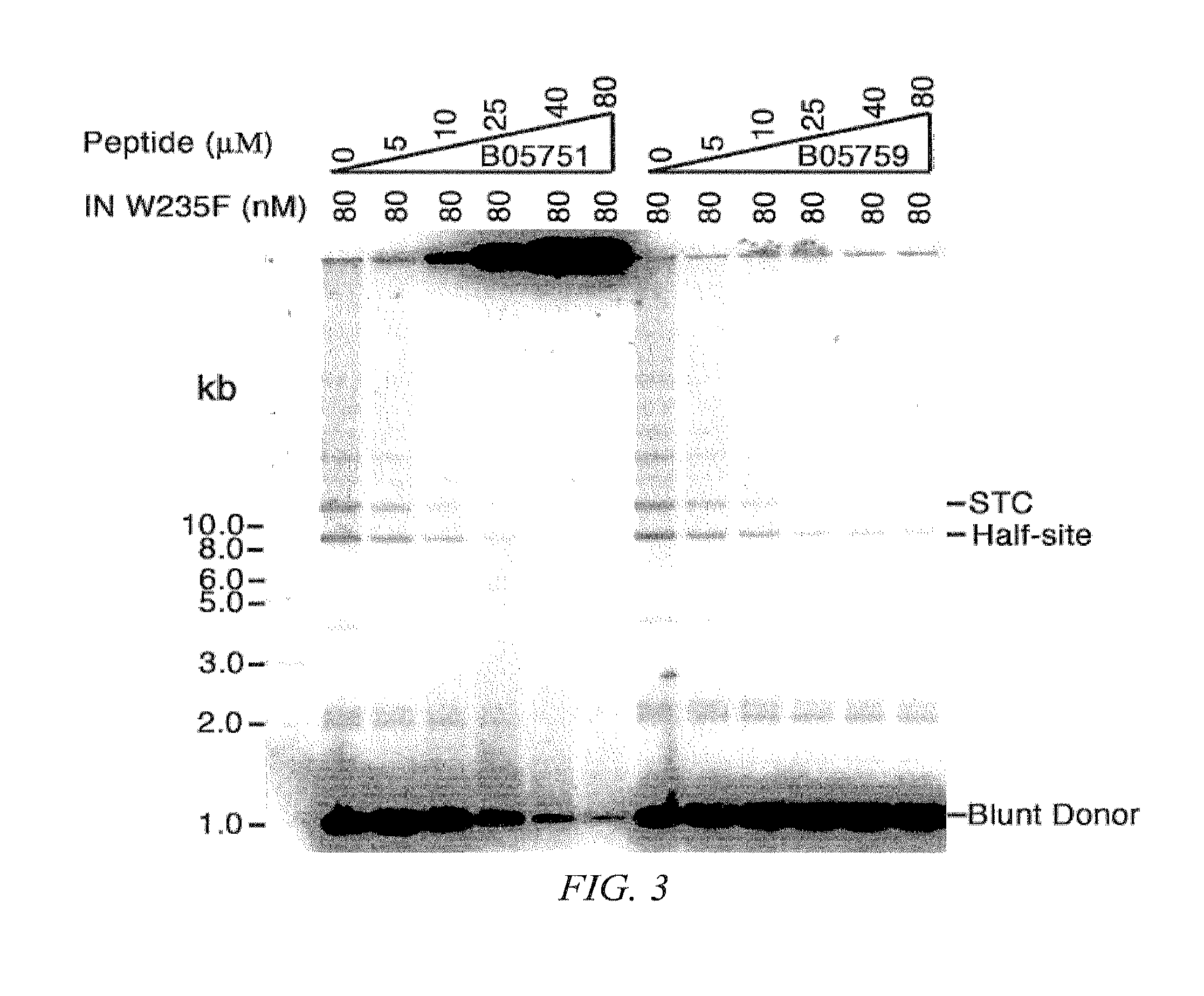
Synthetic nuclear localization signal derived from lentiviral integrase and methods of use thereof
InactiveUS7608699B2Preventing proviral nuclearPolypeptide with localisation/targeting motifSugar derivativesGene deliveryIntegrases
The present invention provides novel peptides, nucleic acids, vectors, compounds, compositions and methods for regulating nuclear import. The present invention also relates to a lentiviral NLS, and methods of use thereof for inhibiting HIV pathogenesis and disease progression, and for gene delivery methods.
Owner:THE ROCKEFELLER UNIV
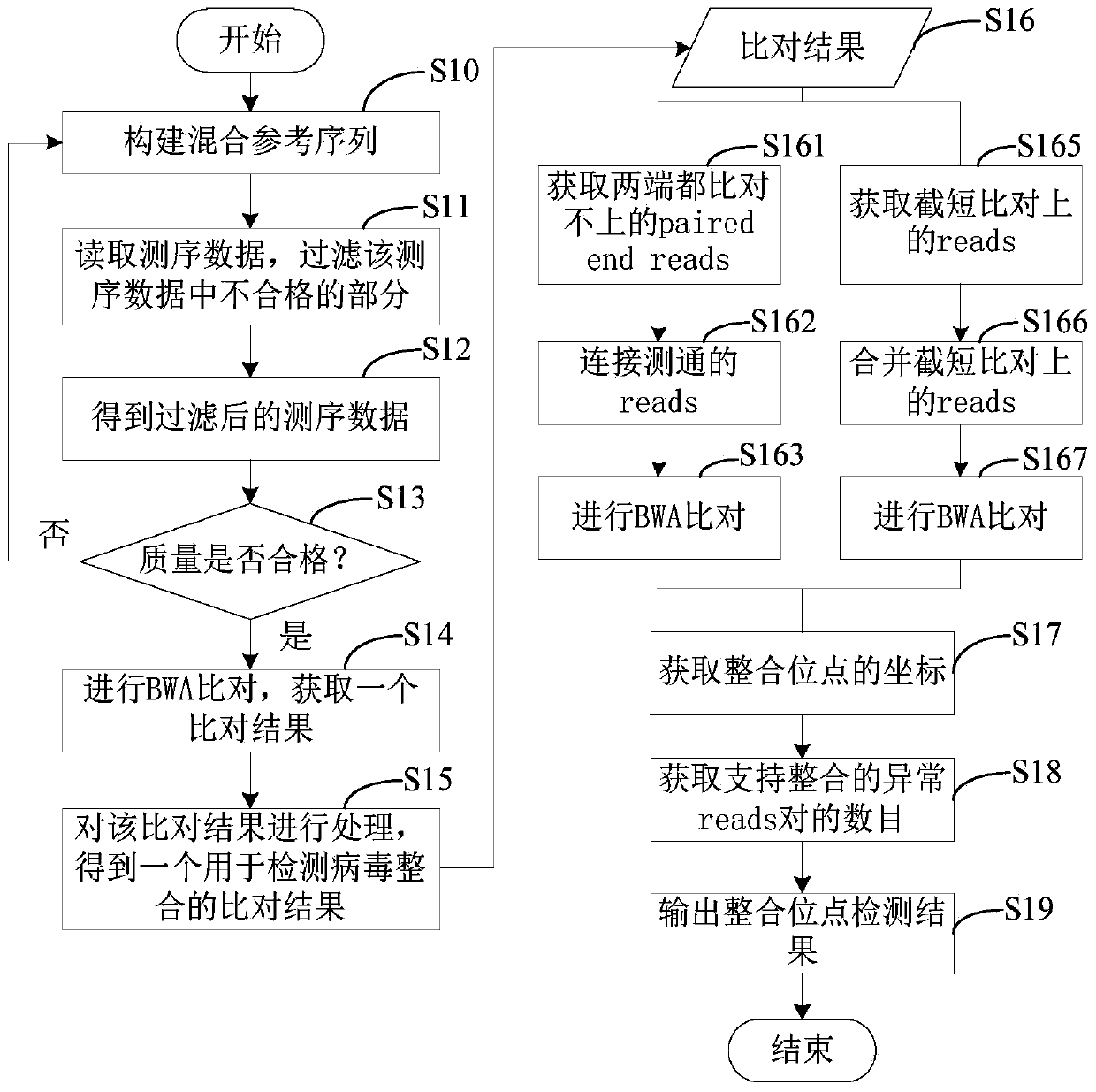
Method and device for detecting human genome virus integration site
ActiveCN110957008AHigh utility valuePrecise positioningBiostatisticsProteomicsHuman DNA sequencingGenome alignment
The invention relates to a method and a device for detecting a human reference genome virus integration site, and belongs to the technical field of gene detection bioinformatics analysis. The method comprises the steps of genome alignment, sliding cutting, short sequence alignment and integration. According to the method, reads which are not compared with a human reference genome and a virus genome at the same time are subjected to sliding cutting, the cut sub-sequences (or short sequences) are compared again, through clustering, correlation and covariance processing of the comparison positions and the sequence of a certain reads segmentation sequence, all highly possible comparison positions can be listed in the analysis process, reads for simultaneously comparing human reference genomesand virus genomes can be accurately found out and accurately positioned, and the error range is within 3bp. The method is low in calculation resource requirement and high in operation speed, and has relatively high practical value.
Owner:GUANGZHOU JINYUDA LOGISTICS CO LTD
HIV integrase inhibitors
The invention encompasses a series bicyclic pyrimidinone compounds of Formula I which inhibit HIV integrase and prevent viral integration into human DNA. This action makes the compounds useful for treating HIV infection and AIDS. The invention also encompasses pharmaceutical compositions and methods for treating those infected with HIV.
Owner:BRISTOL MYERS SQUIBB CO
Inhibitors of viral integrase and methods of use
Described herein are compositions and methods for inhibiting HIV integrase activity. Also described are methods of identifying agents that inhibit HIV integrase for use in treating or preventing HIV. Also disclosed are methods of identifying agents that inhibit HIV viral mutants that are resistant to integrase inhibitors.
Owner:UNITED STATES OF AMERICA +1
HIV Integrase Inhibitors
The disclosure generally relates to the novel compounds of formula I, including their salts, which inhibit HIV integrase and prevent viral integration into human DNA. This action makes the compounds useful for treating HIV infection and AIDS. The invention also encompasses pharmaceutical compositions and methods for treating those infected with HIV.
Owner:BRISTOL MYERS SQUIBB CO
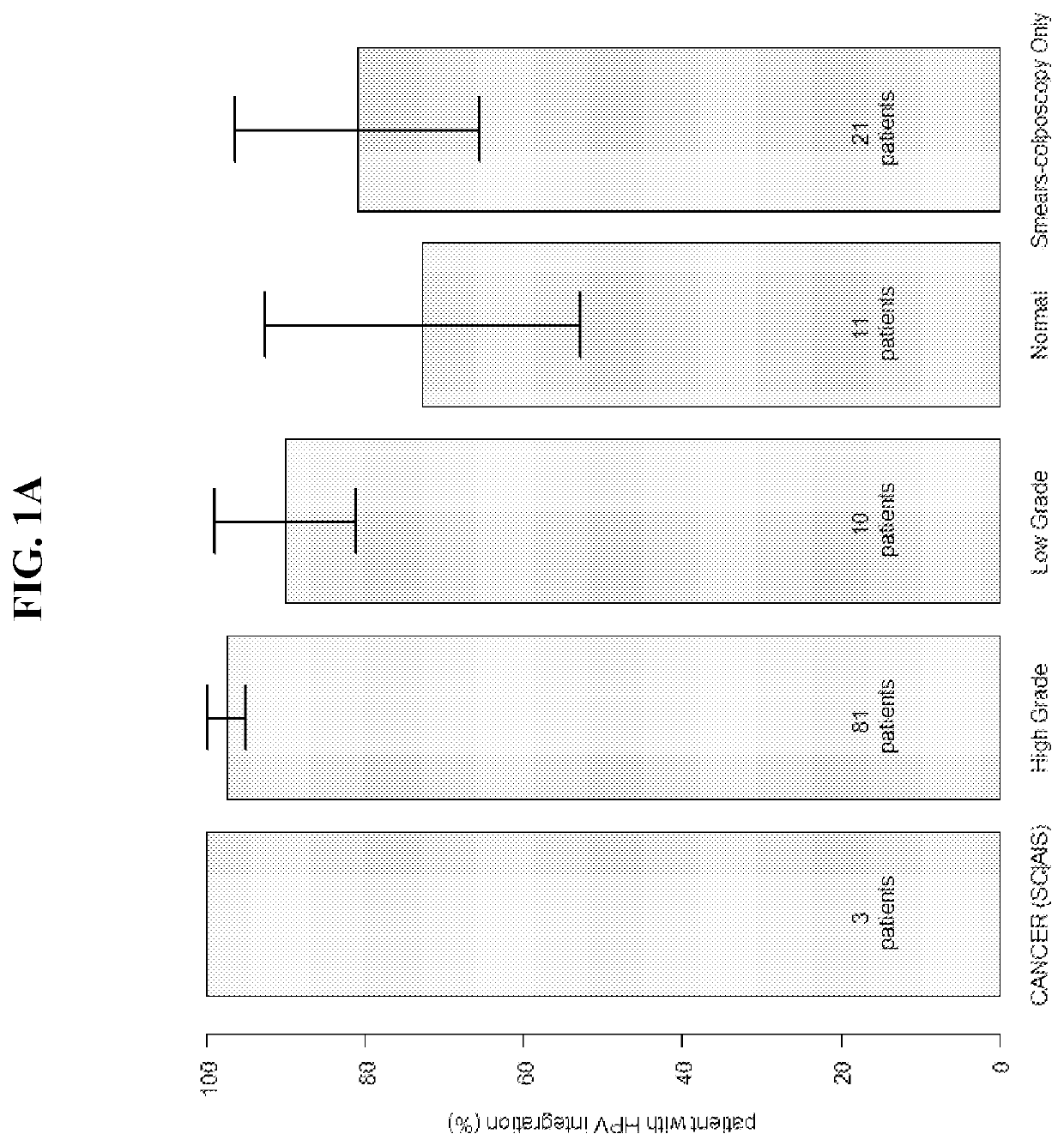
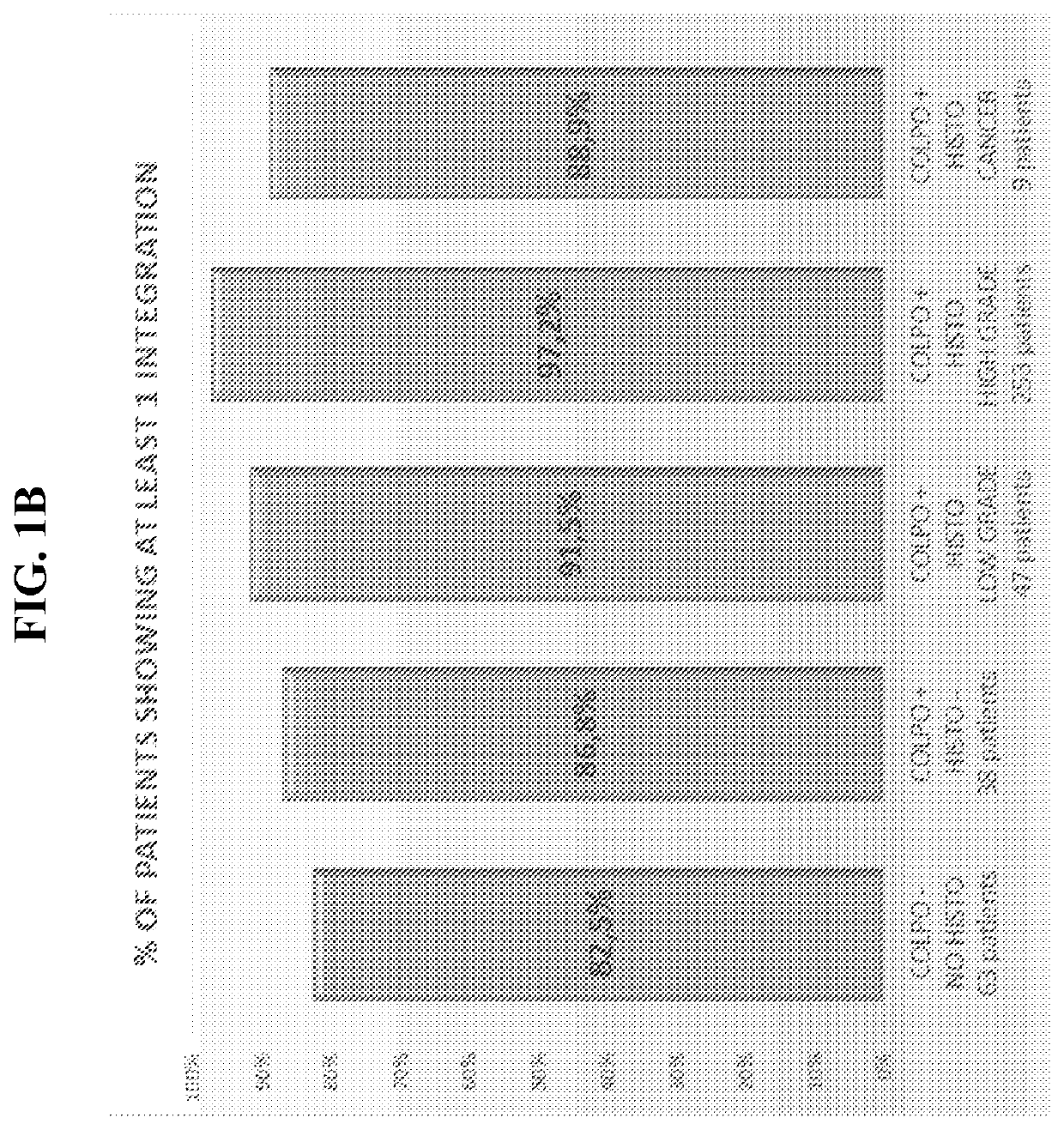
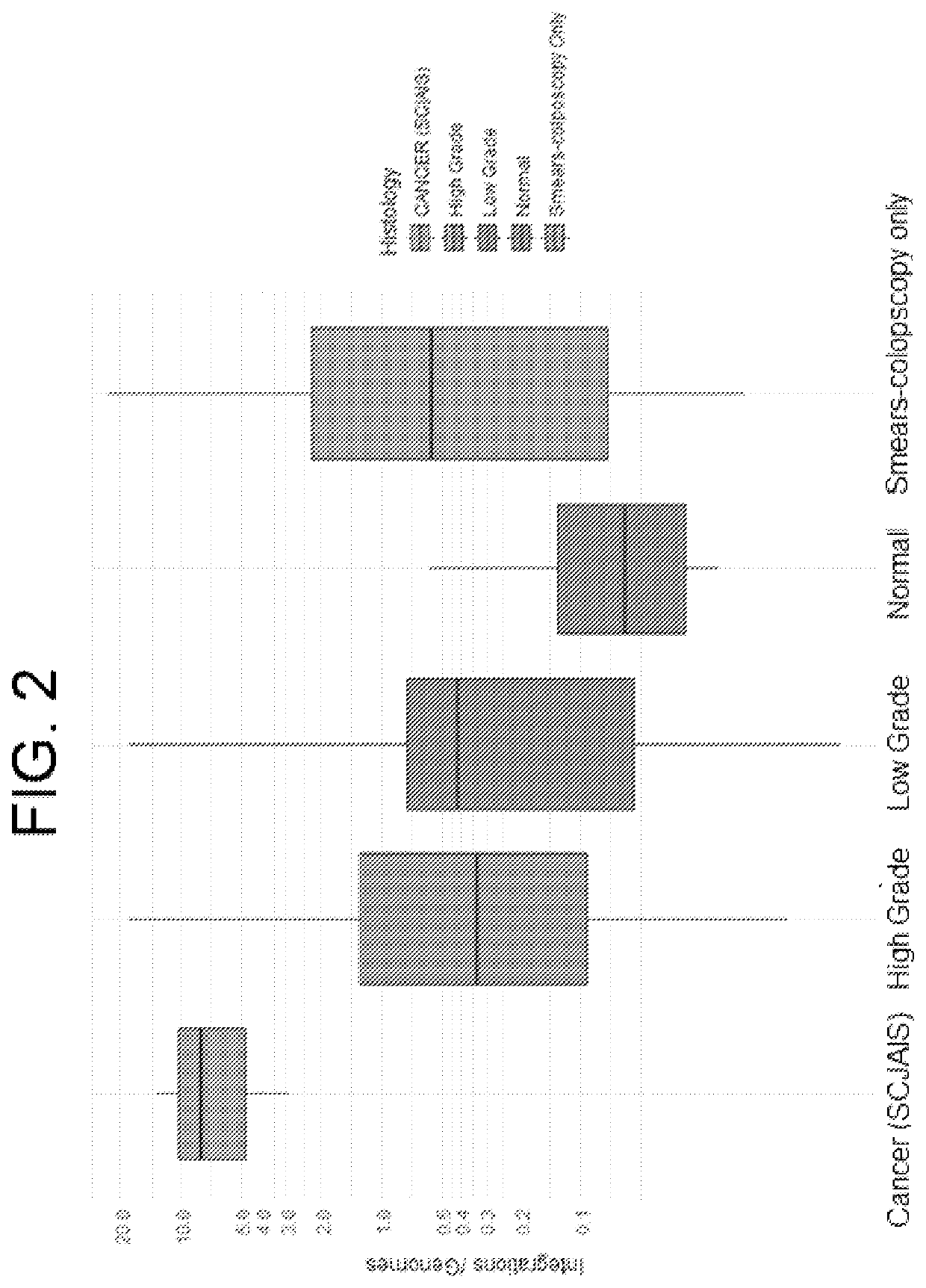
Association between integration of viral as HPV or HIV genomes and the severity and/or clinical outcome of disorders as HPV associated cervical lesions or aids pathology
The invention concerns the detection and the quantification of integrated nucleic acids of viruses and thus the detection and follow-up of reservoir cells harbouring such integrated viral genomes. One aspect of the invention is directed to a method for detecting a level of integrated viral DNA that includes removing episomal viral or vector nucleic acids from genomic DNA in a cell sample, and quantifying a number of integrations of viral DNA into the genomic DNA of the cells by a method of amplification of an integration region in the DNA sample; thereby detecting a level of integrated viral DNA in the genomic DNA from a cell sample, such as a biological sample containing HPV virus and DNA.
Owner:GENOMIC VISION
Method for detecting integrated copy number of porcine endogenous retrovirus (PERV) through fluorescence quantitative polymerase chain reaction (PCR) and application thereof
InactiveCN101845518AAccurate detectionHigh sensitivityMicrobiological testing/measurementFluorescence/phosphorescencePol genesFluorescence
The invention discloses a method for detecting the integrated copy number of a porcine endogenous retrovirus (PERV) through a fluorescence quantitative polymerase chain reaction (PCR) and application thereof. In the method, the copy number of the porcine endogenous retrovirus which is integrated in a genome is detected by using SYBR Green I dye-based fluorescence quantitative PCR technology, and a PERV pol gene conservative region is taken as a detection object. The detection method has the advantages of high sensitivity, high specificity, good repeatability, rapidness, simpleness and convenience, low cost, capability of rapidly and correctly detecting the copy number of the PERV and estimating the copy number of the PERV which is integrated in a single cell genome, suitability for rapidly screening the copy number of the PERV integrated in a heteroplastic small pig donor and a porcine biological material genome and performing long-term monitoring and forward evaluation on the spreading of heteroplastic PERV, deep practical meaning and wide application prospect.
Owner:FIELD OPERATION BLOOD TRANSFUSION INST OF PLA SCI ACAD OF MILITARY +1
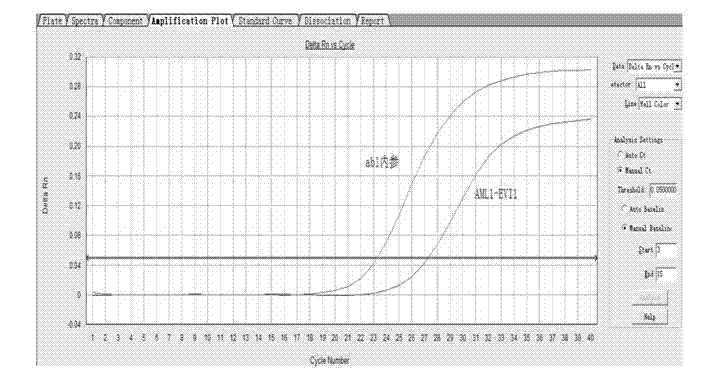
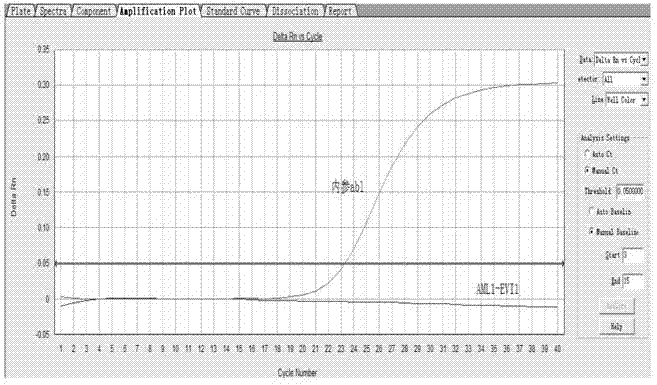
Kit for detecting relevant expression quantity of AML-EVI1 (acute myelocytic leukemia-ecotropic virus integration site 1) fusion gene
ActiveCN102776282AHigh precisionEasy to readMicrobiological testing/measurementGene expression levelMyelocytic leukemia
The invention discloses a kit for detecting relevant expression quantity of an AML-EVI1 (acute myelocytic leukemia-ecotropic virus integration site-1) fusion gene. The kit comprises red blood cell lysis buffer, TRIzol, chloroform, absolute ethyl alcohol, RevertraAceqPCRRTKit, detecting system PCR (polymerase chain reaction) liquid, a positive reference substance and a negative reference substance. The kit is characterized in that the detecting system PCR reaction liquid comprises THUNDERBIRDqPCRMIX; a target gene is detected via upstream and downstream primers AML1-EVI1-F and AML1-EVI1-R and a probe AML1-EVI1-Probe; and an internal control gene Abl is detected via primers abl-F and abl-R and a probe abl-Probe. The kit disclosed by the invention can detect the expression level of the AML1-EVI1 fusion engine in a patient body subjected to CML (chronicmyelognousleukemia), thus, the detecting time can be effectively salved, and the detecting precision can be increased.
Owner:WUHAN ADICON CLINICAL LAB
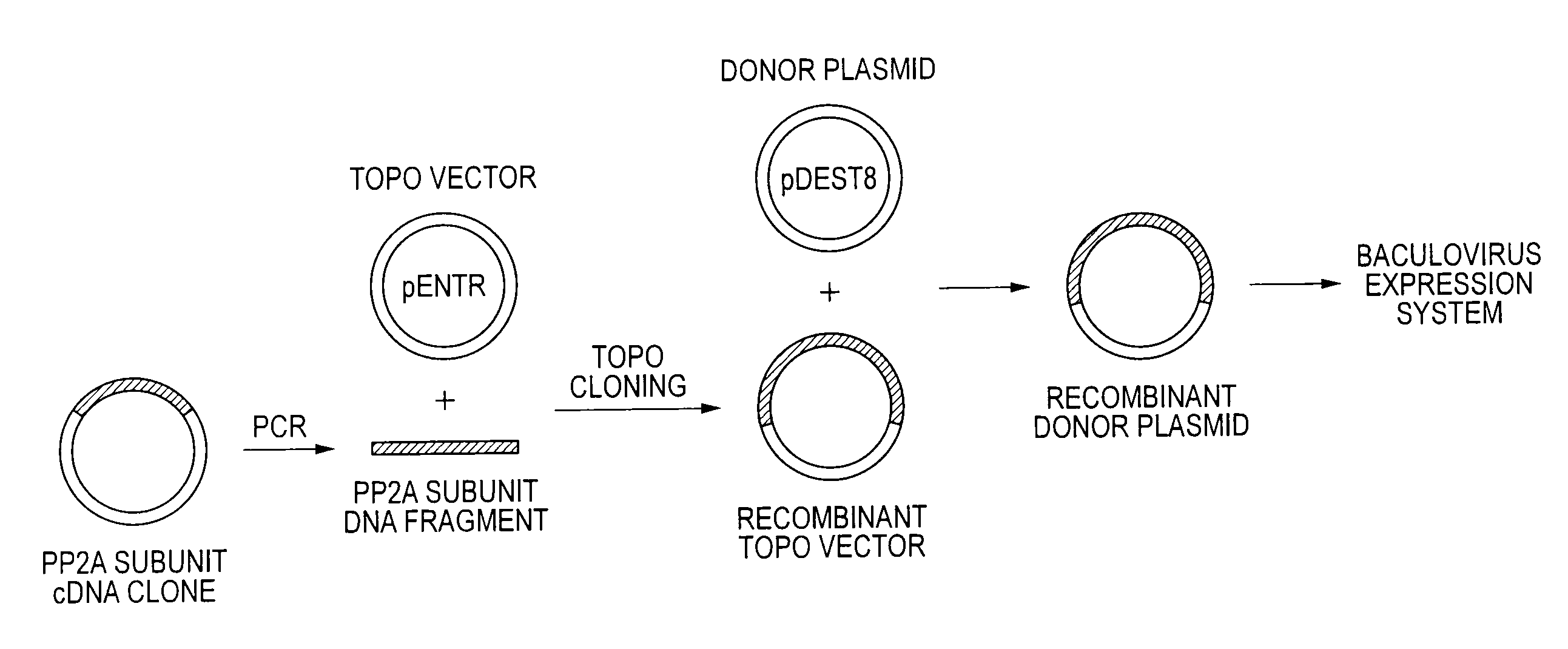
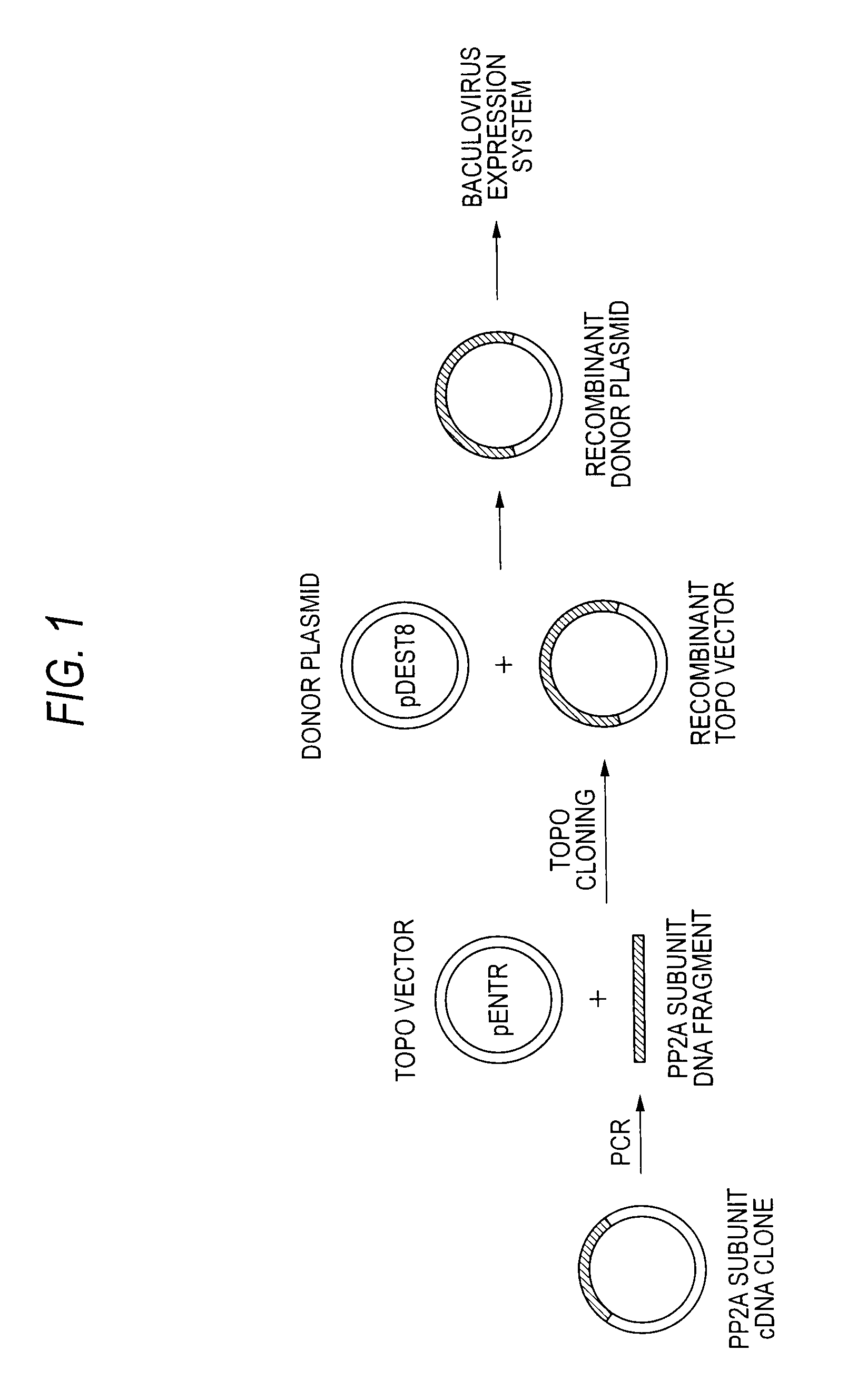
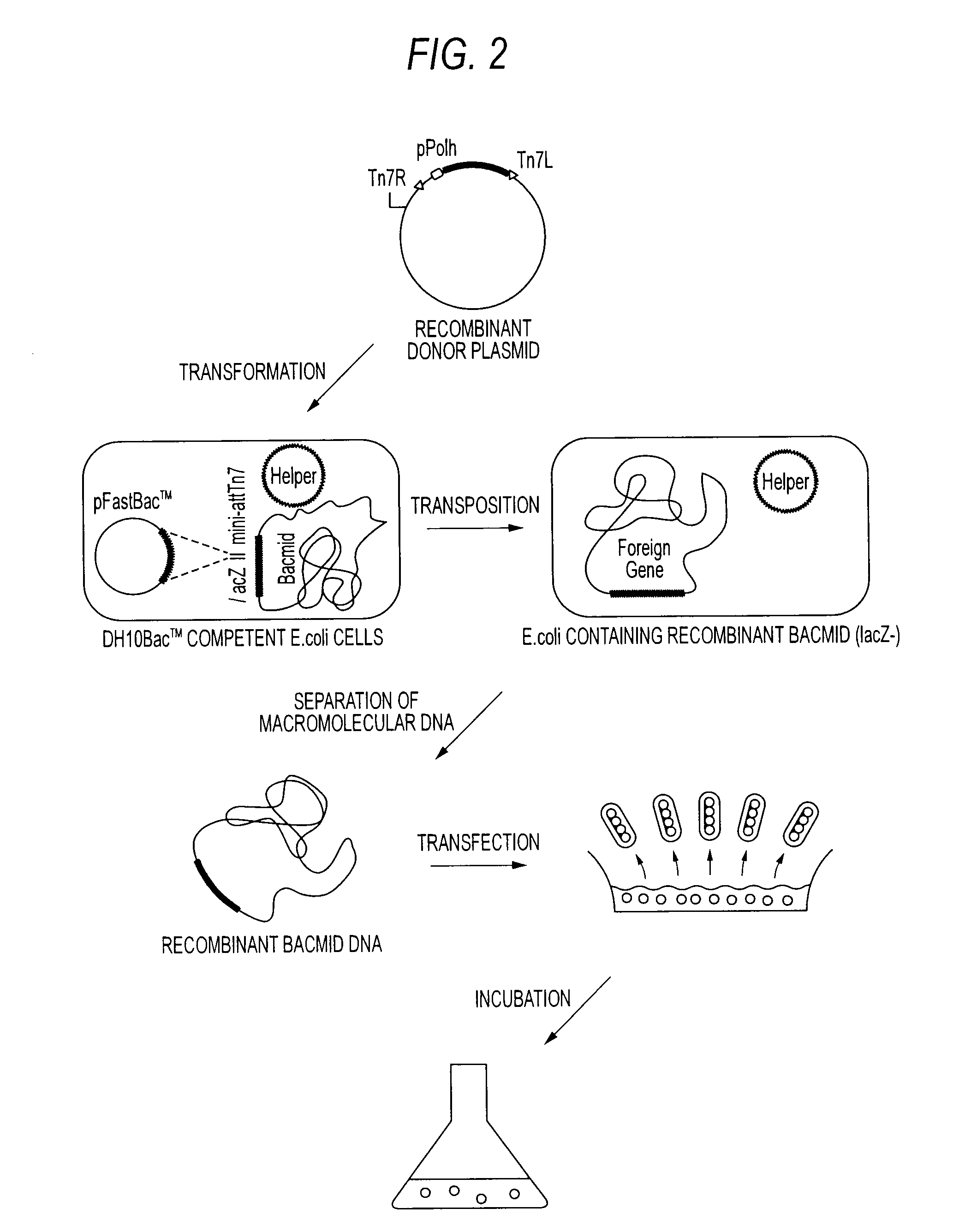
Method of producing heterodimer derivative of protein phosphatase type 2a enzyme
The purpose of the invention is to provide an activated protein phosphatase 2A (PP2A) in large quantities with high purity by a genetic engineering and to provide a method for producing a heterodimer derivative of PP2A which comprises infecting insect cultured cells with a baculovirus in which a cDNA encoding the catalytic subunit of PP2A carrying a first tag is integrated together with another baculovirus in which a cDNA encoding the A subunit of PP2A carrying a second tag is integrated, incubating the infected cells, disrupting the incubated cells to obtain a disrupted cell suspension, and then purifying the disrupted cell suspension with a solid phase carrying a substance capable of binding to the first tag and another solid phase carrying a substance capable of binding to the second tag, characterized in that the insect cells infected with the baculovirus are incubated at a temperature of from 18 to 22° C.
Owner:TROPICAL TECH CENT LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com




![Substituted imidazo[1,2-a]pyrimidines as HIV viral DNA integrase inhibitors Substituted imidazo[1,2-a]pyrimidines as HIV viral DNA integrase inhibitors](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/6adf7269-b42f-4b83-9357-b8de6749ea21/US07494984-20090224-C00001.png)
![Substituted imidazo[1,2-a]pyrimidines as HIV viral DNA integrase inhibitors Substituted imidazo[1,2-a]pyrimidines as HIV viral DNA integrase inhibitors](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/6adf7269-b42f-4b83-9357-b8de6749ea21/US07494984-20090224-C00002.png)
![Substituted imidazo[1,2-a]pyrimidines as HIV viral DNA integrase inhibitors Substituted imidazo[1,2-a]pyrimidines as HIV viral DNA integrase inhibitors](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/6adf7269-b42f-4b83-9357-b8de6749ea21/US07494984-20090224-C00003.png)