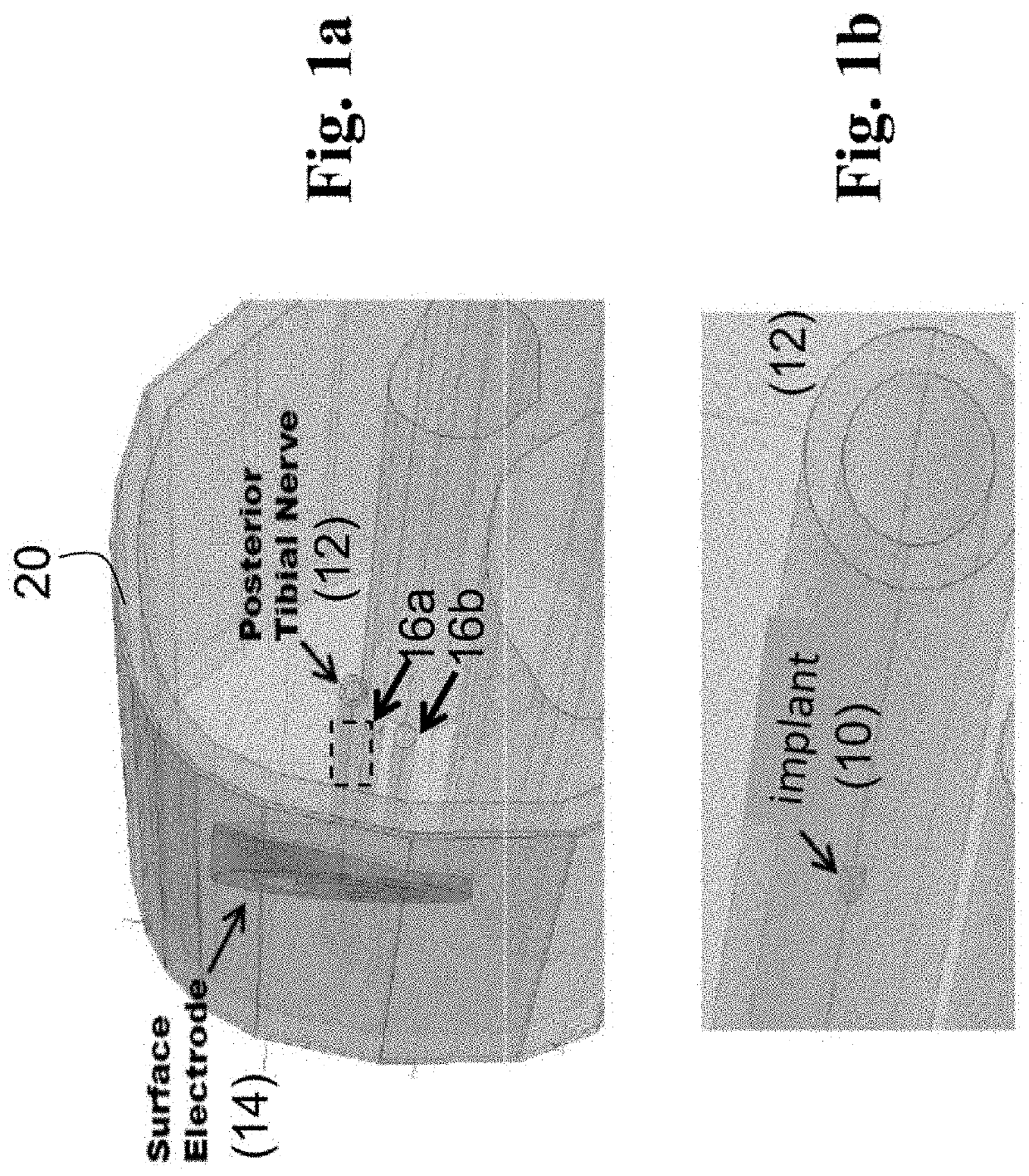
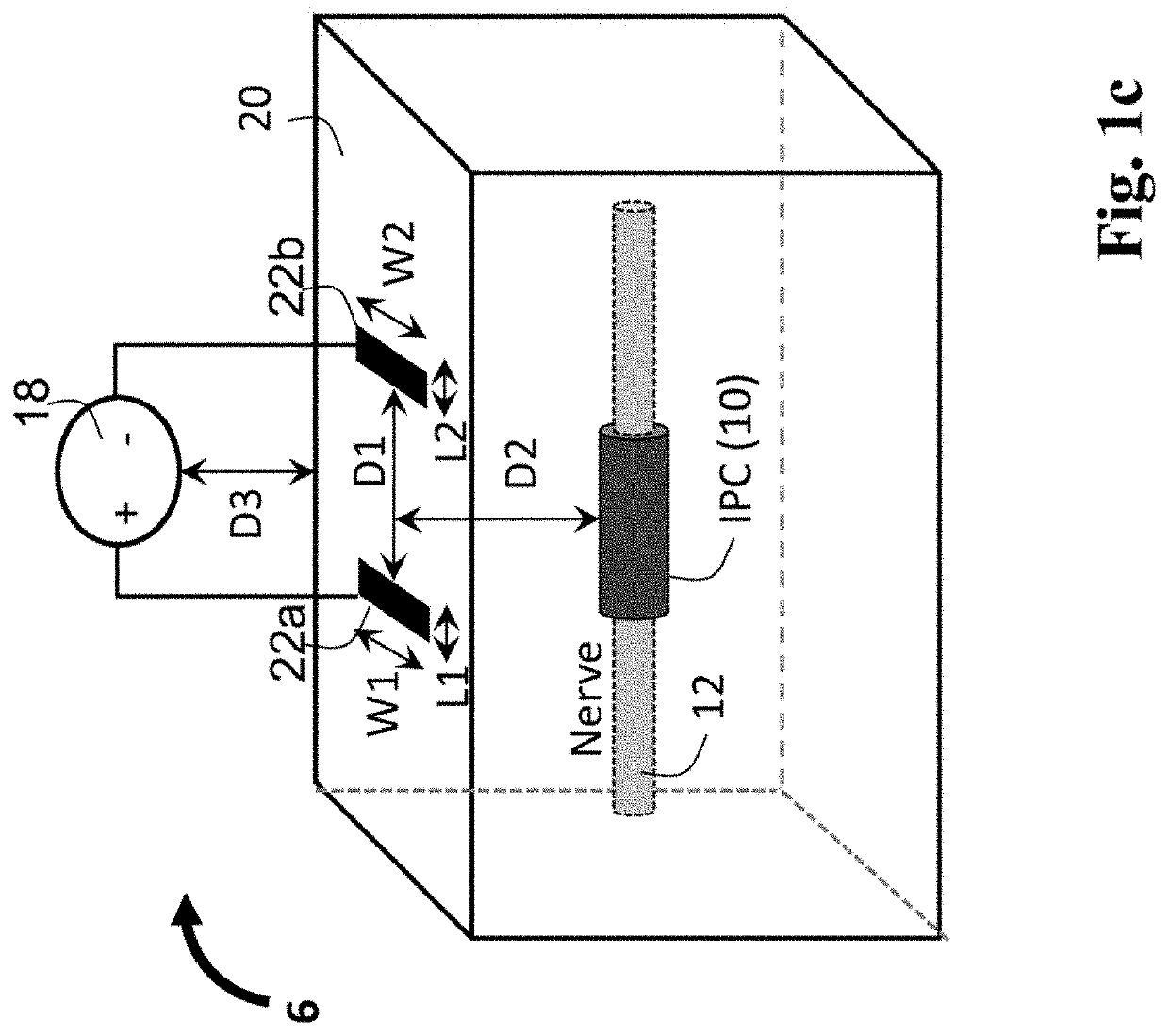
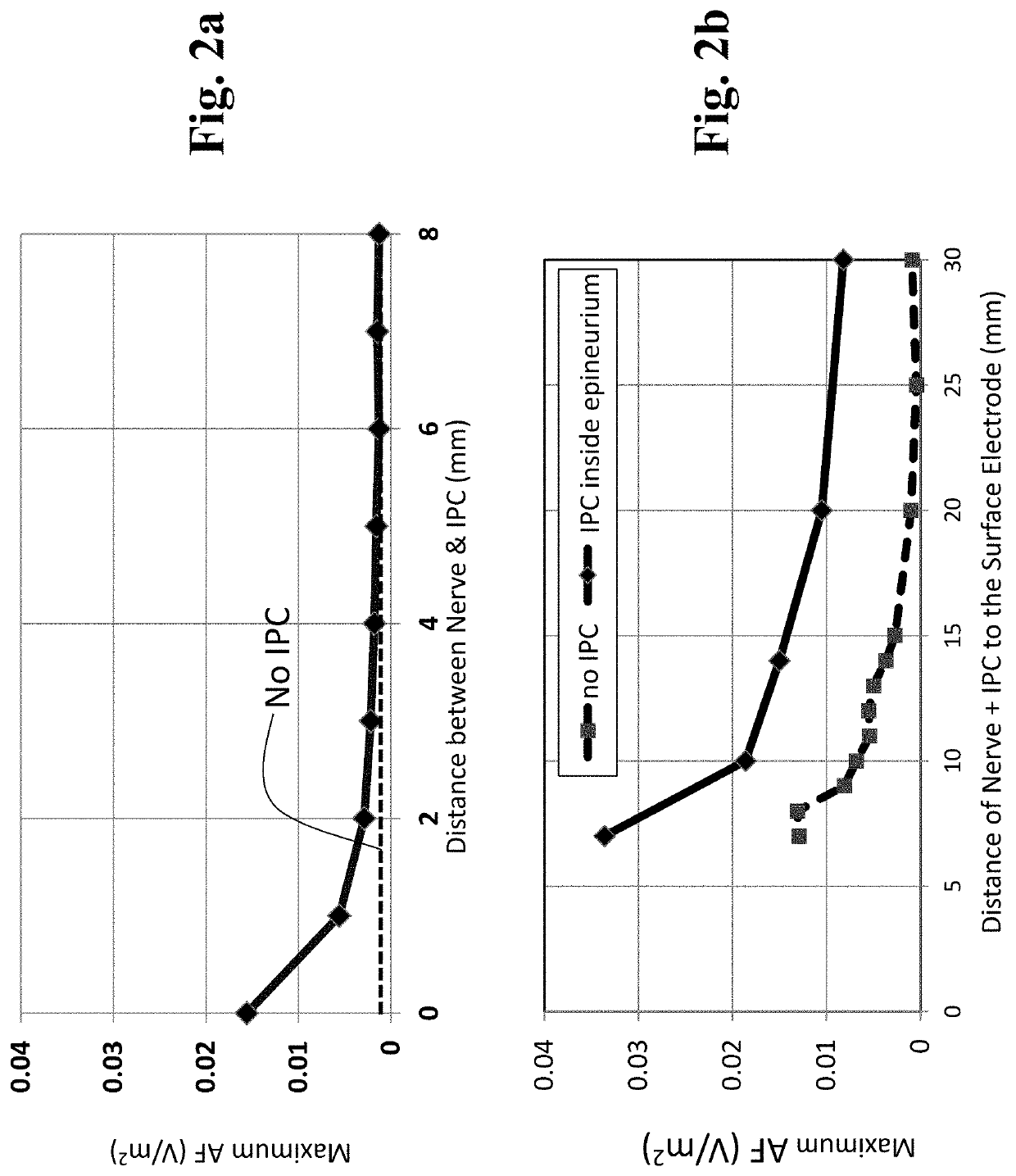
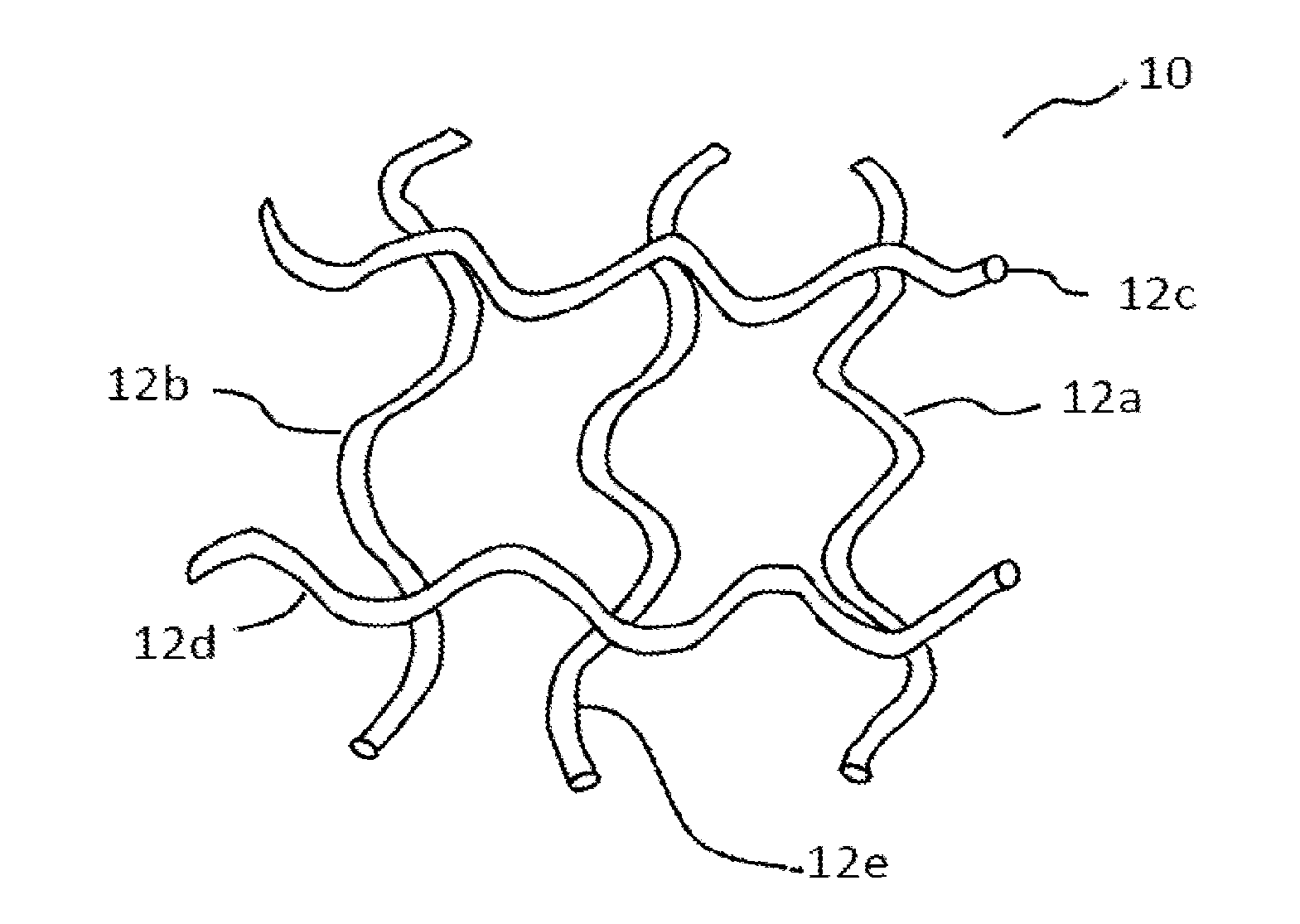
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
44 results about "Pelvic Floor Disorders" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
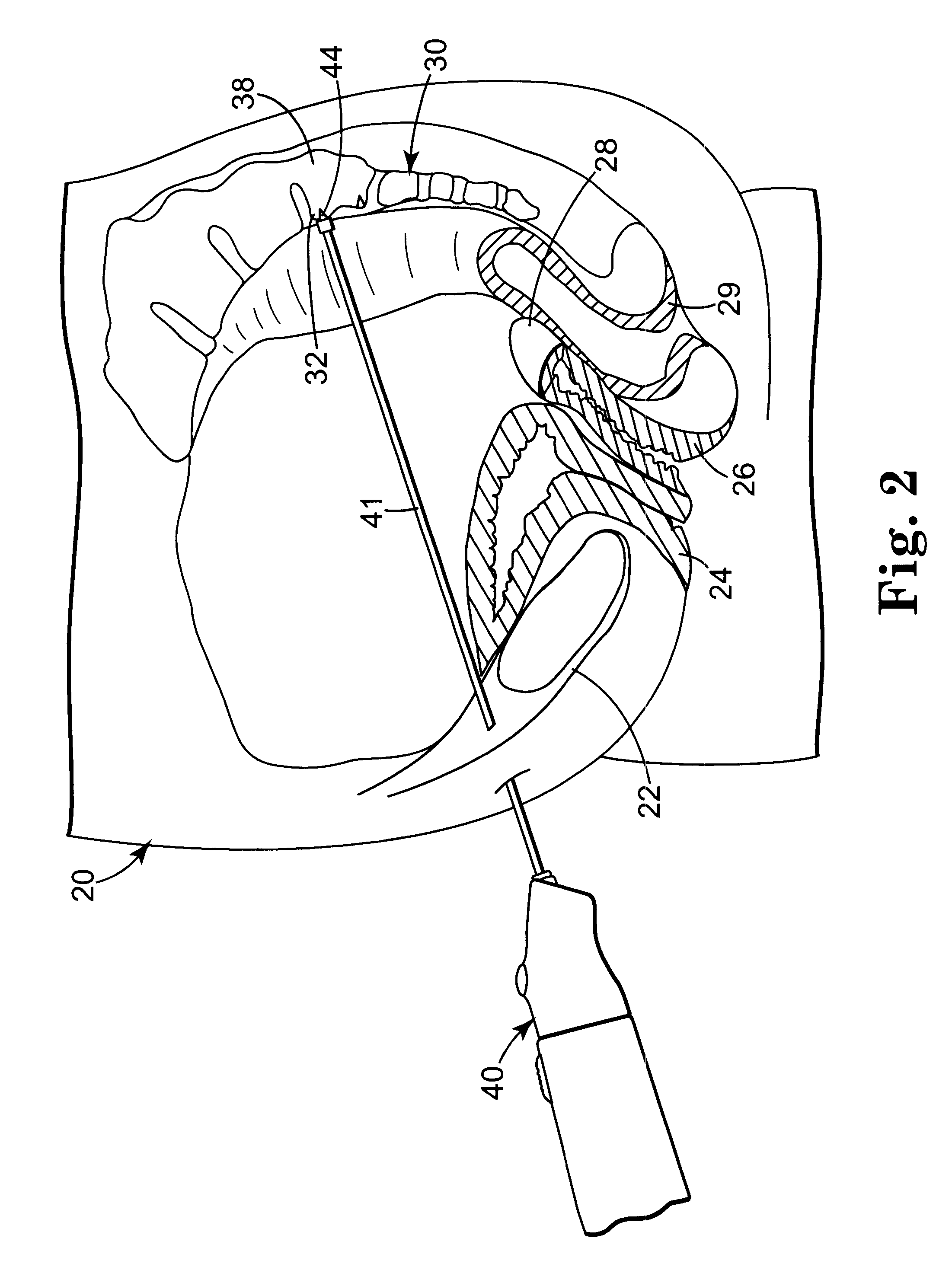
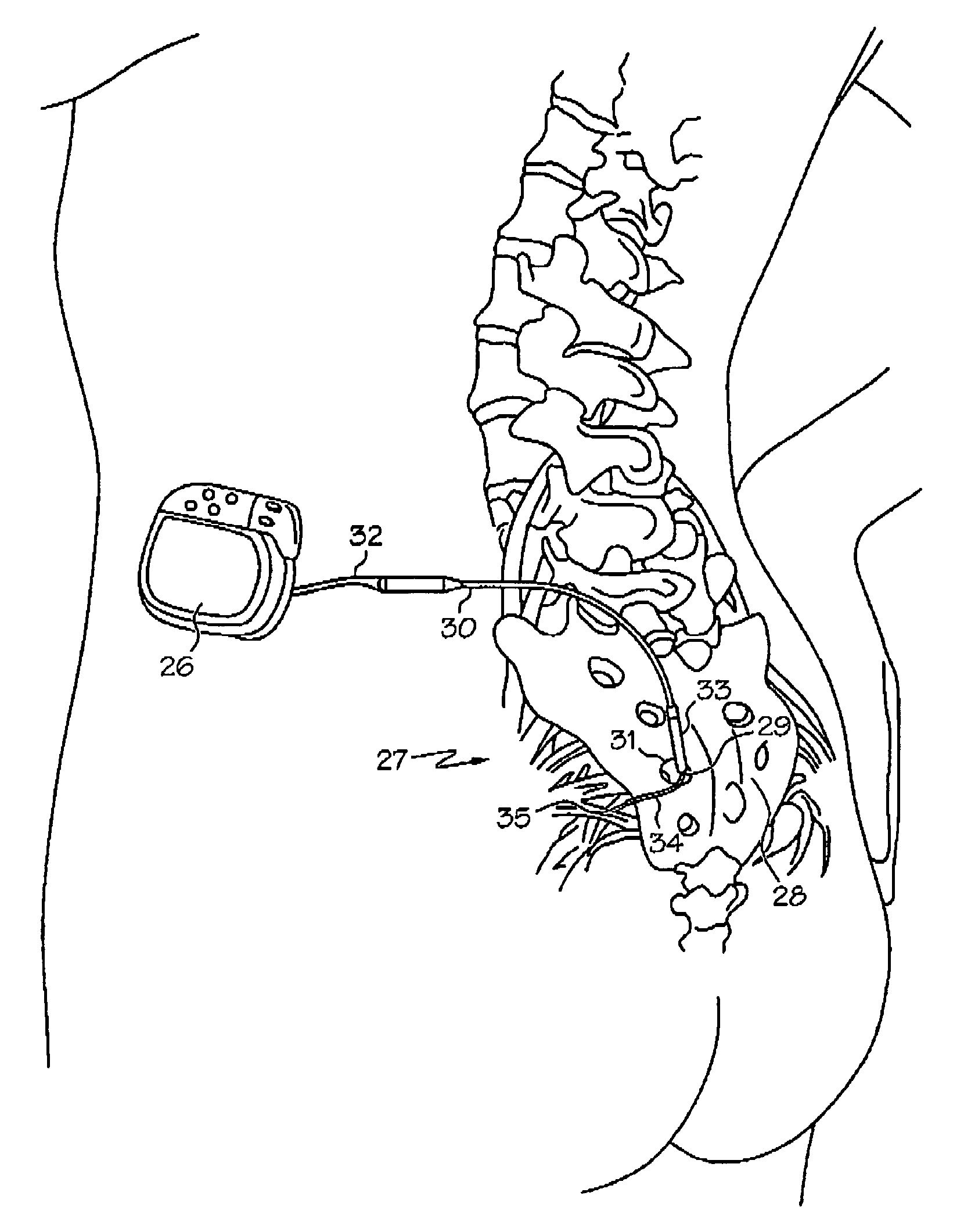
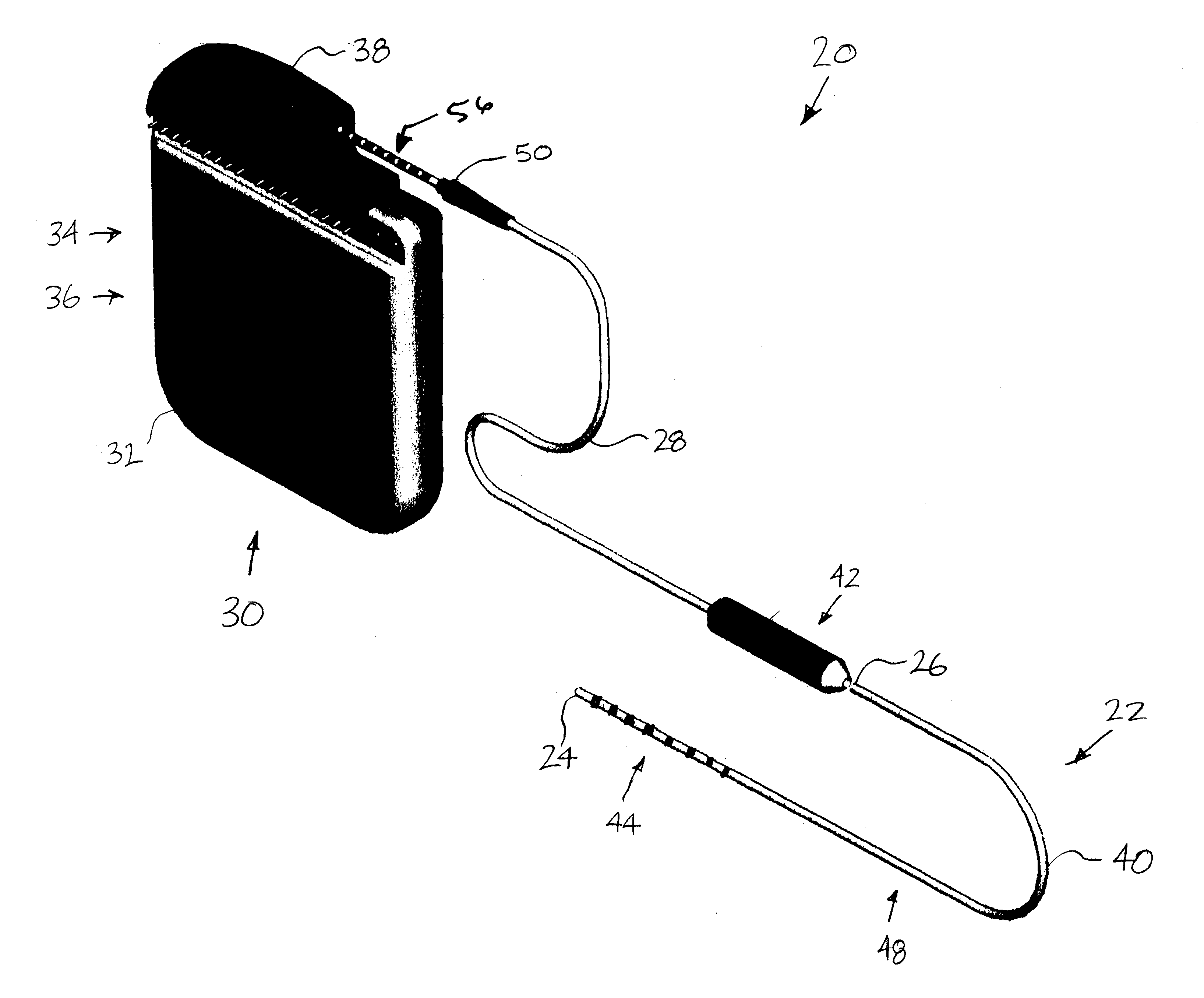
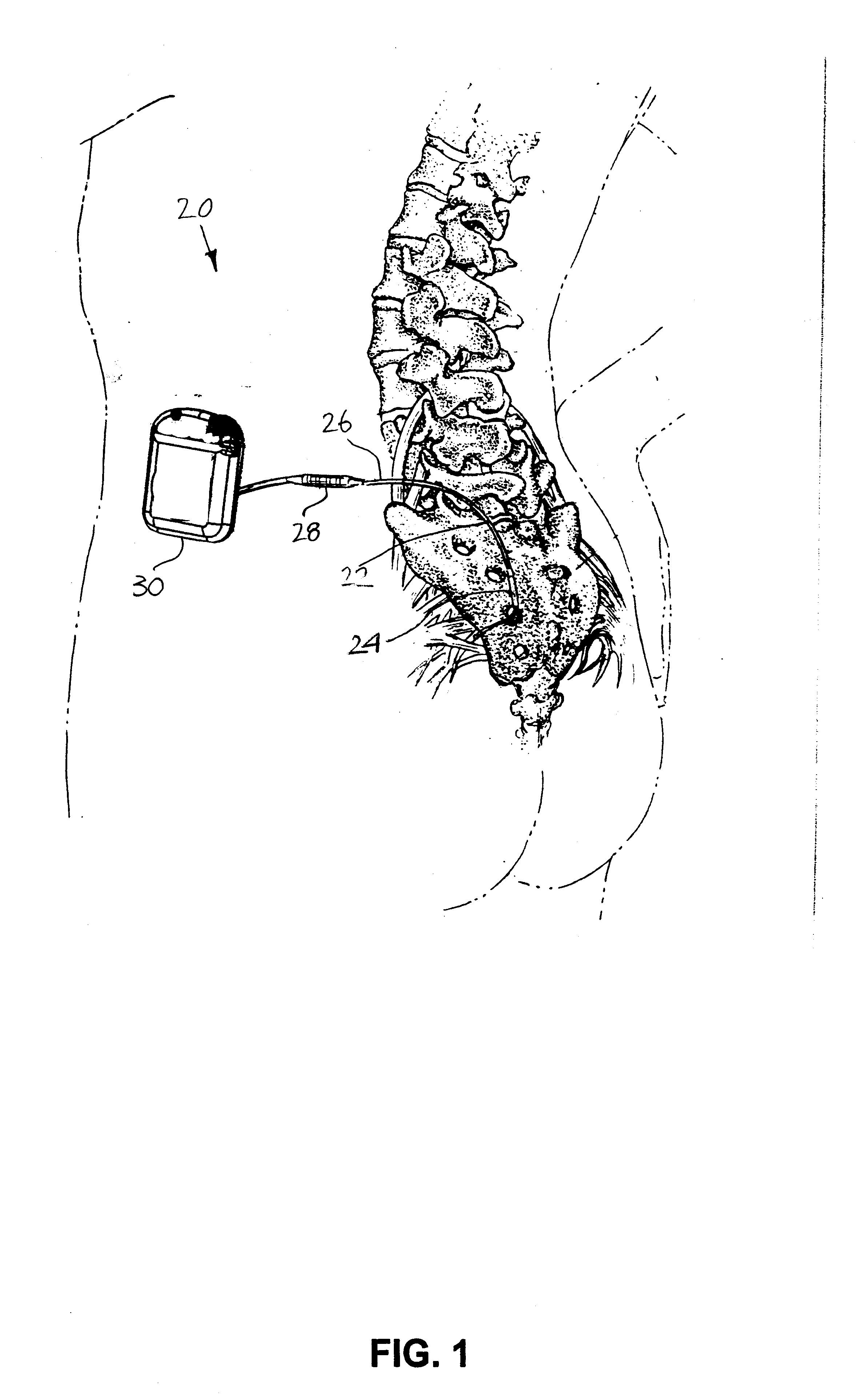
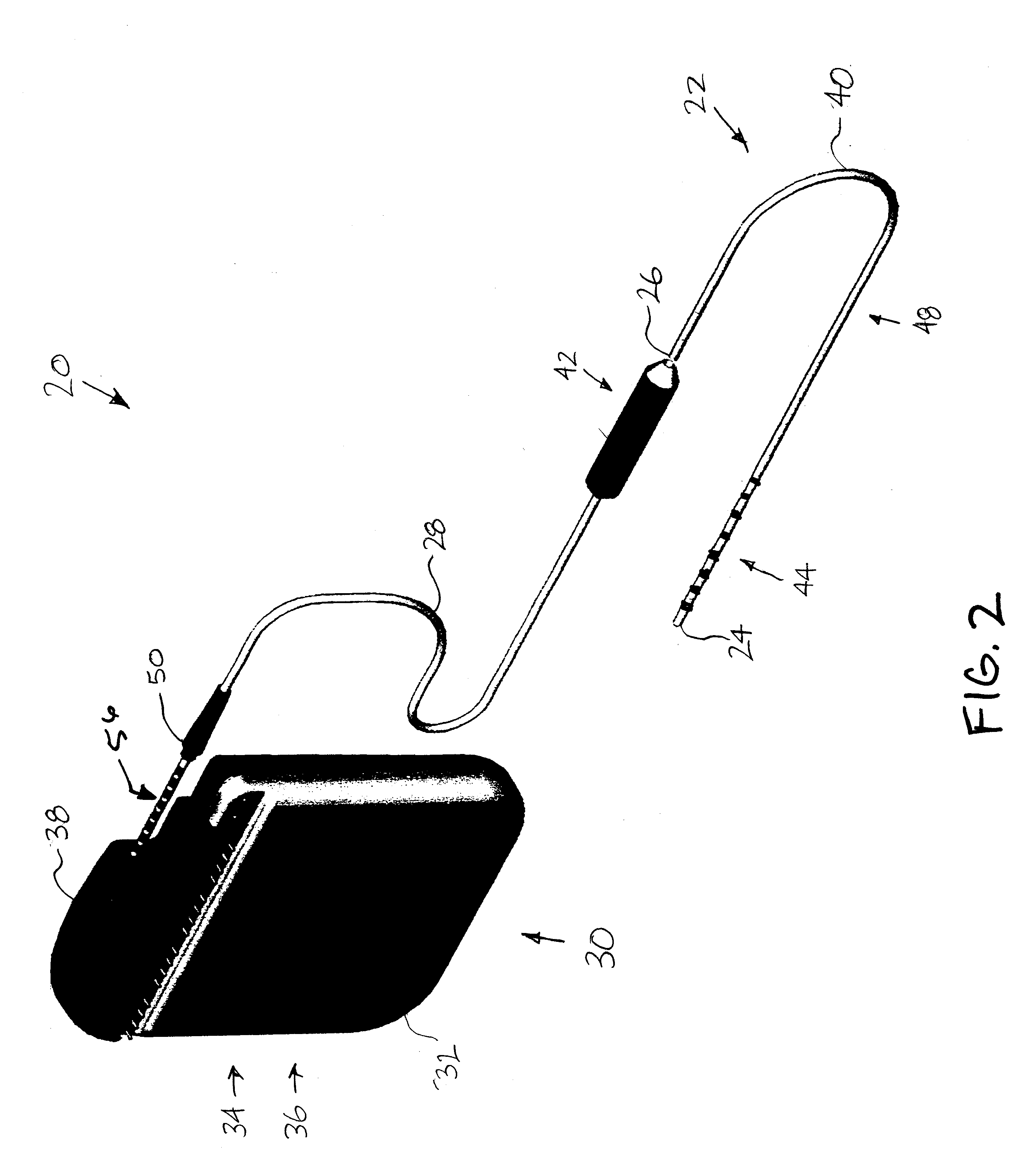
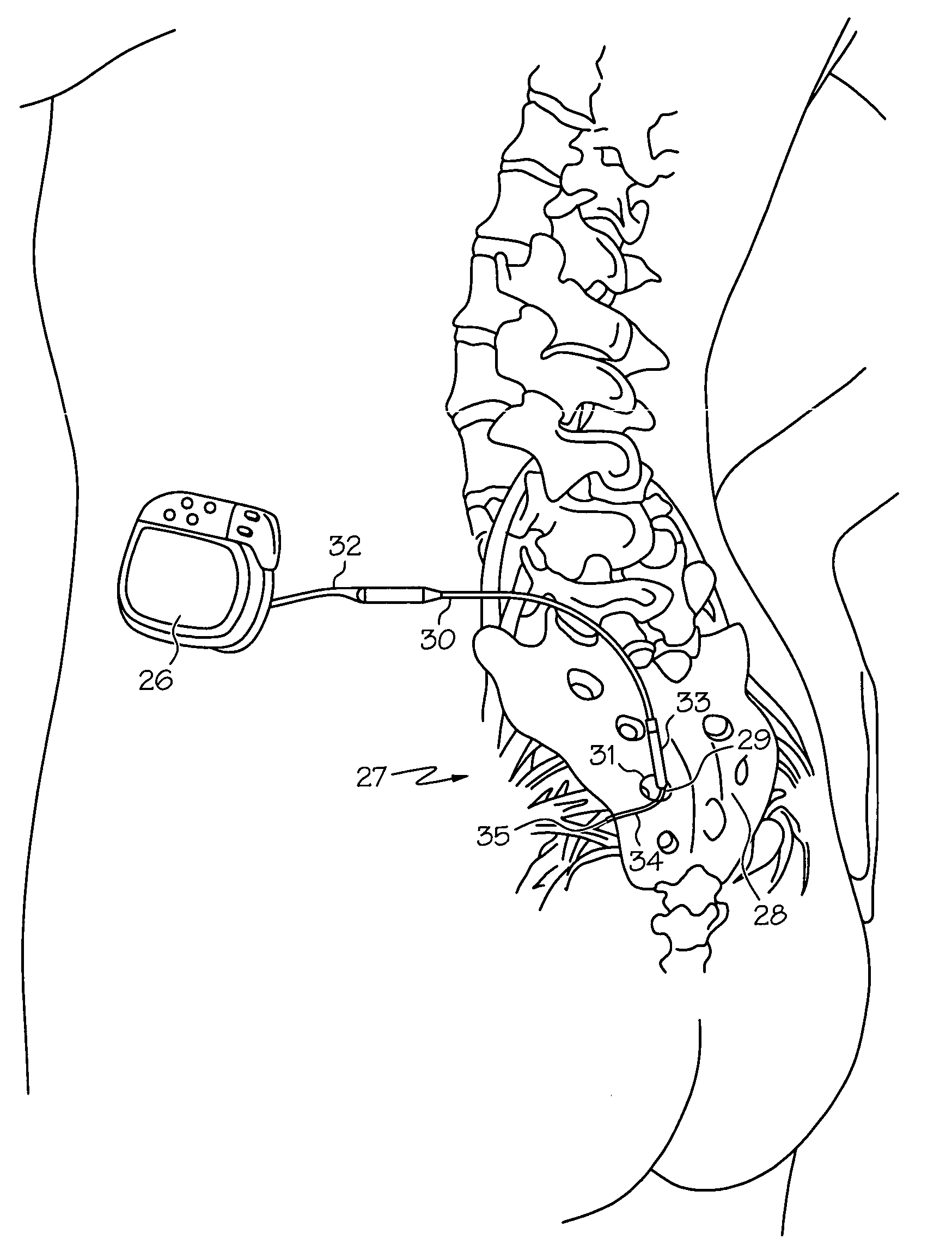
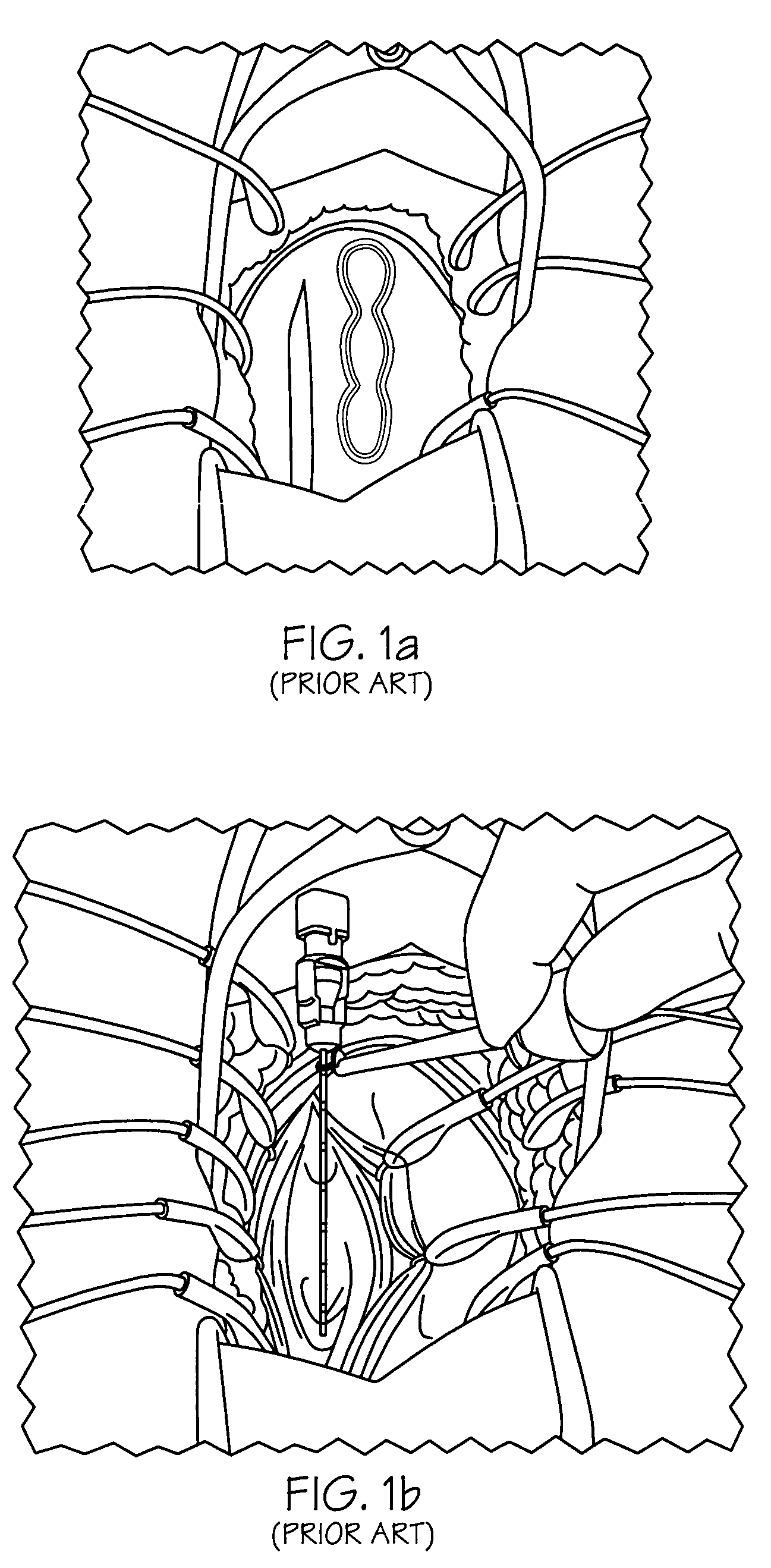
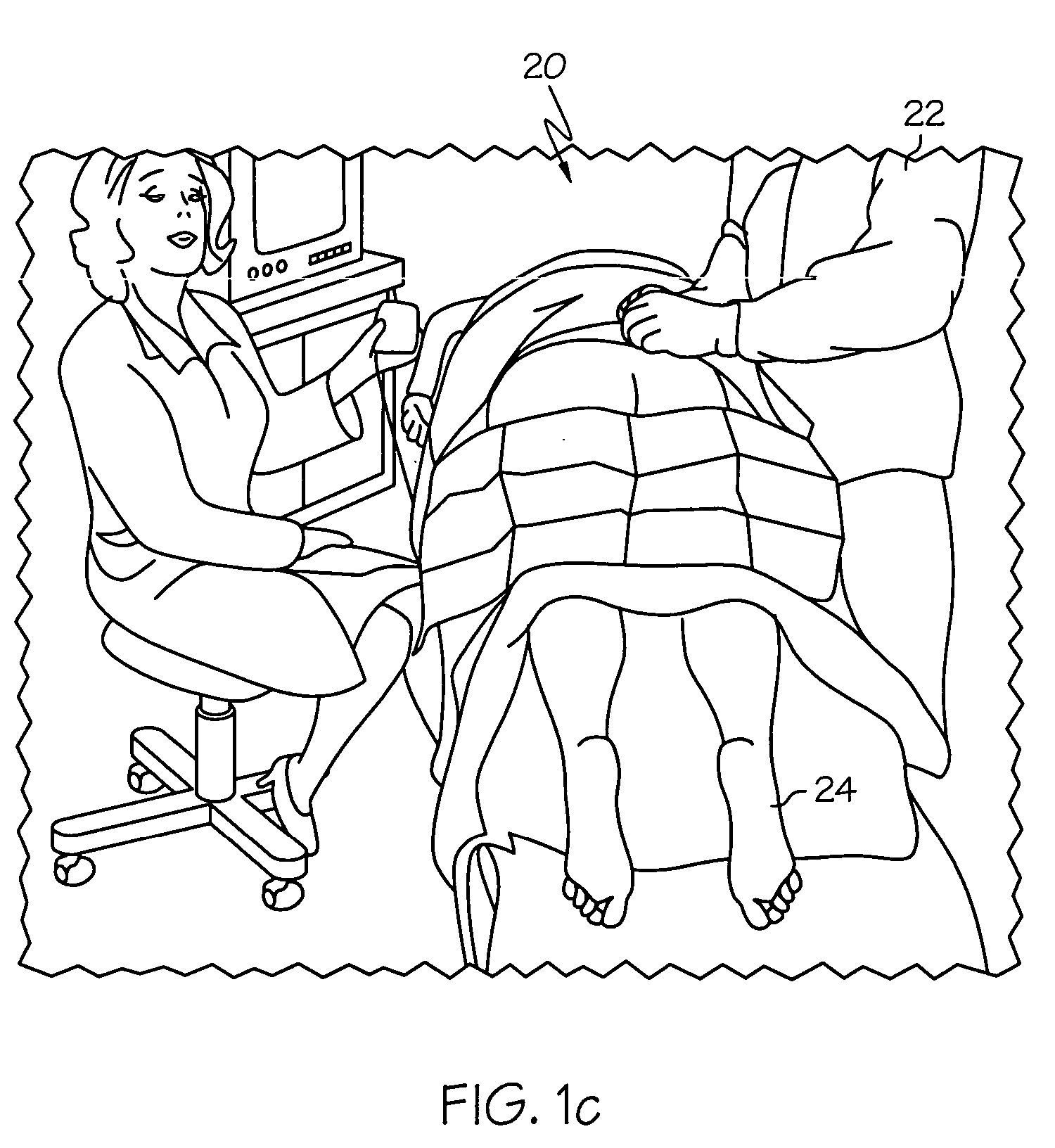
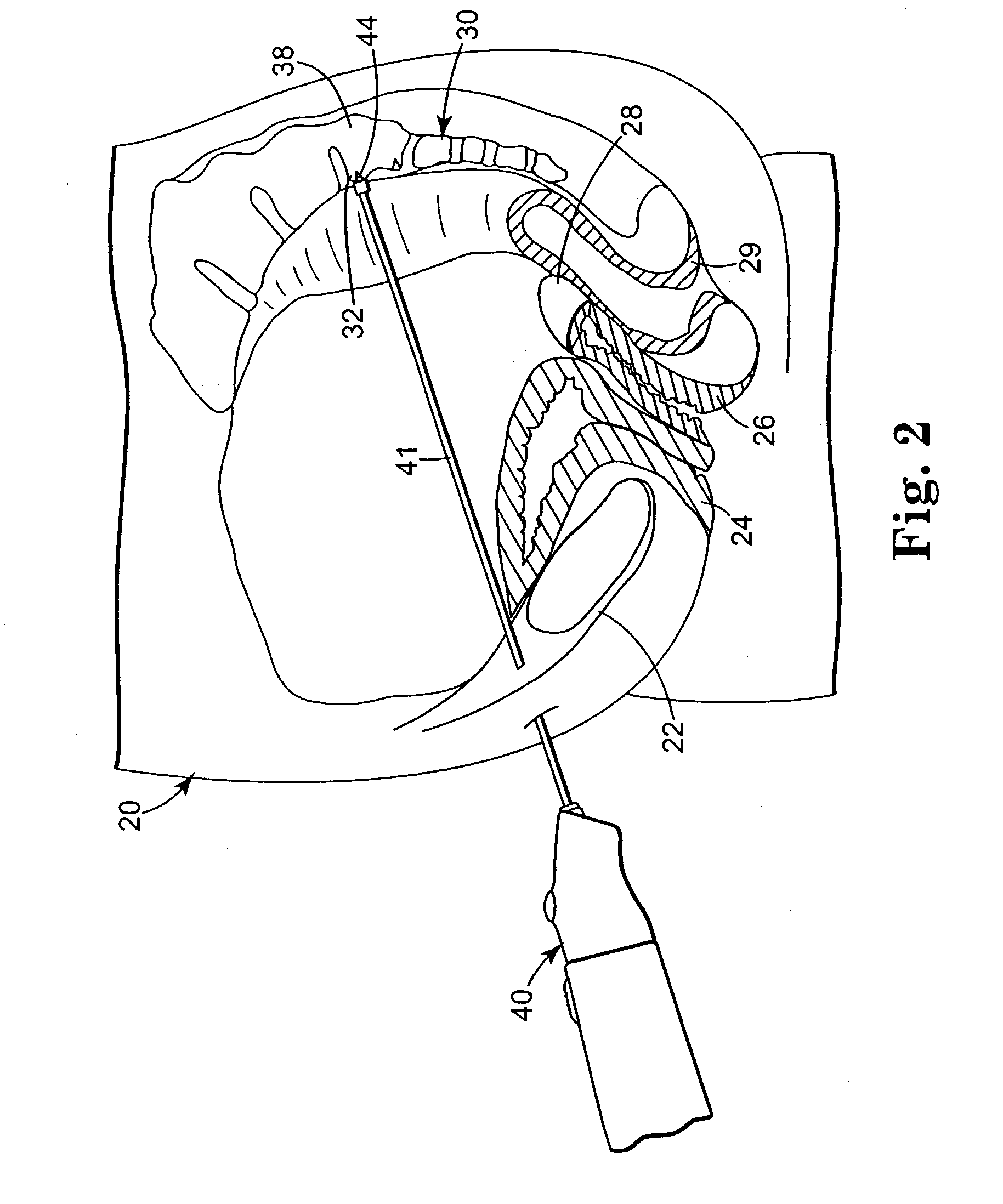
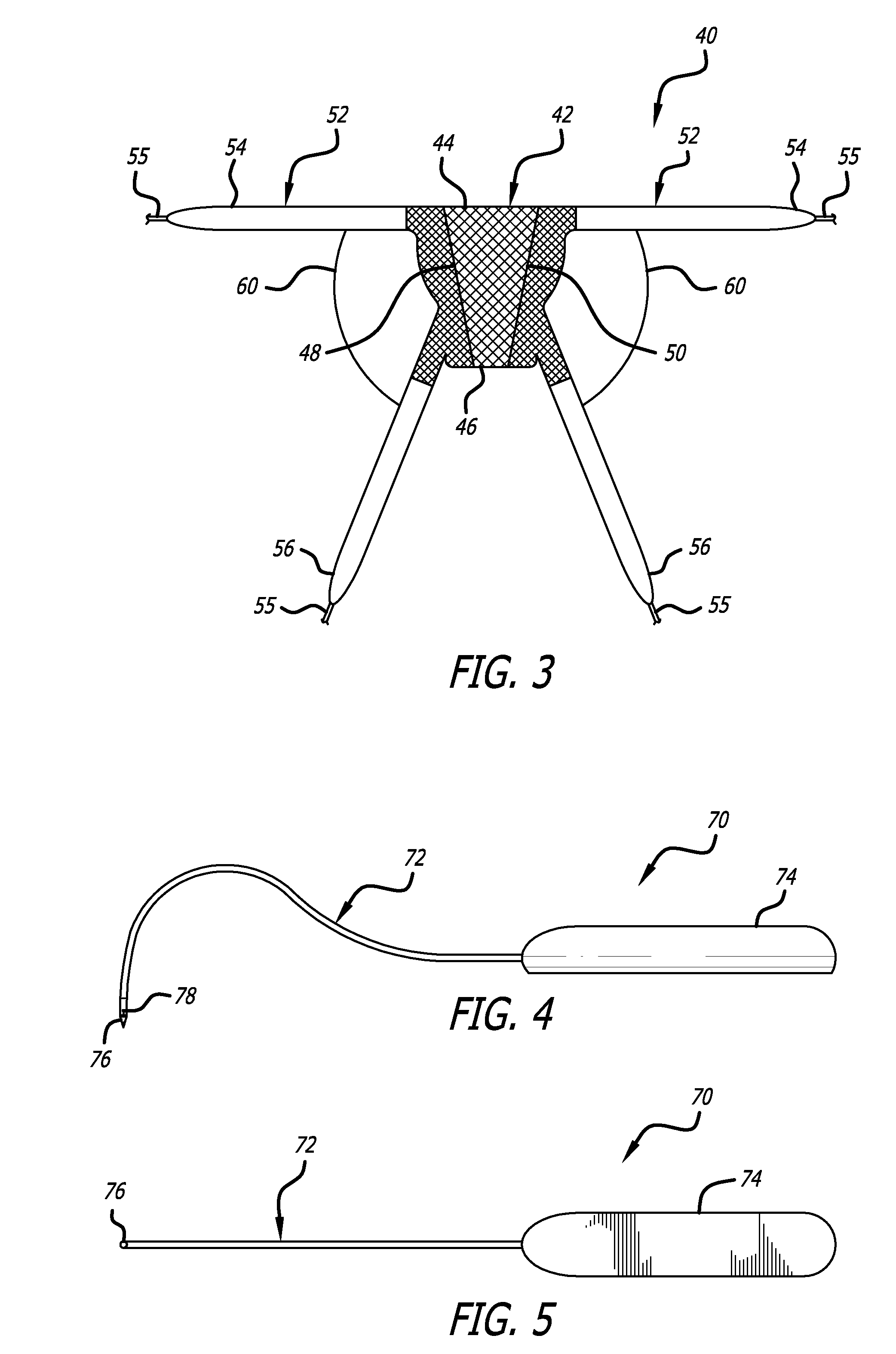
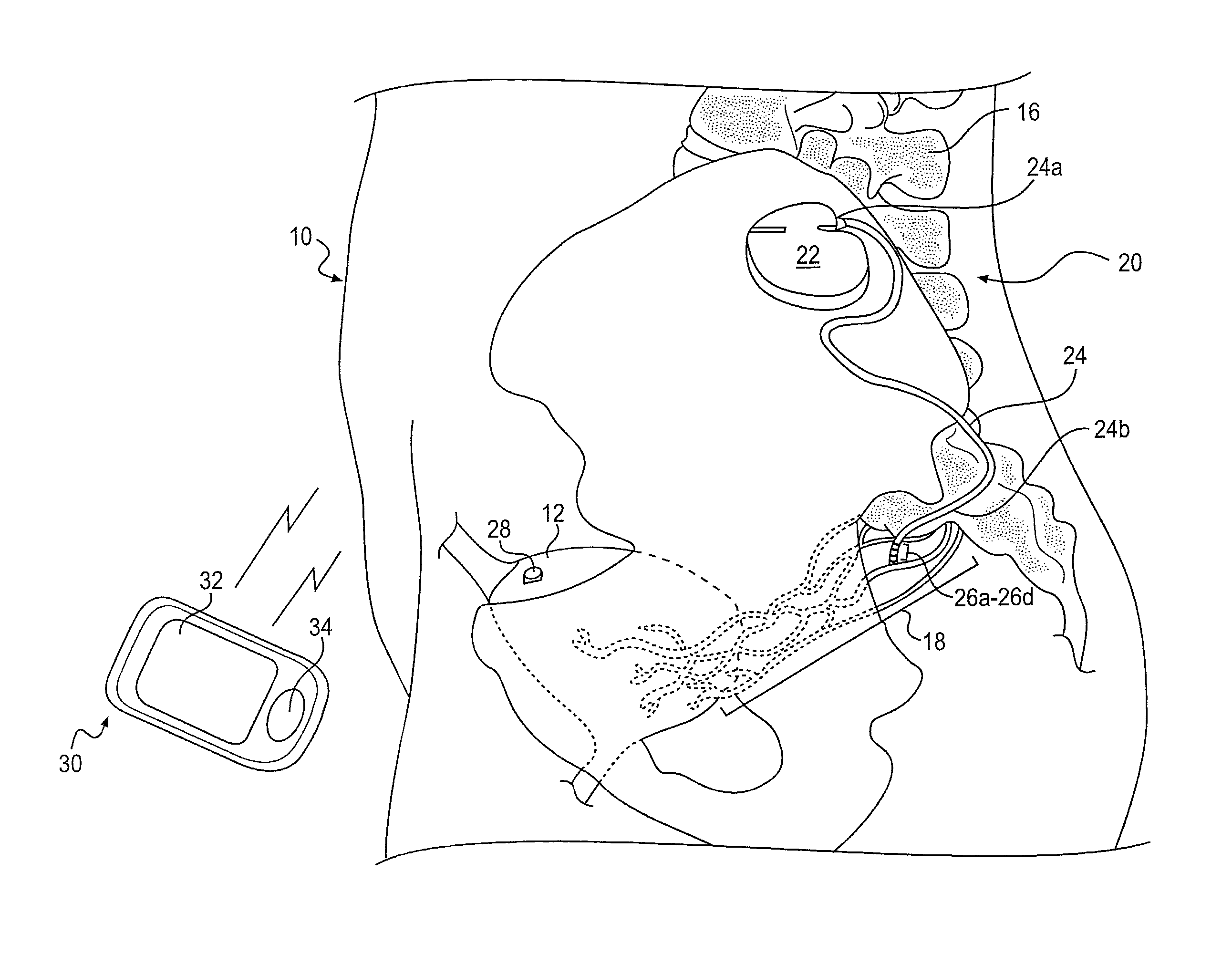
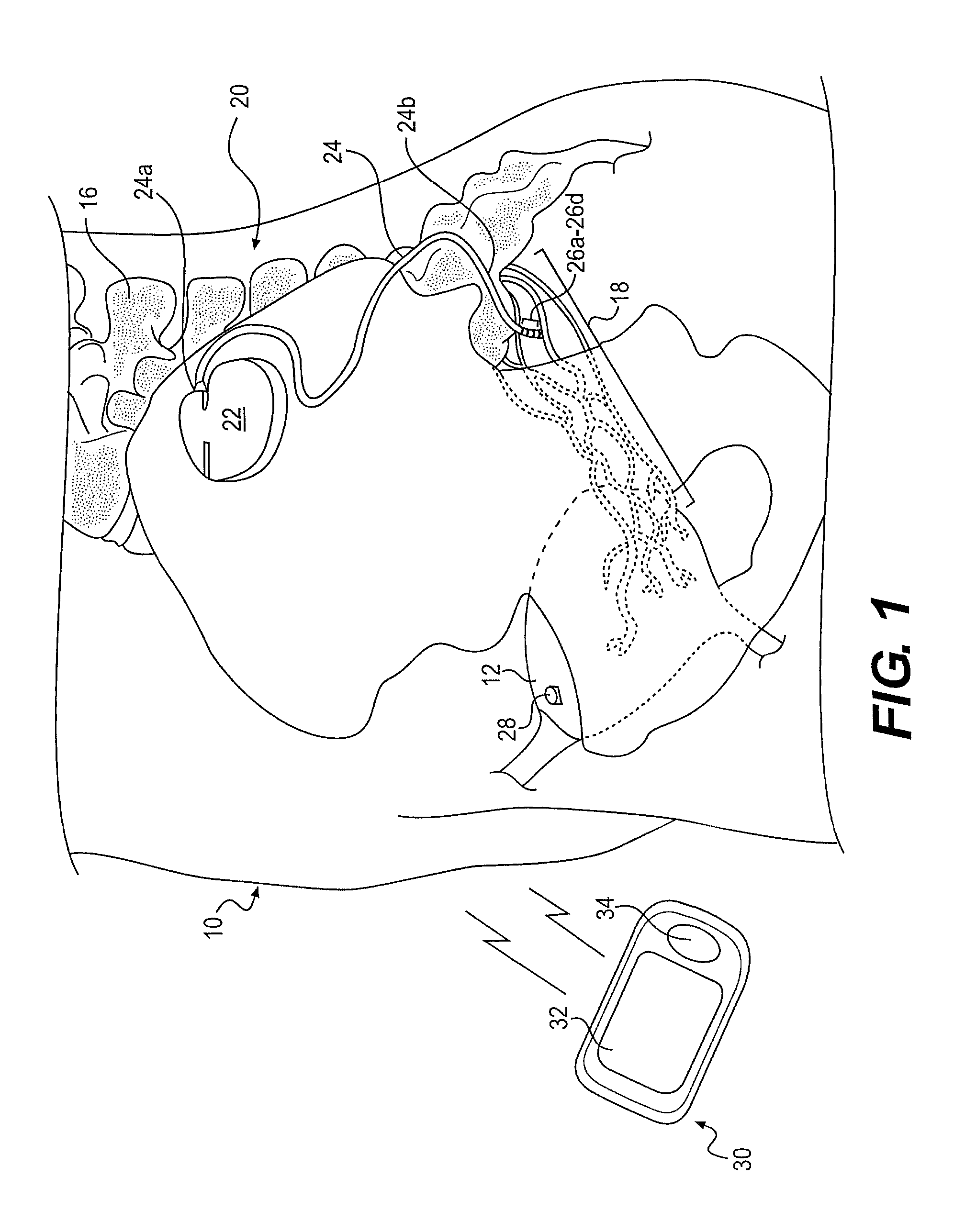
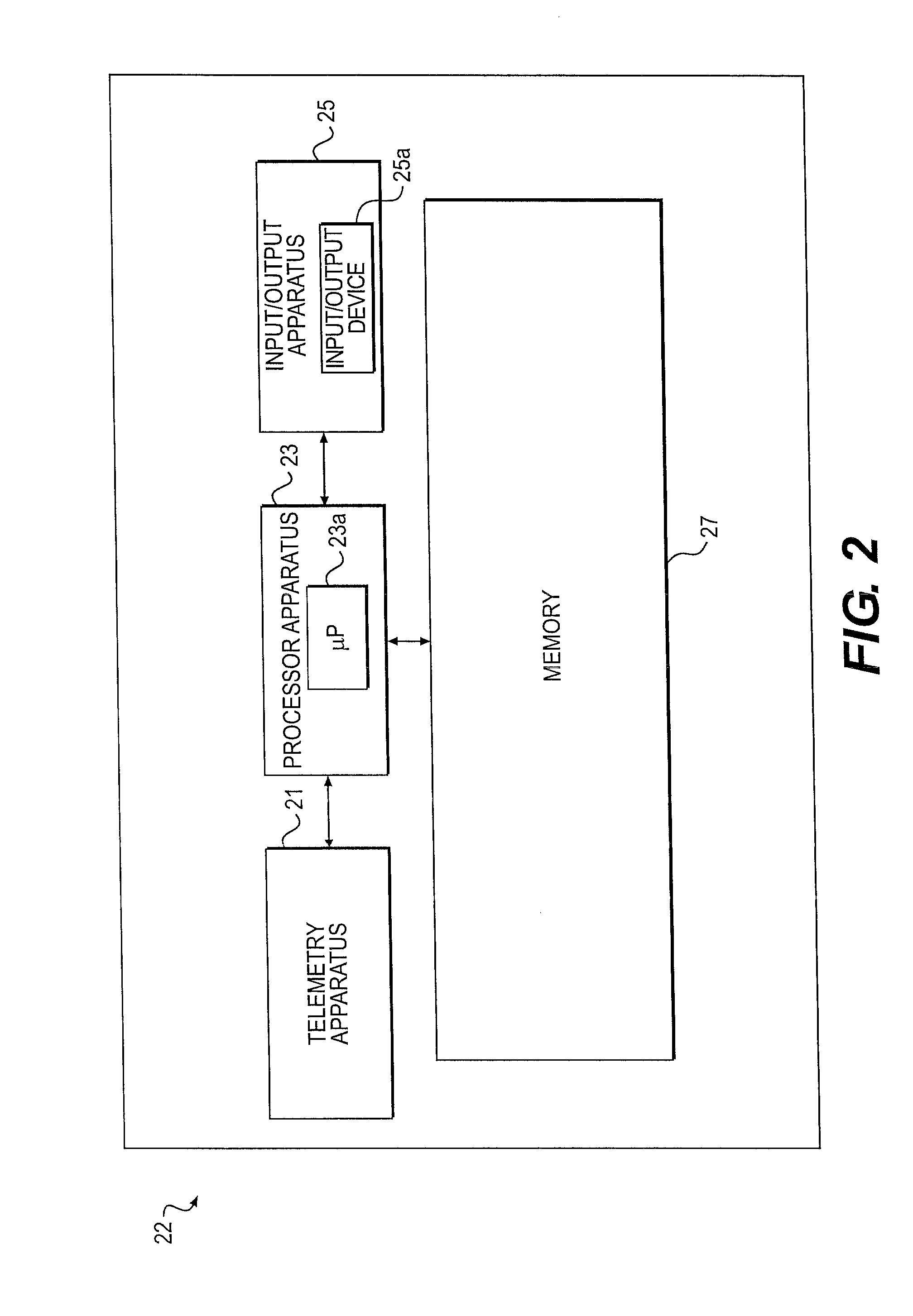
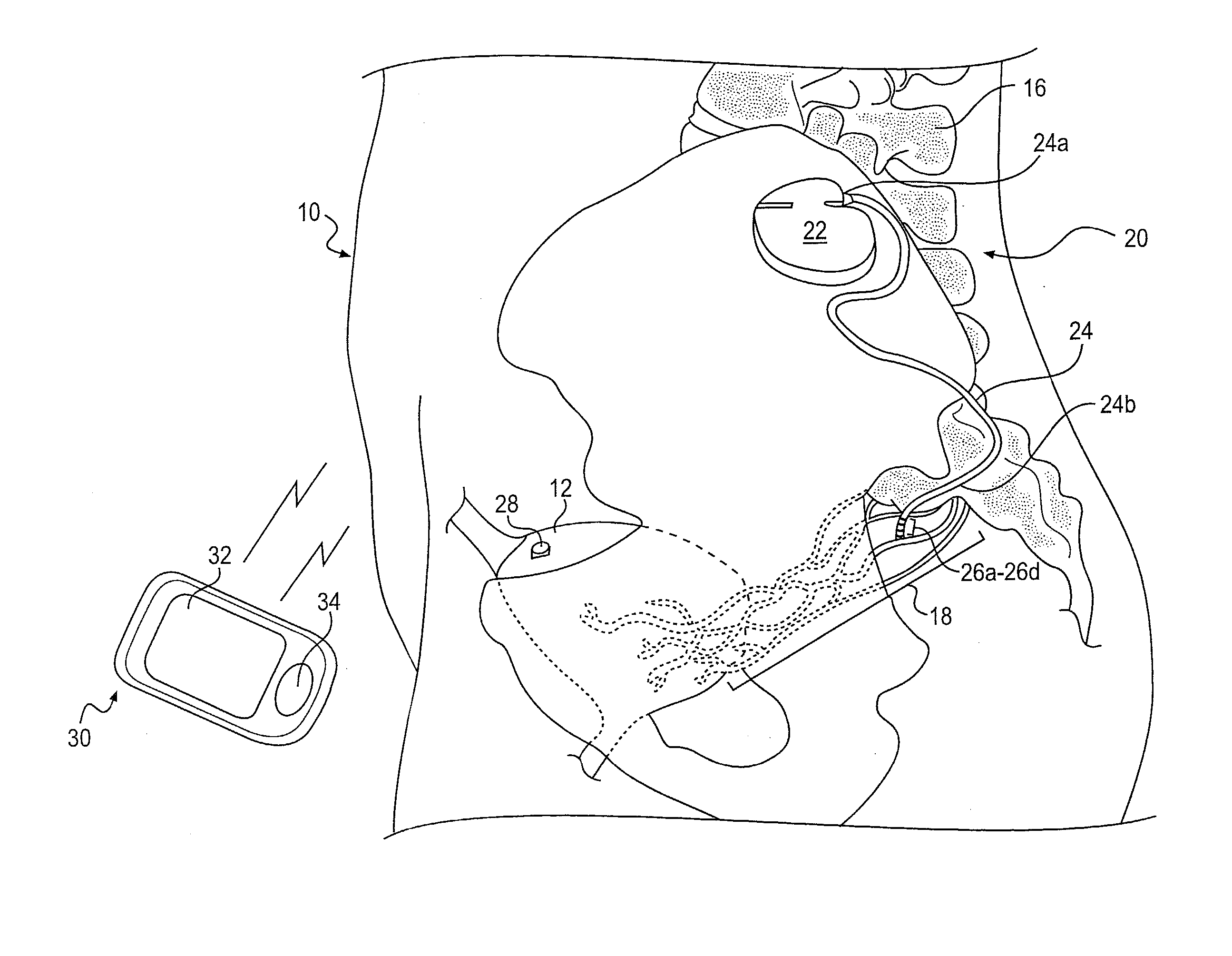
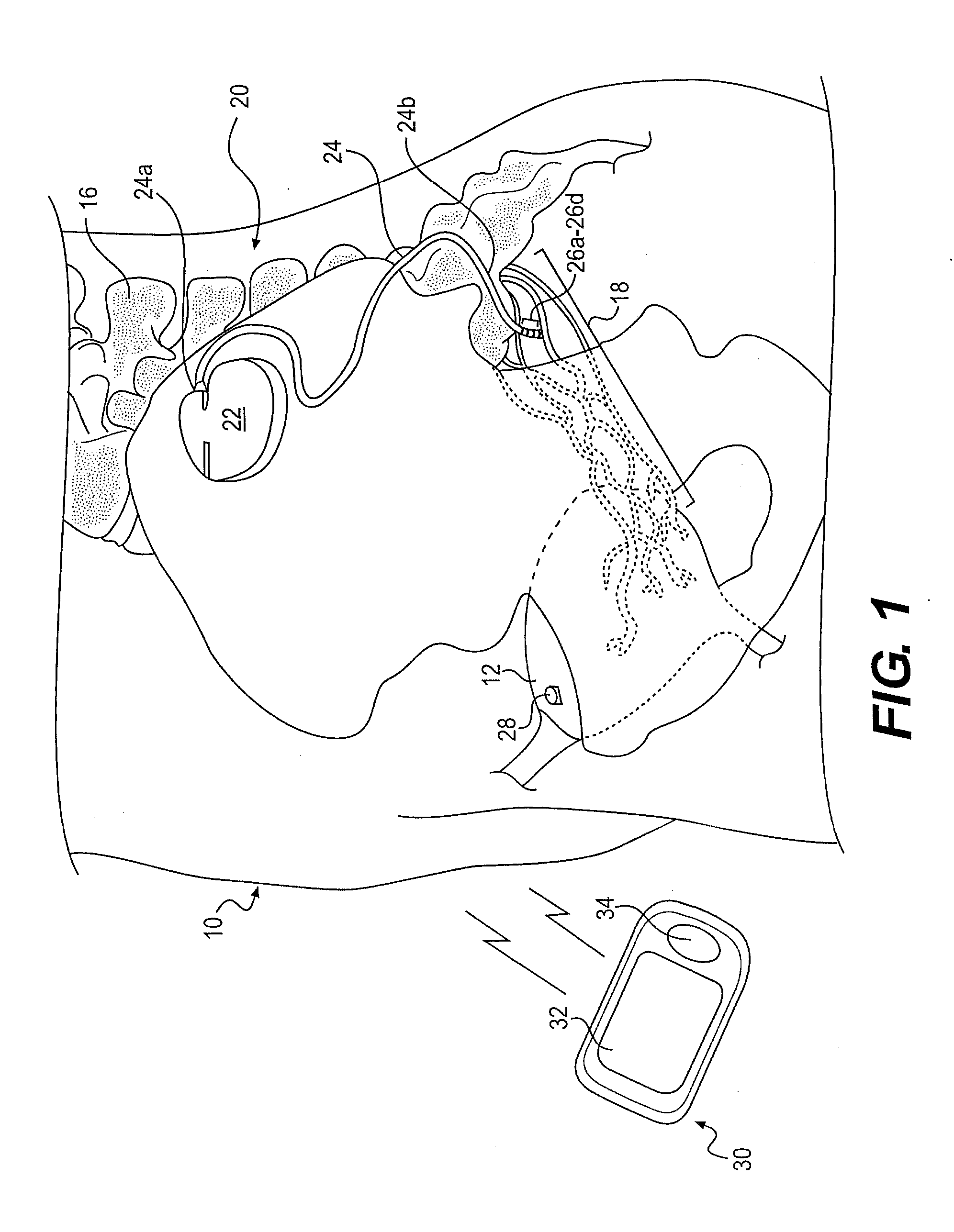
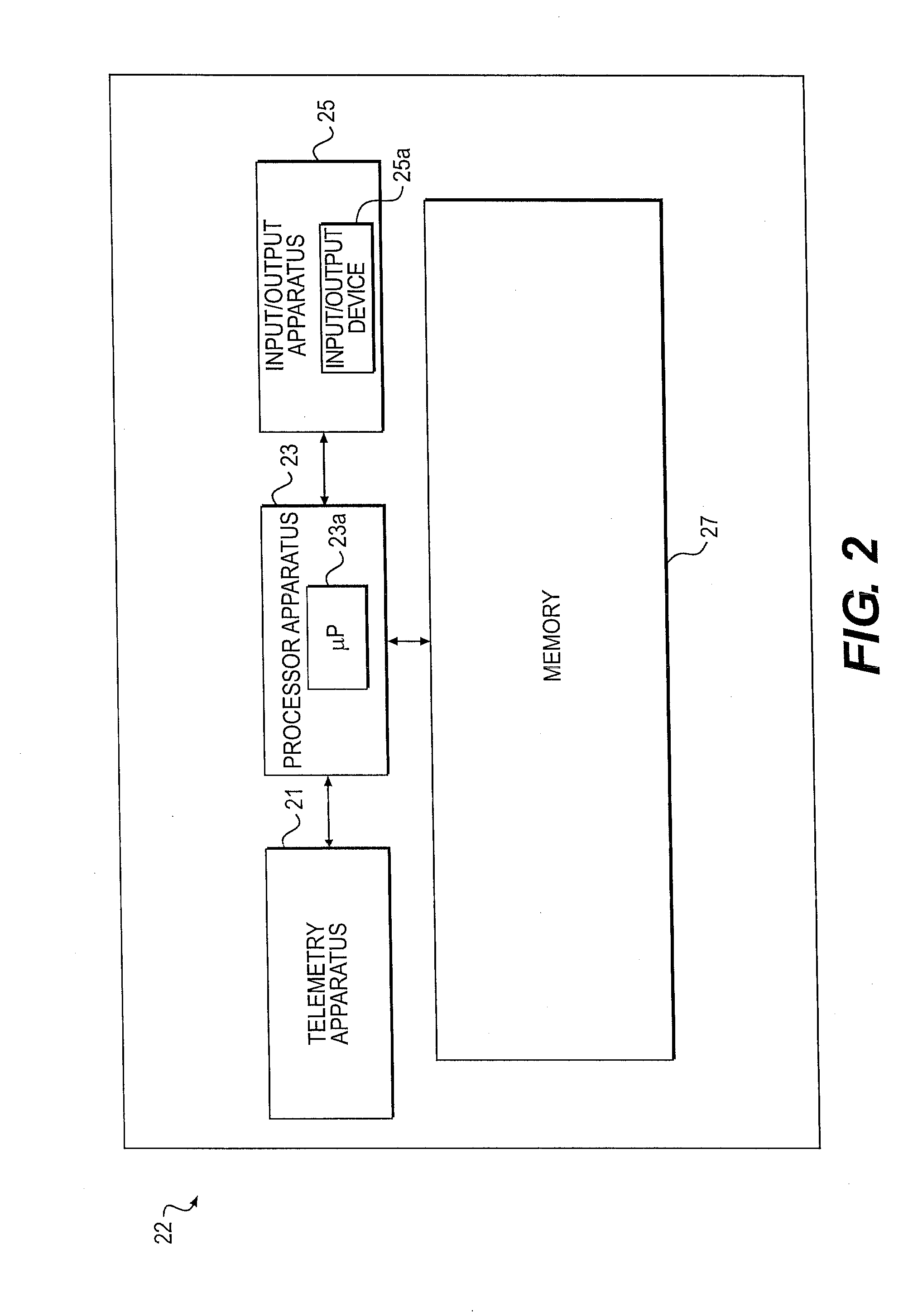
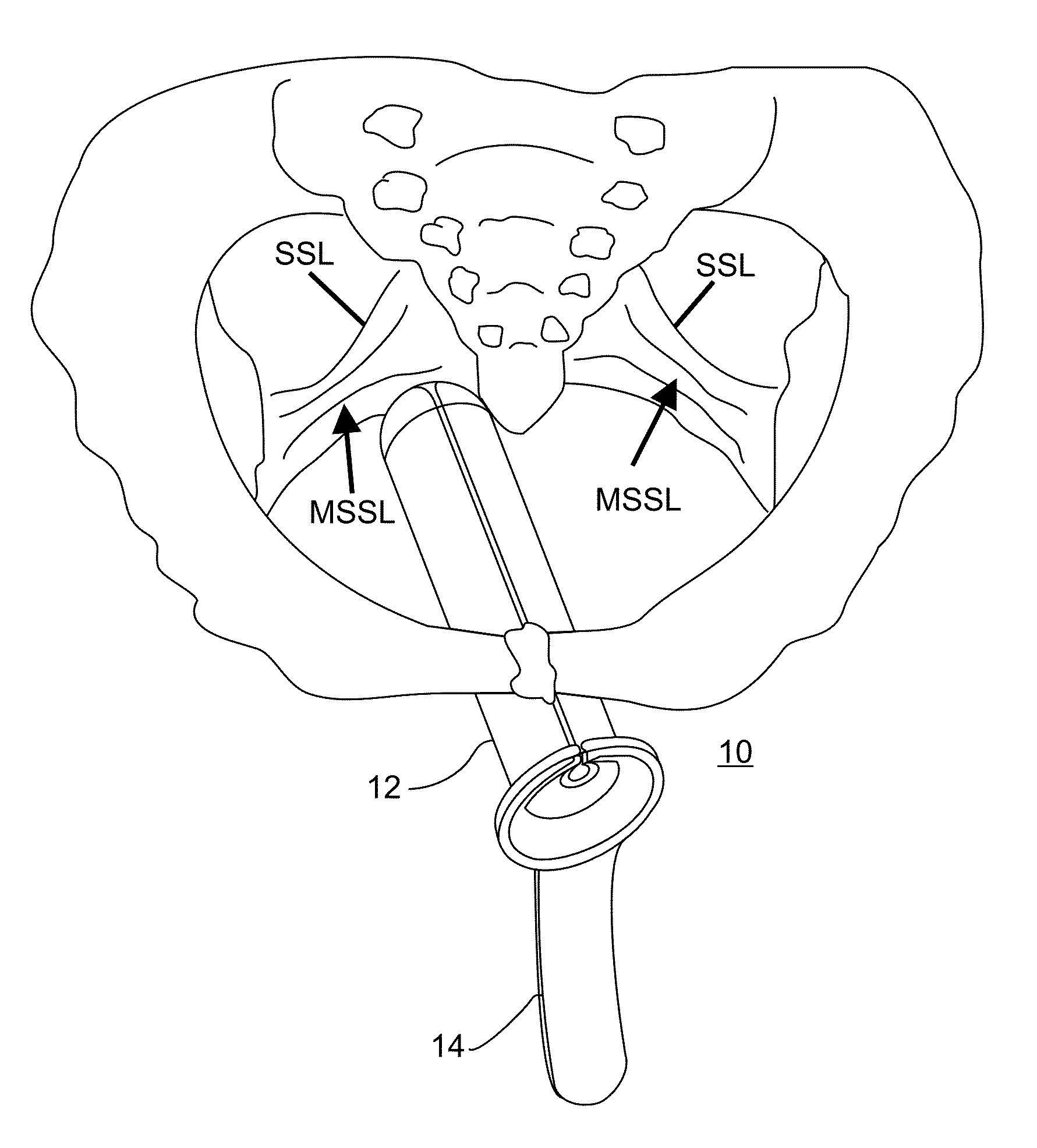
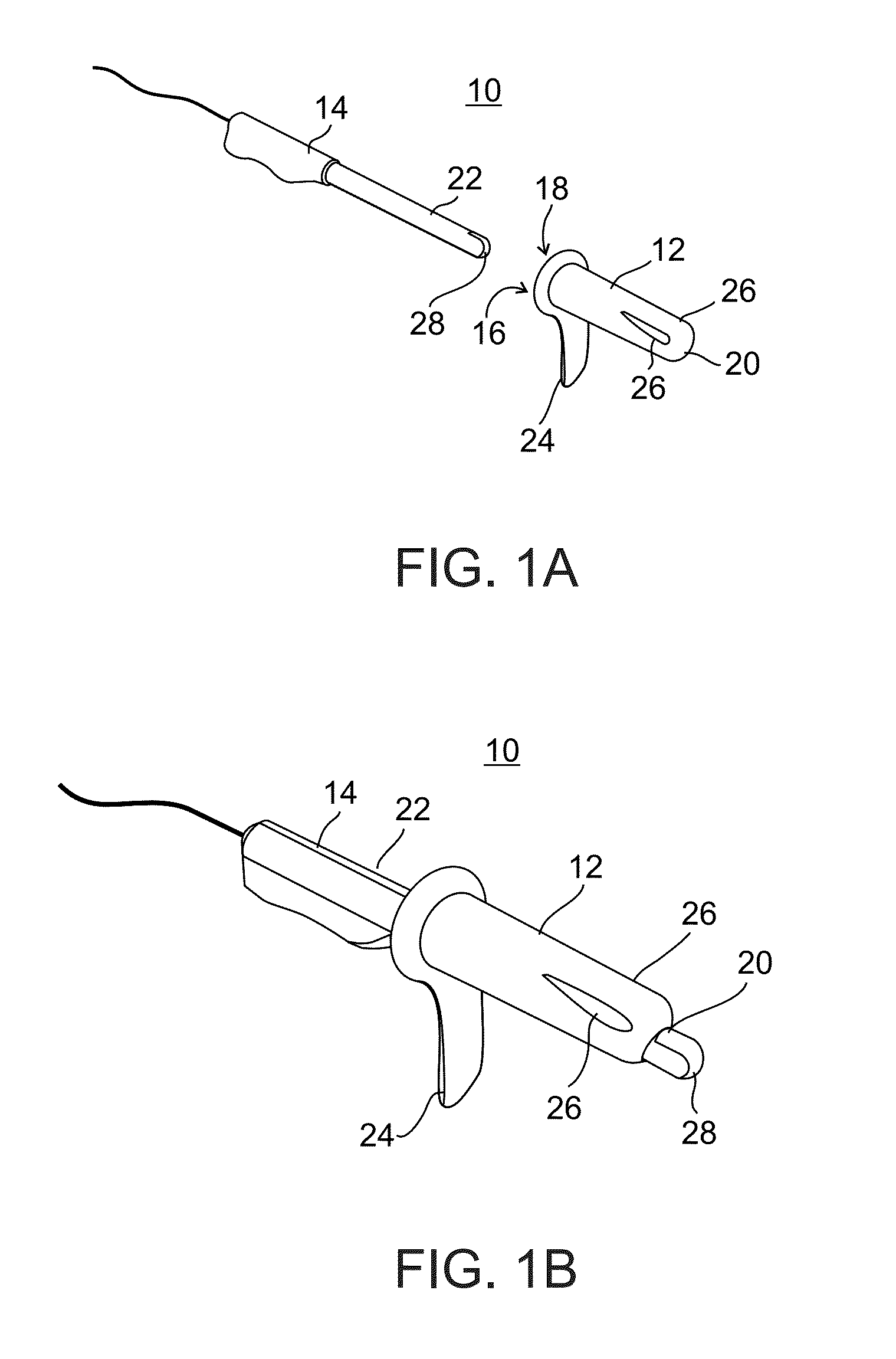
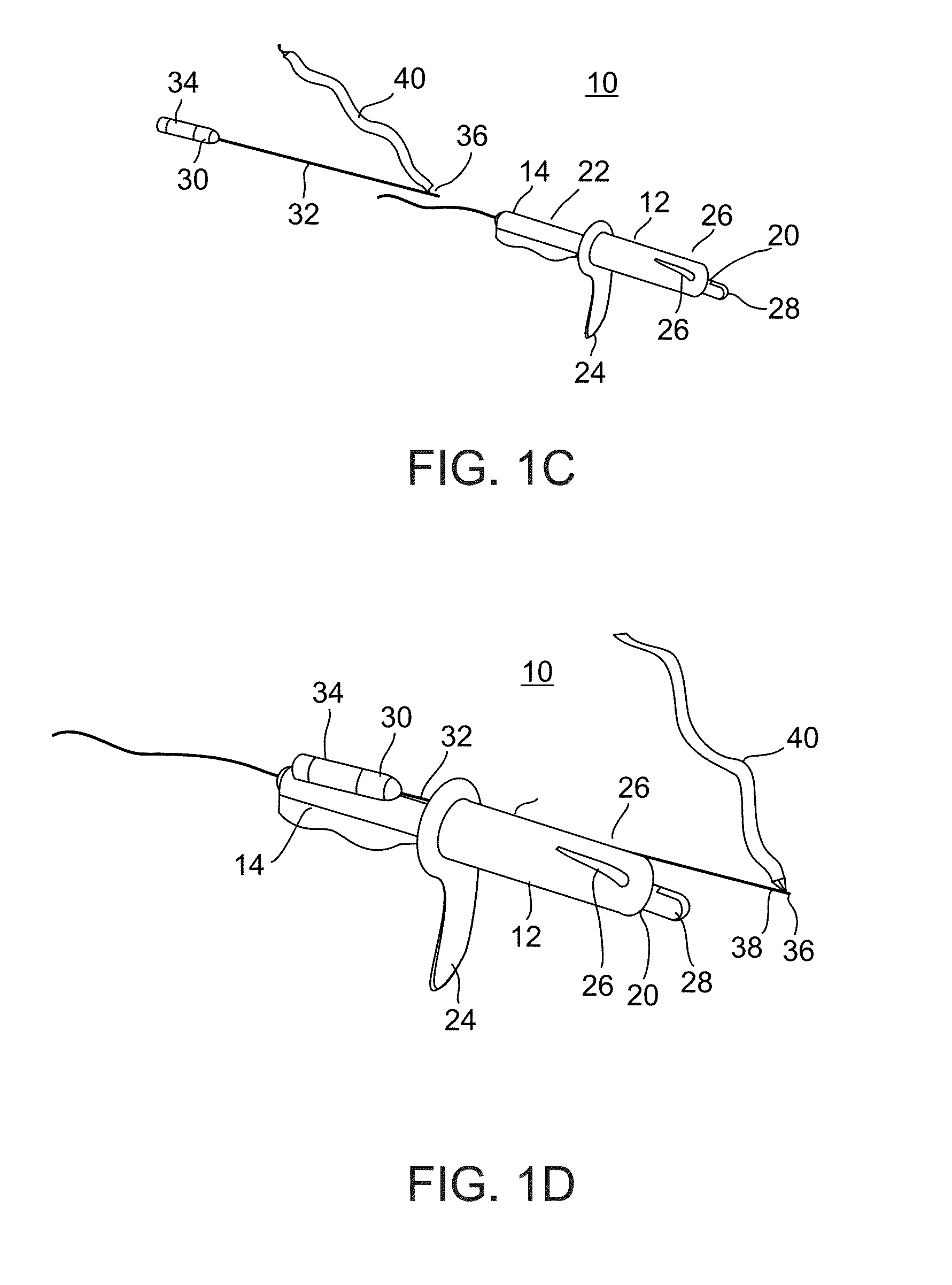
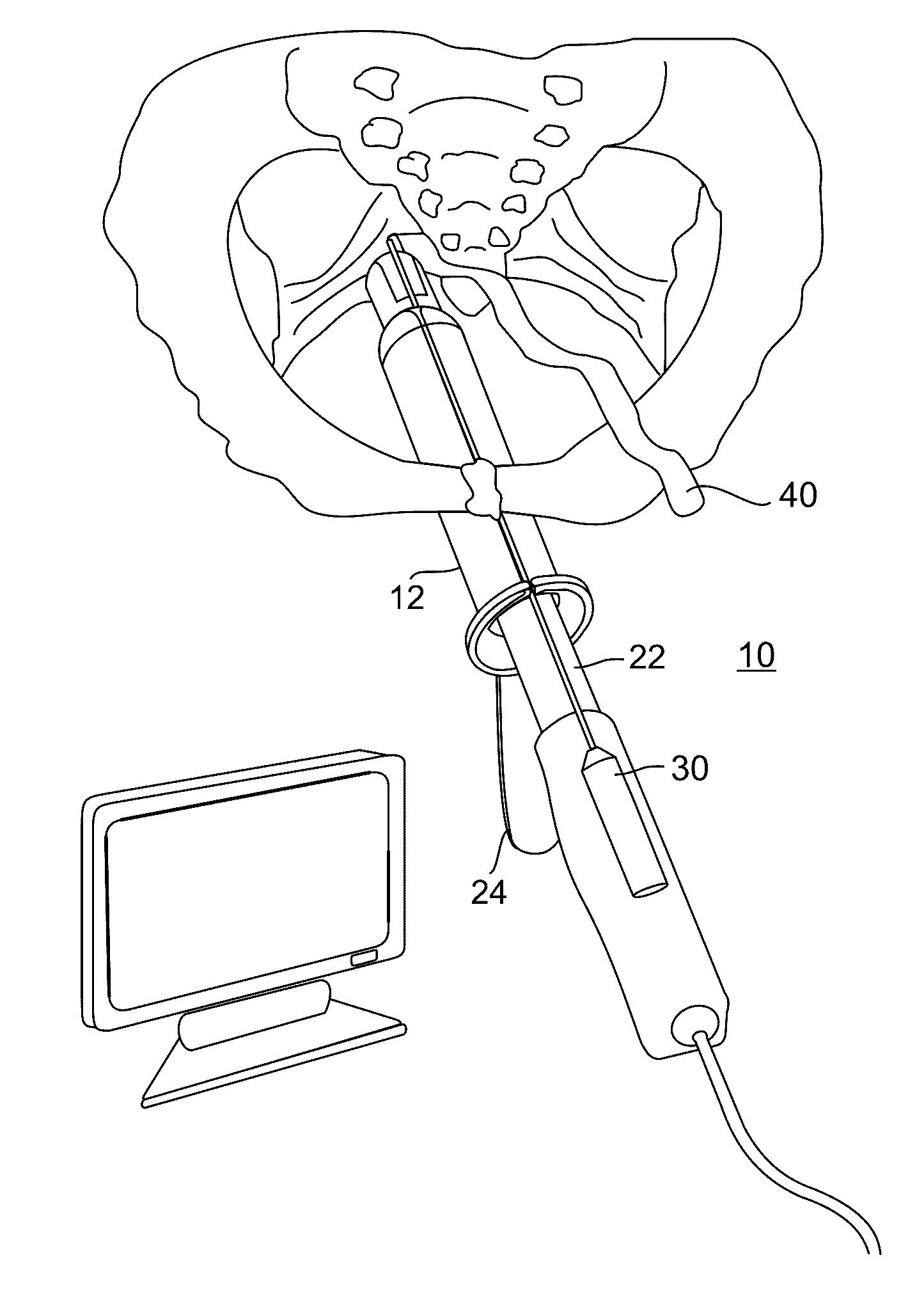
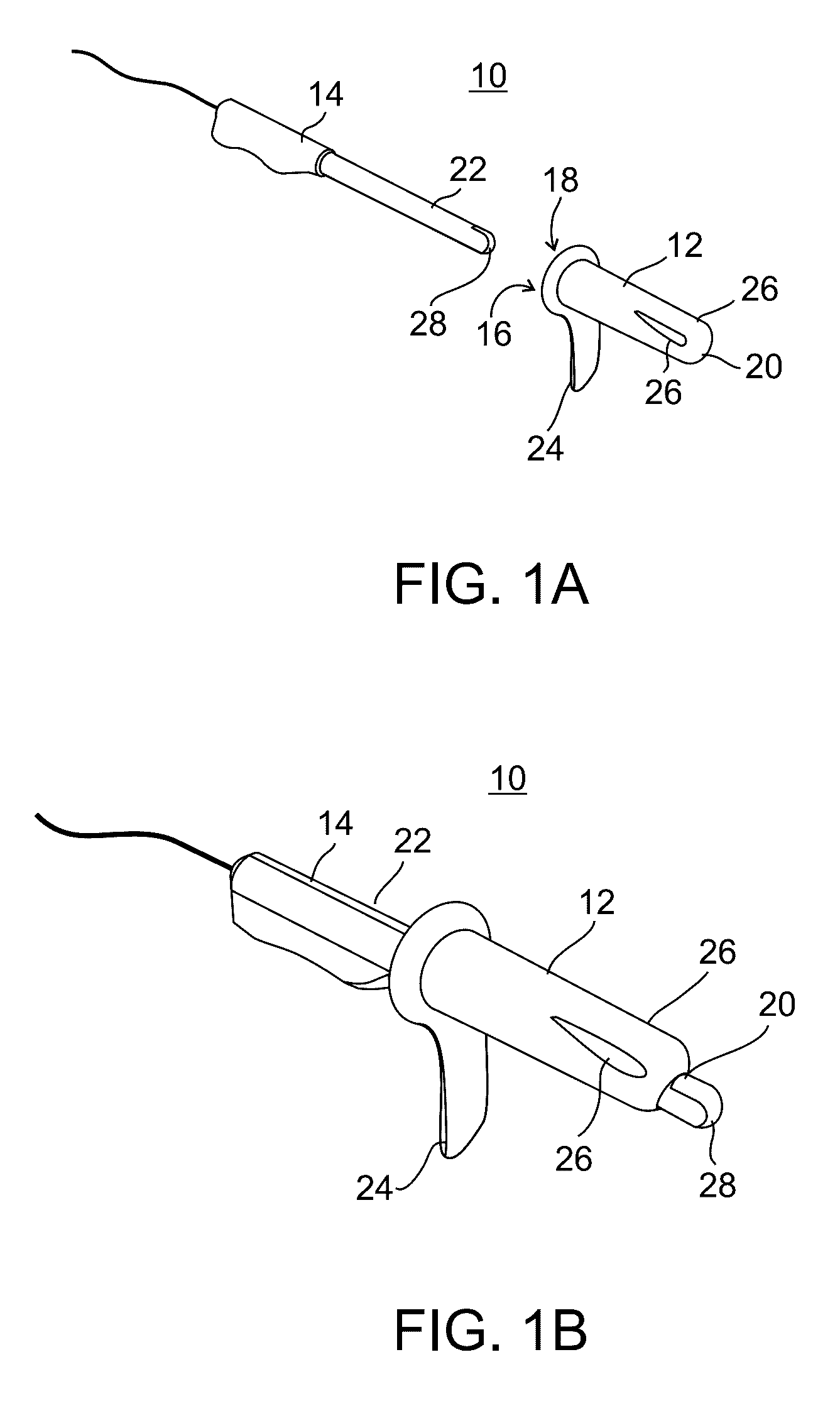
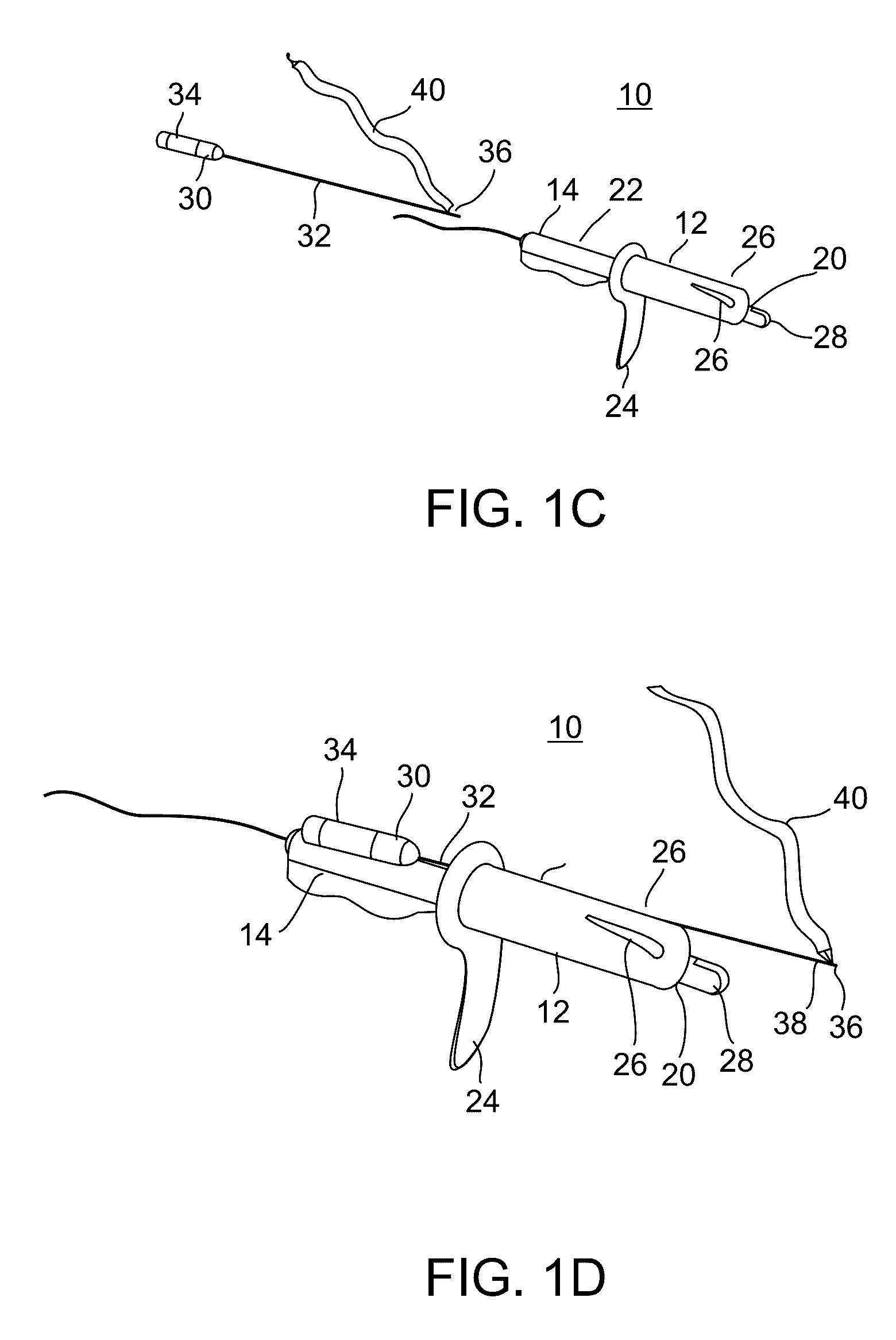
Minimally invasive apparatus for implanting a sacral stimulation lead
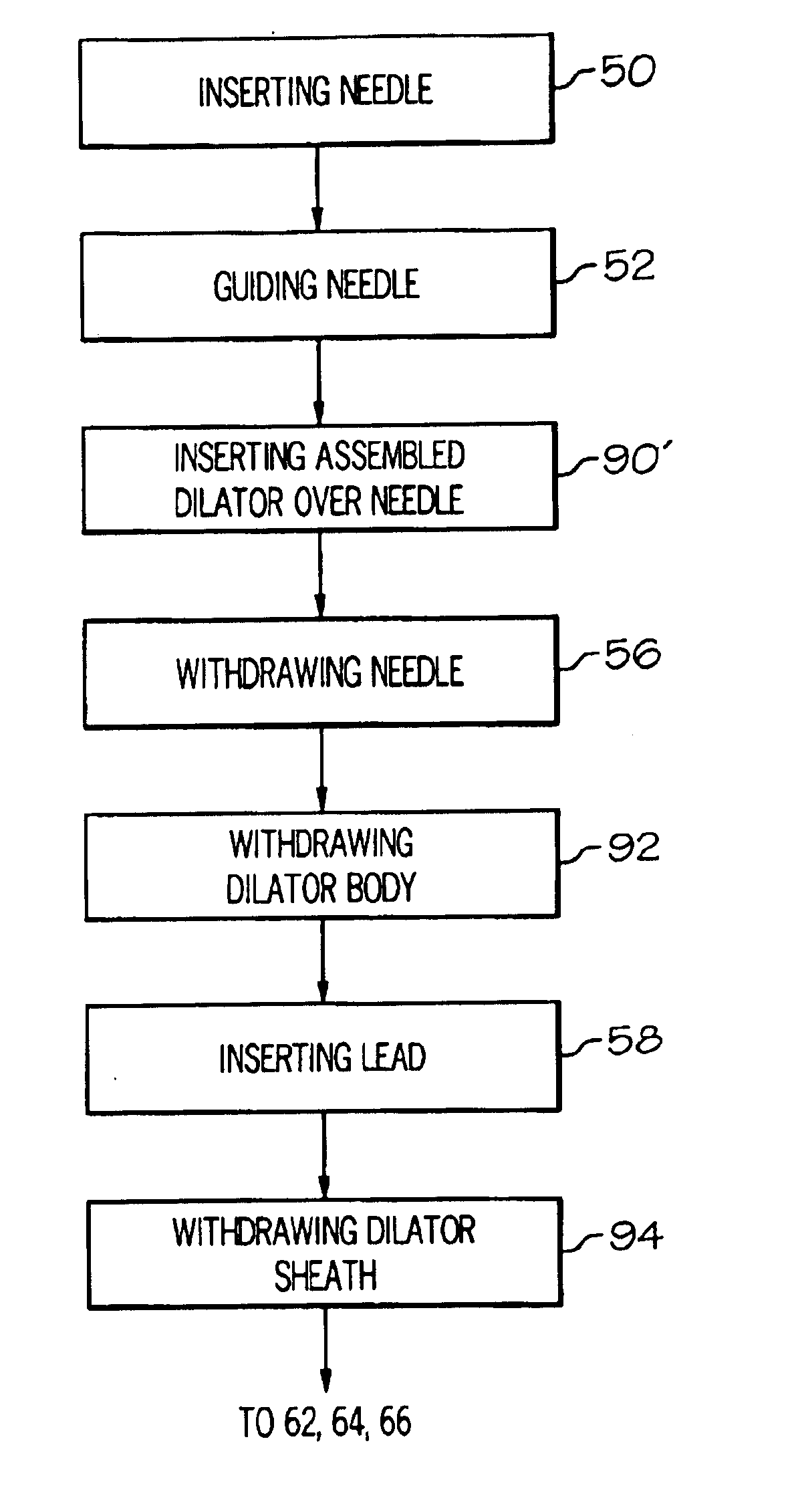
Methods and apparatus for implanting a stimulation lead in a patient's sacrum to deliver neurostimulation therapy that can reduce patient surgical complications, reduce patient recovery time, and reduce healthcare costs. A surgical instrumentation kit for minimally invasive implantation of a sacral stimulation lead through a foramen of the sacrum in a patient to electrically stimulate a sacral nerve comprises a needle and a dilator and optionally includes a guide wire. The needle is adapted to be inserted posterior to the sacrum through an entry point and guided into a foramen along an insertion path to a desired location. In one variation, a guide wire is inserted through a needle lumen, and the needle is withdrawn. The insertion path is dilated with a dilator inserted over the needle or over the guide wire to a diameter sufficient for inserting a stimulation lead, and the needle or guide wire is removed from the insertion path. The dilator optionally includes a dilator body and a dilator sheath fitted over the dilator body. The stimulation lead is inserted to the desired location through the dilator body lumen or the dilator sheath lumen after removal of the dilator body, and the dilator sheath or body is removed from the insertion path. If the clinician desires to separately anchor the stimulation lead, an incision is created through the entry point from an epidermis to a fascia layer, and the stimulation lead is anchored to the fascia layer. The stimulation lead can be connected to the neurostimulator to delivery therapies to treat pelvic floor disorders such as urinary control disorders, fecal control disorders, sexual dysfunction, and pelvic pain.
Owner:MEDTRONIC INC +1
Implantable article and method
InactiveUS20020028980A1Good treatment effectEncourage tissue ingrowthSuture equipmentsAnti-incontinence devicesDiseaseVaginal vault
An implantable article and method are disclosed for treating pelvic floor disorders such as vaginal vault prolase. A surgical kit useful for performing a surgical procedure such as a sacral colpopexy is also described.
Owner:ASTORA WOMENS HEALTH
Implantable article and method
InactiveUS6592515B2Alleviate challengeGood treatment effectSuture equipmentsAnti-incontinence devicesDiseaseVaginal vault
An implantable article and method are disclosed for treating pelvic floor disorders such as vaginal vault prolase. A surgical kit useful for performing a surgical procedure such as a sacral colpopexy is also described.
Owner:ASTORA WOMENS HEALTH
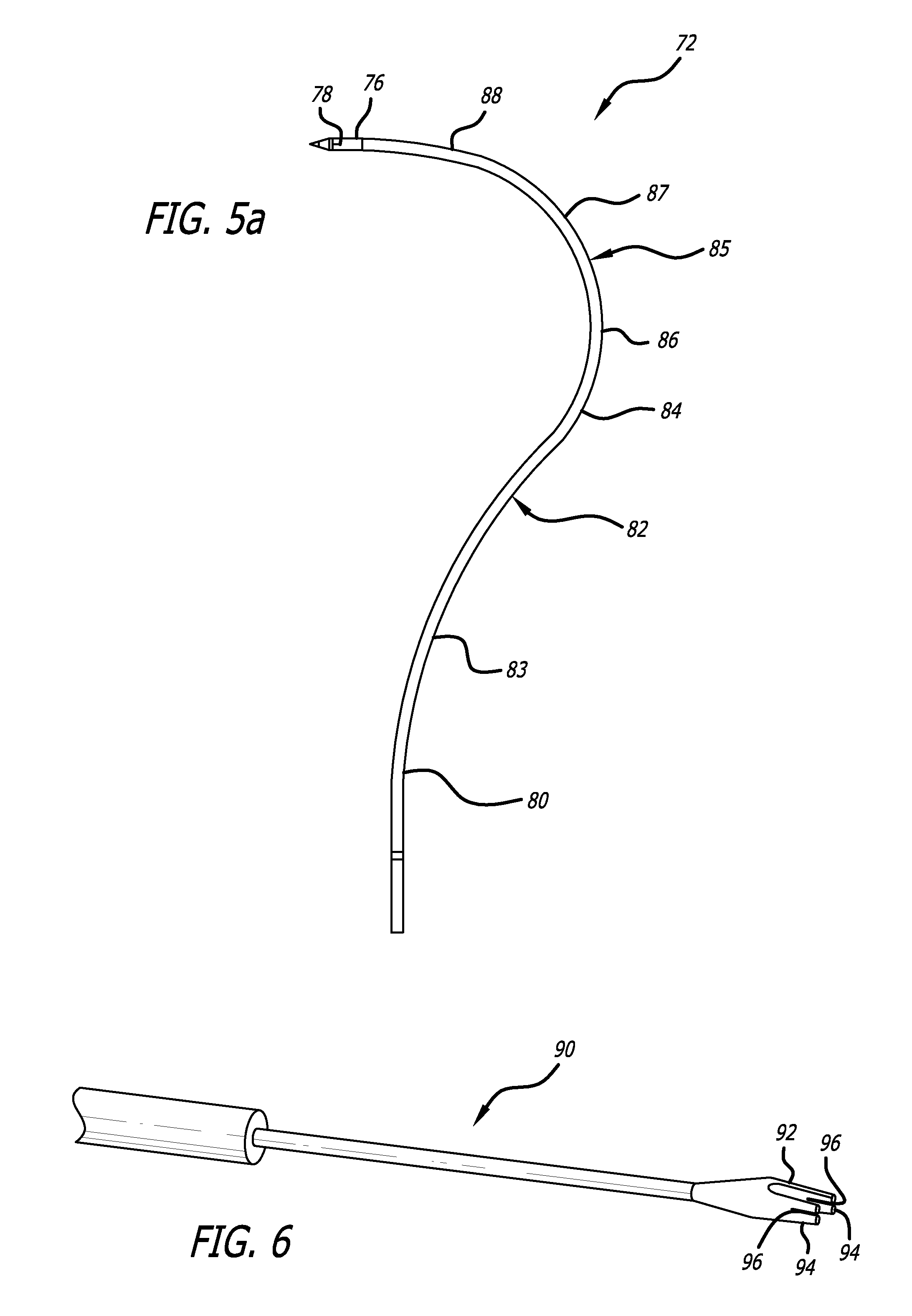
Minimally invasive method for implanting a sacral stimulation lead
Method embodiments to implant a stimulation lead in a patient's sacrum to deliver neurostimulation therapy can reduce patient surgical complications, reduce patient recovery time, and reduce healthcare costs. A method embodiment begins by inserting a needle posterior to the sacrum through an entry point. The needle is guided into a foramen along an insertion path to a desired location. The insertion path is dilated with a dilator to a diameter sufficient for inserting a stimulation lead. The needle is removed from the insertion path. The stimulation lead is inserted to the desired location. The dilator is removed from the insertion path. Additionally if the clinician desires to separately anchor the stimulation lead, an incision is created through the entry point from an epidermis to a fascia layer. The stimulation lead is anchored to the fascia layer. After the stimulation lead has been anchored, the incision can be closed, or the stimulation lead proximal end can be tunneled to where an implantable neurostimulator is located and then the incision can be closed. A implanted sacral stimulation lead can be connected to the neurostimulator to delivery therapies to treat pelvic floor disorders such as urinary control disorders, fecal control disorders, sexual dysfunction, and pelvic pain.
Owner:MEDTRONIC INC +1
Low impedance implantable extension for a neurological electrical stimulator
InactiveUS6671544B2Reduce energy consumptionLower impedanceSpinal electrodesExternal electrodesElectrical conductorMedical treatment
A medical device known as an implantable neurostimulation system is configured for implanting in humans to deliver a therapeutic electrical stimulation to tissue to treat a variety of medical conditions such as pain, movement disorders, pelvic floor disorders, and many other conditions. The implantable neurostimulation has a housing, a power supply carried in the housing, stimulation electronics coupled to the battery and coupled to a neurostimulator connector block, a stimulation lead, and a lead extension. The lead extension is electrically coupleable between the neurostimulation connector block and the stimulation lead. The extension conductor is composed of an outer surface and an inner core. The outer surface has an outer impedance and the inner core has a core impedance that is substantially lower than the outer impedance. Many embodiments of the low impedance lead extension are possible.
Owner:MEDTRONIC INC
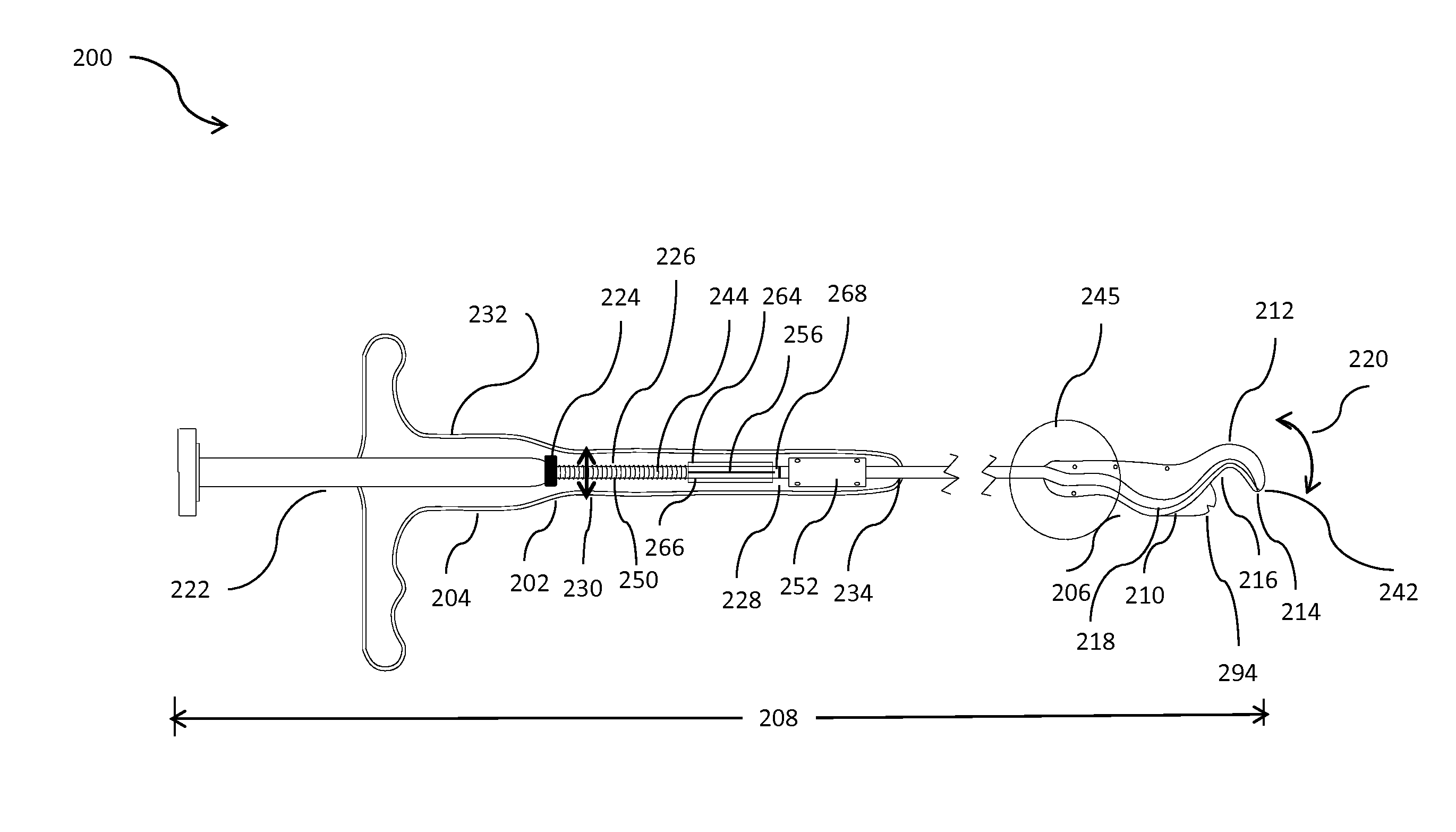
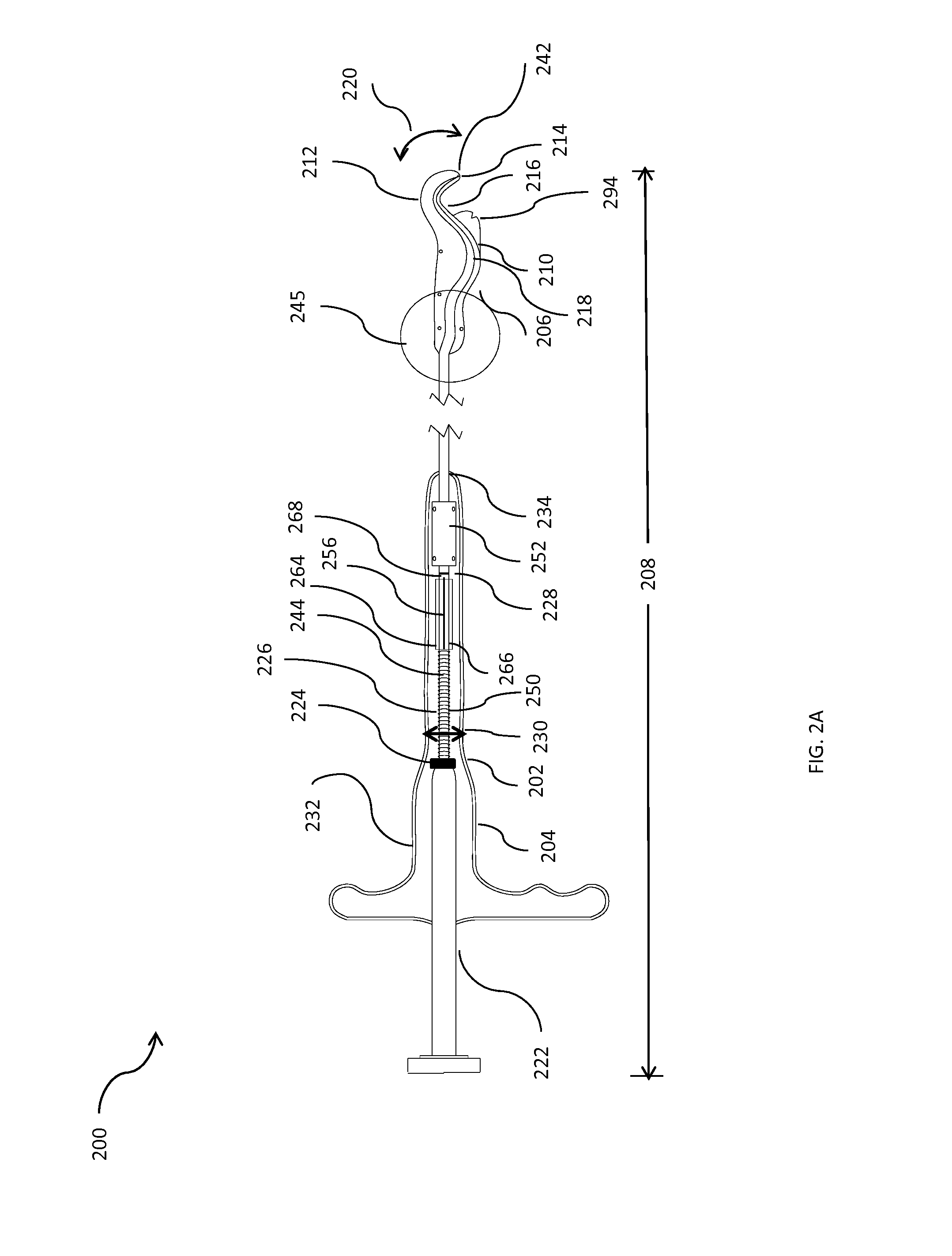
Minimally invasive apparatus for implanting a sacral stimulation lead
Methods and apparatus for implanting a stimulation lead in a patient's sacrum to deliver neurostimulation therapy that can reduce patient surgical complications, reduce patient recovery time, and reduce healthcare costs. A surgical instrumentation kit for minimally invasive implantation of a sacral stimulation lead through a foramen of the sacrum in a patient to electrically stimulate a sacral nerve comprises a needle and a dilator and optionally includes a guide wire. The needle is adapted to be inserted posterior to the sacrum through an entry point and guided into a foramen along an insertion path to a desired location. In one variation, a guide wire is inserted through a needle lumen, and the needle is withdrawn. The insertion path is dilated with a dilator inserted over the needle or over the guide wire to a diameter sufficient for inserting a stimulation lead, and the needle or guide wire is removed from the insertion path. The dilator optionally includes a dilator body and a dilator sheath fitted over the dilator body. The stimulation lead is inserted to the desired location through the dilator body lumen or the dilator sheath lumen after removal of the dilator body, and the dilator sheath or body is removed from the insertion path. If the clinician desires to separately anchor the stimulation lead, an incision is created through the entry point from an epidermis to a fascia layer, and the stimulation lead is anchored to the fascia layer. The stimulation lead can be connected to the neurostimulator to delivery therapies to treat pelvic floor disorders such as urinary control disorders, fecal control disorders, sexual dysfunction, and pelvic pain.
Owner:MEDTRONIC INC
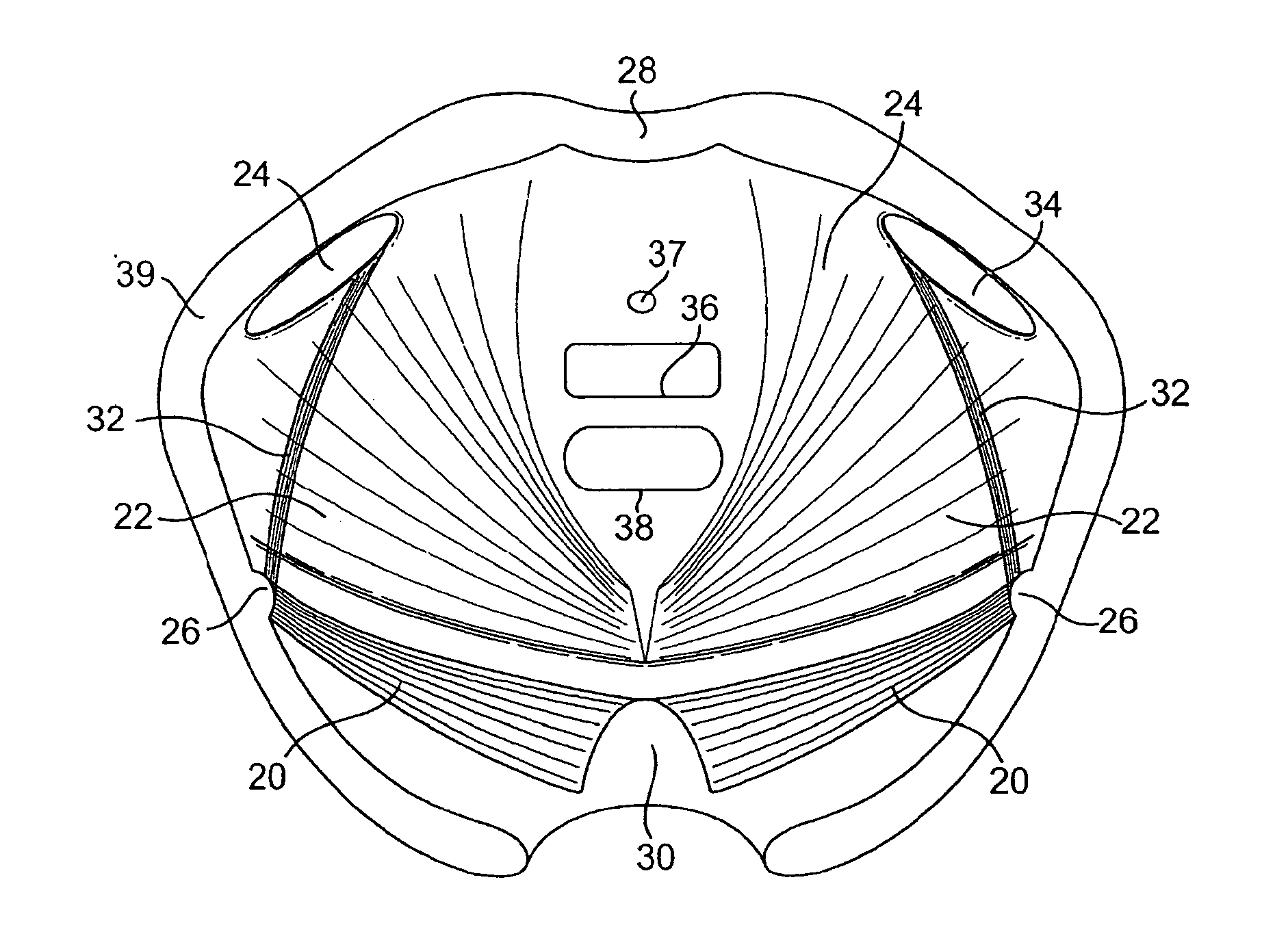
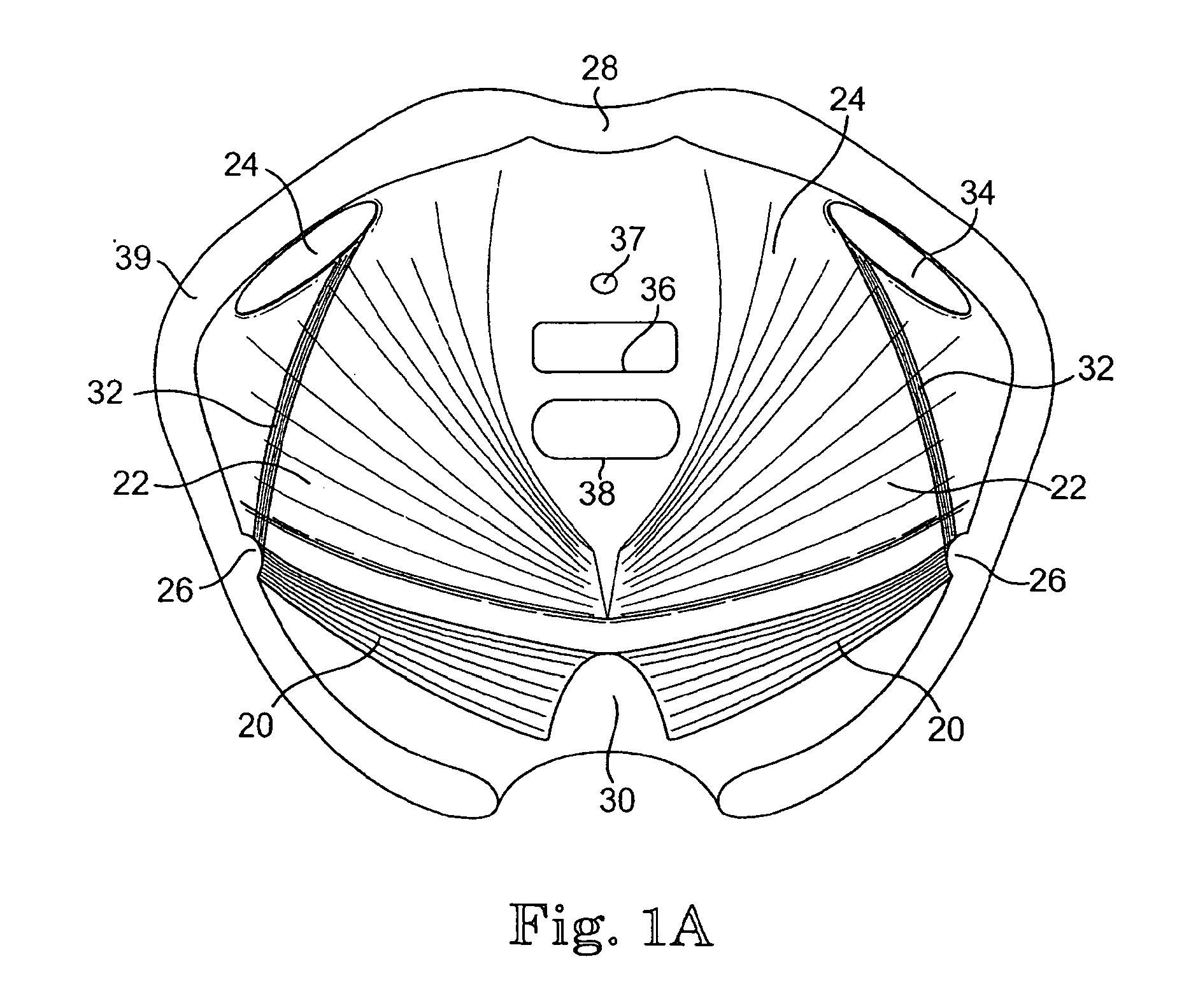
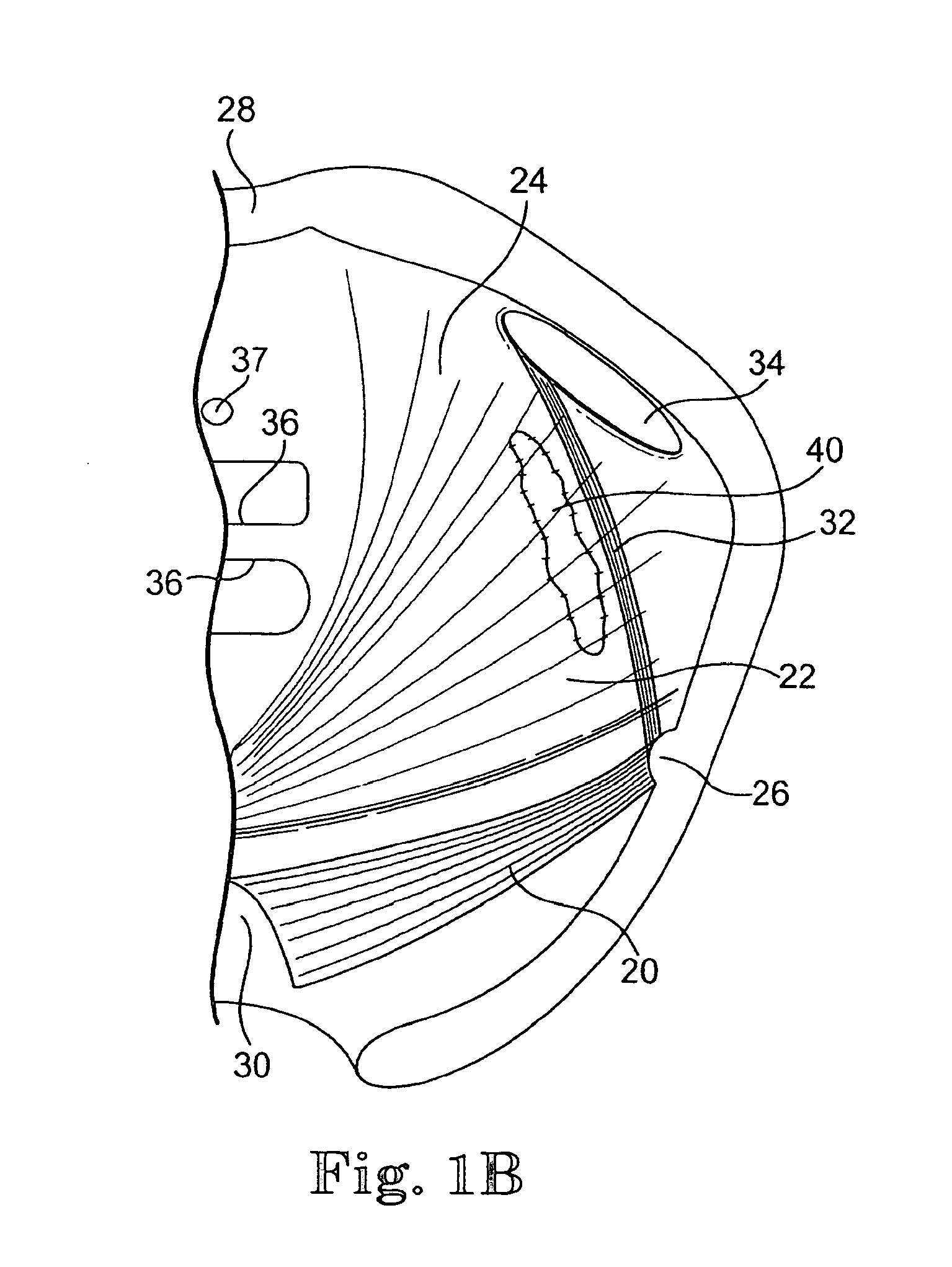
Implants for the treatment of pelvic floor disorders
An implant for repairing pelvic floor disorders, such as urinary incontinence or prolapse, by lifting the prolapsed tissue or organ into a more anatomically correct position is provided. The prosthesis is shaped with a central support section and one or more lateral anchoring sections or arms extending outwardly from the central section. The support section lifts the prolapsed organ to a more anatomically correct position, whereas the anchoring secton(s) are positioned through soft tissue away from the organ being supported to hold the implant in place by allowing tissue ingrowth into the anchoring sections. The implant may be formed of both synthetic and more natural materials.
Owner:CR BARD INC
Implantable article and method
InactiveUS20030195386A1Alleviate challengeSuture equipmentsAnti-incontinence devicesDiseaseVaginal vault
An implantable article and method are disclosed for treating pelvic floor disorders such as vaginal vault prolase. A surgical kit useful for performing a surgical procedure such as a sacral colpopexy is also described.
Owner:STASKIN DAVID MD DR
Low impedance implantable extension for a neurological electrical stimulator
InactiveUS6950709B2Reduce energy consumptionSpinal electrodesExternal electrodesElectrical conductorMedical treatment
Owner:MEDTRONIC INC
Implants And Procedures For Treatment Of Pelvic Floor Disorders
InactiveUS20080177132A1Preserve natural lengthNot to damageSuture equipmentsAnti-incontinence devicesDiseasePelvic diaphragm muscle
Implants for the treatment of pelvic support conditions and methods of implementing the same. The implants comprise relatively soft, flexible bodies and relatively strong arms extending in predetermined orientation therefrom. Methods and devices for placing the implants minimize trauma to the pelvic floor and provide well-anchored support to pelvic organs without interfering with sexual or other bodily functions.
Owner:CALDERA MEDICAL
Method for Vaginal Skin Biomechanical Evaluation
A method and technique to measure vaginal skin biomechanical properties in vivo by applying a temporary deforming force to the vaginal skin while using sensors to monitor and record the vaginal skins response to the deforming force as well as how it responds after the deforming force is removed. By placing a female participant in the correct anatomical position and employing a vaginal biomechanical evaluation tool to temporarily distort and measure the vaginal skin an examiner is able to obtain measurements for biomechanical properties of the vaginal skin. These measurements may allow clinicians and medical researchers to understand a woman's vaginal tissue quality, response to treatment, and risk for future pelvic floor disorders more completely in order to take appropriate prophylactic or corrective action.
Owner:EPSTEIN LEE BRANDON +2
Apparatus for treating pelvic floor disorders and related methods of use
A method of optimizing the electrical stimulation of a bladder of a patient including selecting a first subset of electrodes from a set of electrodes positioned adjacent to a set of nerves associated with the bladder. The set of electrodes may include one or more electrodes, each of which may be configured to deliver electrical stimulation pulses generated by a stimulator device to the nerves. The method may further include delivering an electrical stimulation pulse through the selected first subset of electrodes and recording at least one parameter of the electrical stimulation pulse after receiving patient feedback.
Owner:BOSTON SCI SCIMED INC
Apparatus for treating pelvic floor disorders and related methods of use
A method of optimizing the electrical stimulation of a bladder of a patient including selecting a first subset of electrodes from a set of electrodes positioned adjacent to a set of nerves associated with the bladder. The set of electrodes may include one or more electrodes, each of which may be configured to deliver electrical stimulation pulses generated by a stimulator device to the nerves. The method may further include delivering an electrical stimulation pulse through the selected first subset of electrodes and recording at least one parameter of the electrical stimulation pulse after receiving patient feedback.
Owner:BOSTON SCI SCIMED INC
Treatment of pelvic floor disorders with an adipose-derived cell composition
InactiveUS20110008299A1BiocideMammal material medical ingredientsPelvic Floor DiseasesBiomedical engineering
A method for treating a pelvic floor disease comprises removing adipose tissue from a patient, processing a first portion of the adipose tissue to obtain a heterogeneous mixture of cells that includes adipose-derived stem cells, combining the heterogeneous mixture of cells with a second, unprocessed portion of the adipose tissue in a ratio of from approximately 1:1 to 1:4 to produce a cell composition, wherein the second portion of the adipose tissue is structured to provide a natural scaffold, and administering the cell composition to the patient to treat a pelvic floor disease.
Owner:BOSTON SCI SCIMED INC
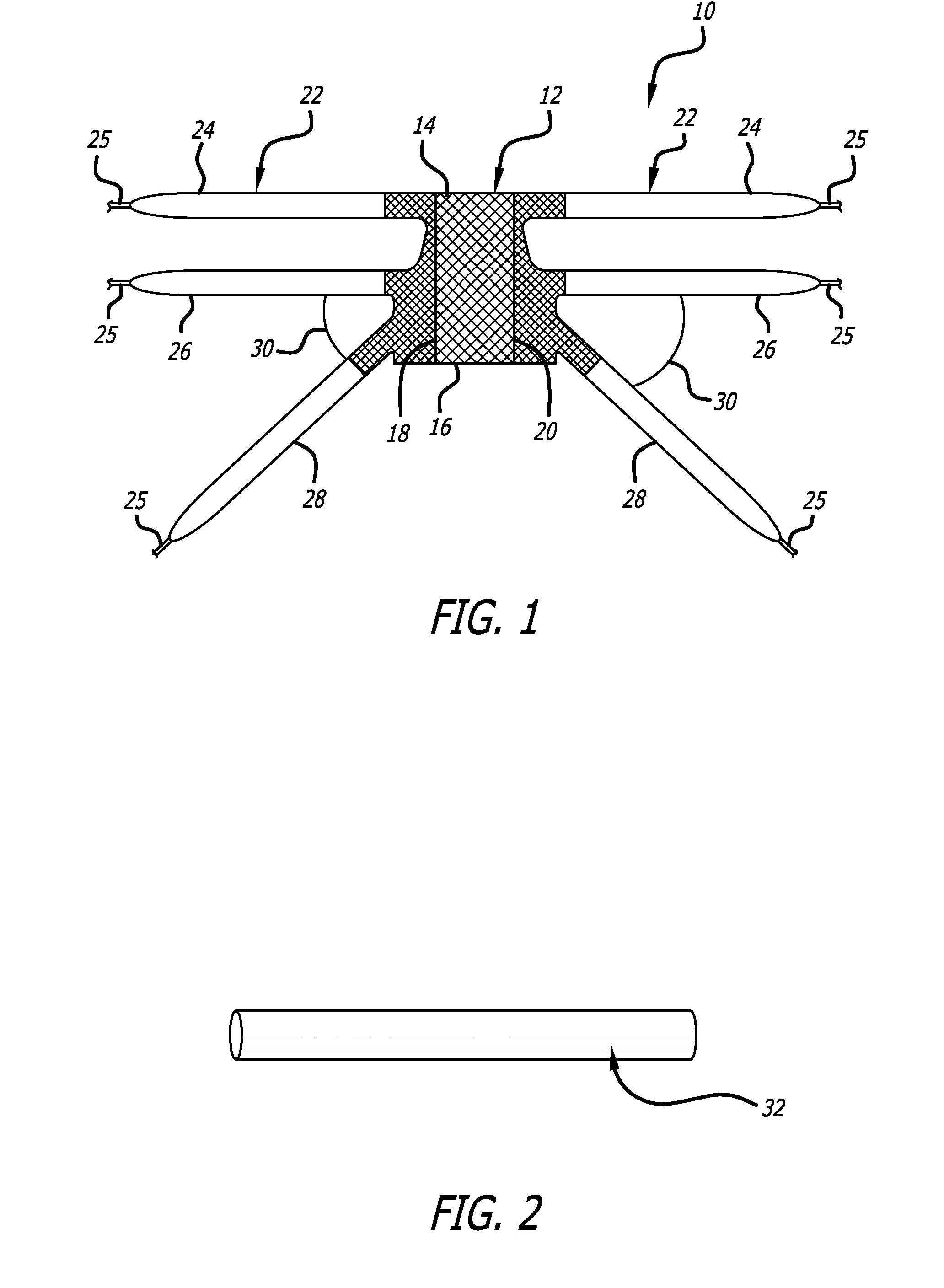
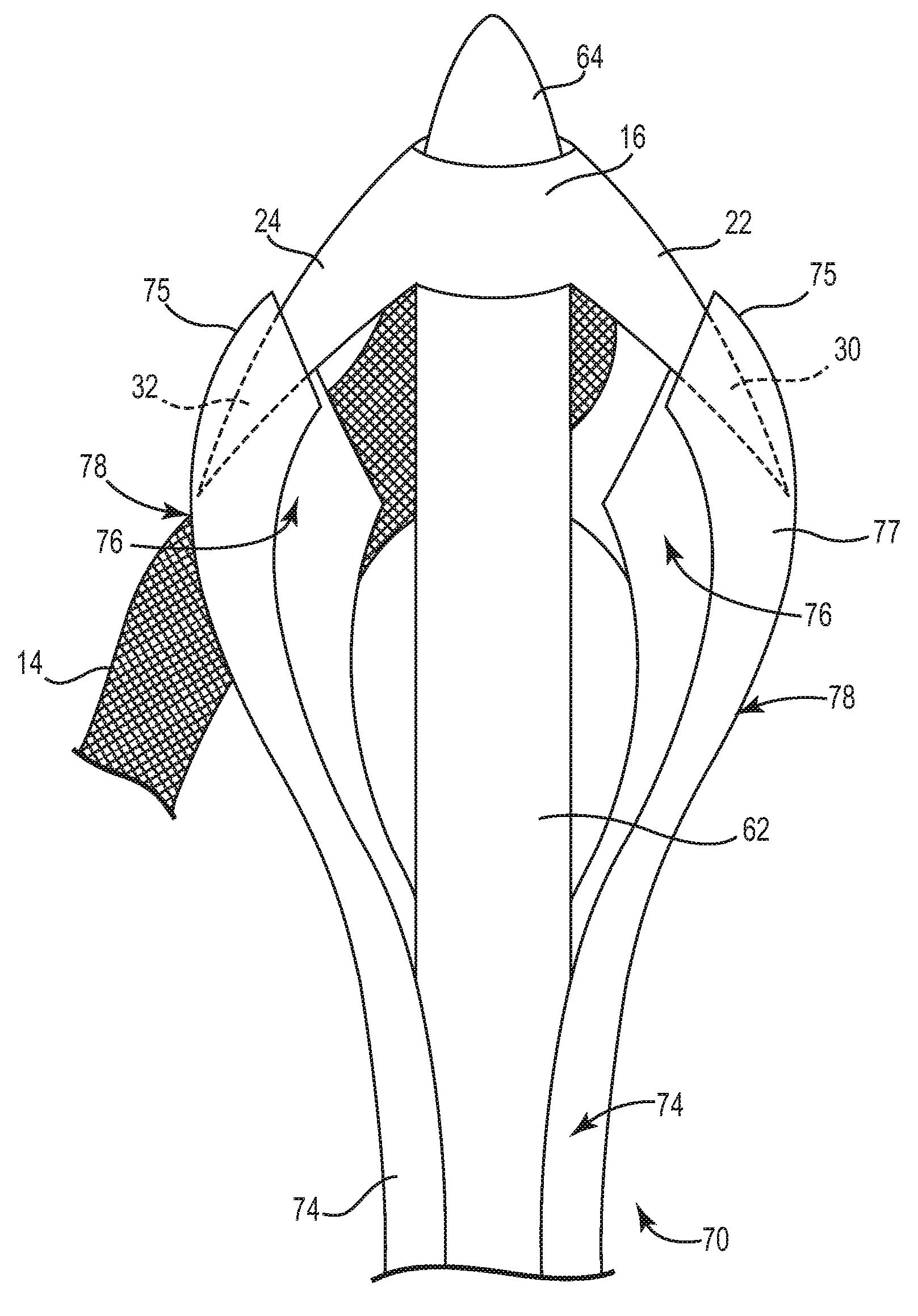
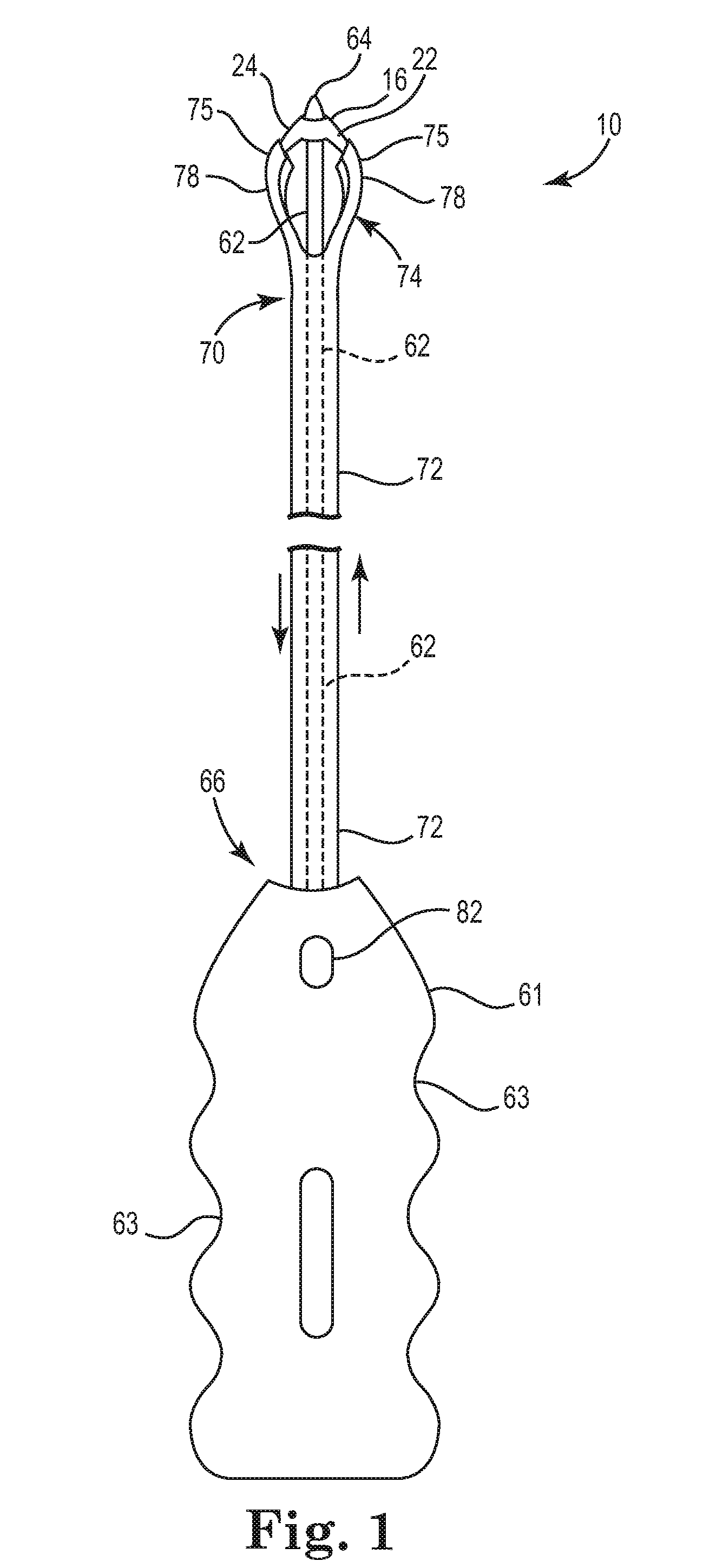
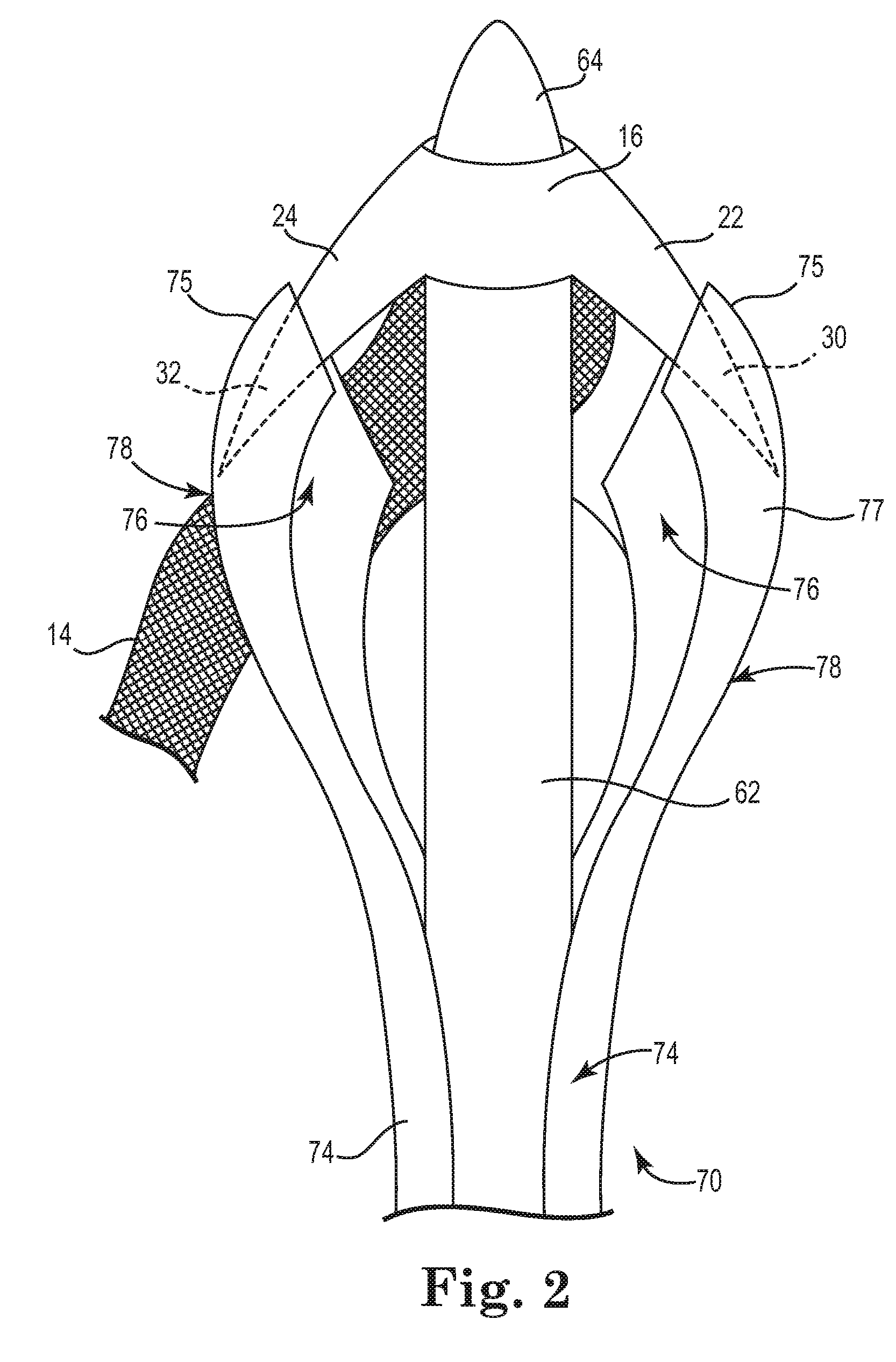
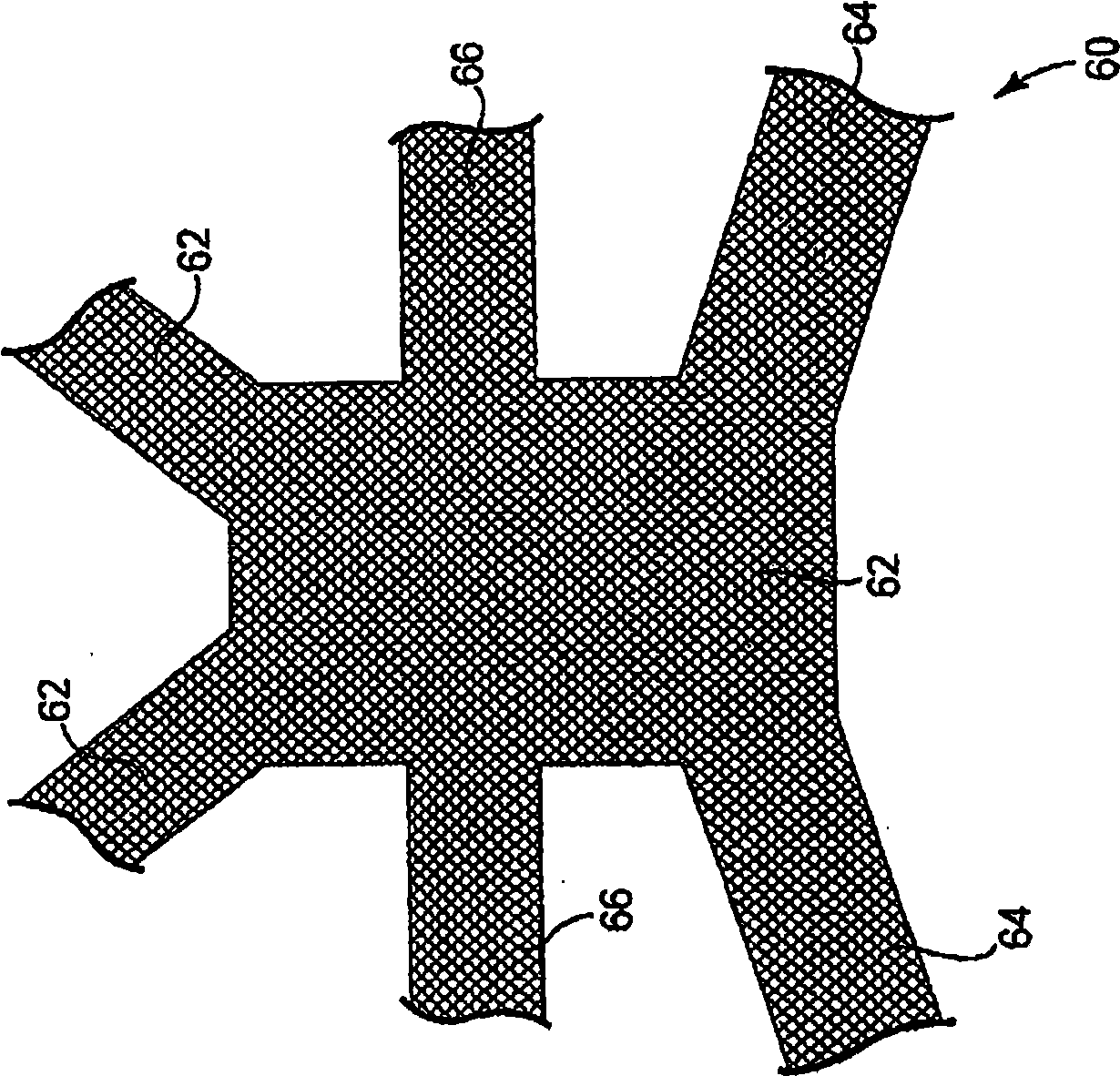
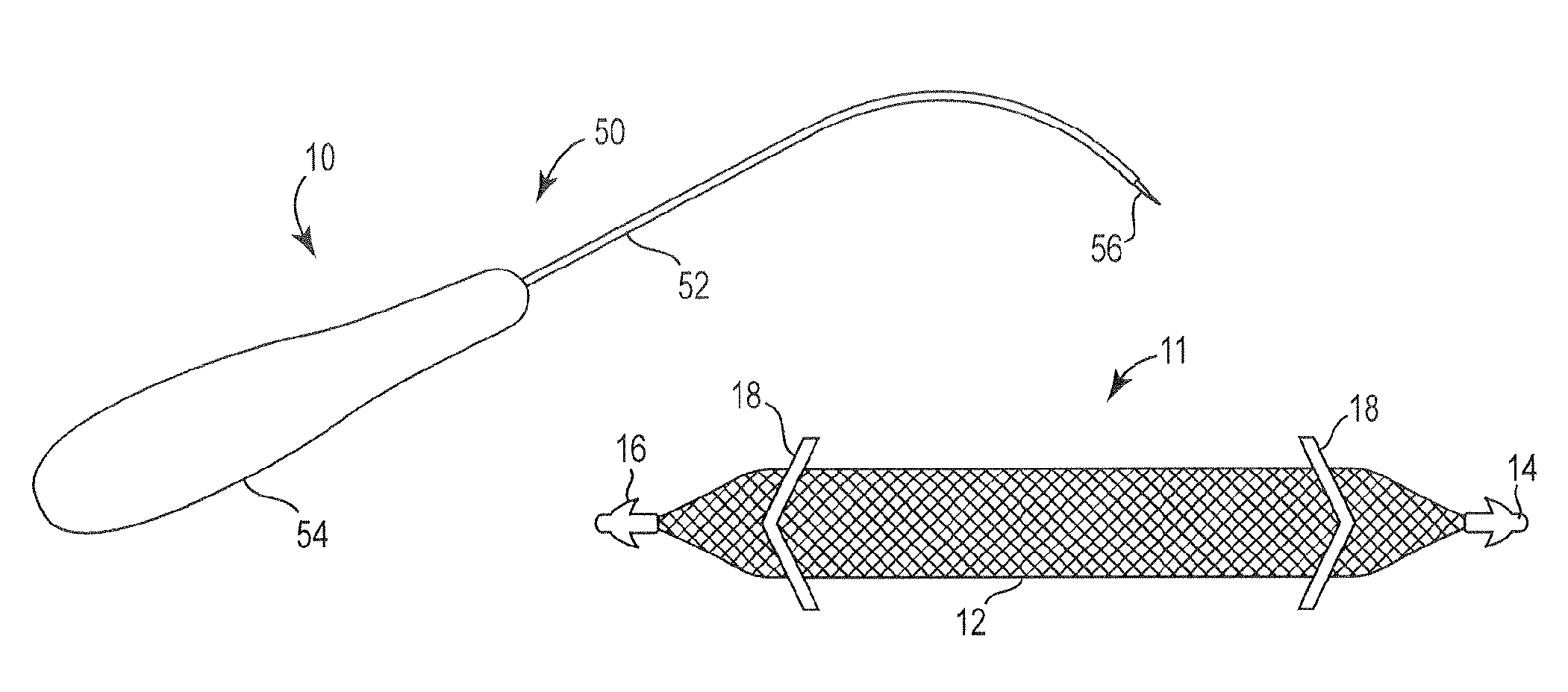
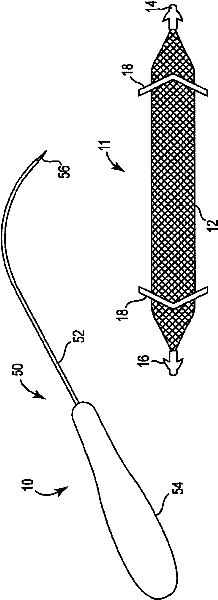
Pelvic implant and delivery system
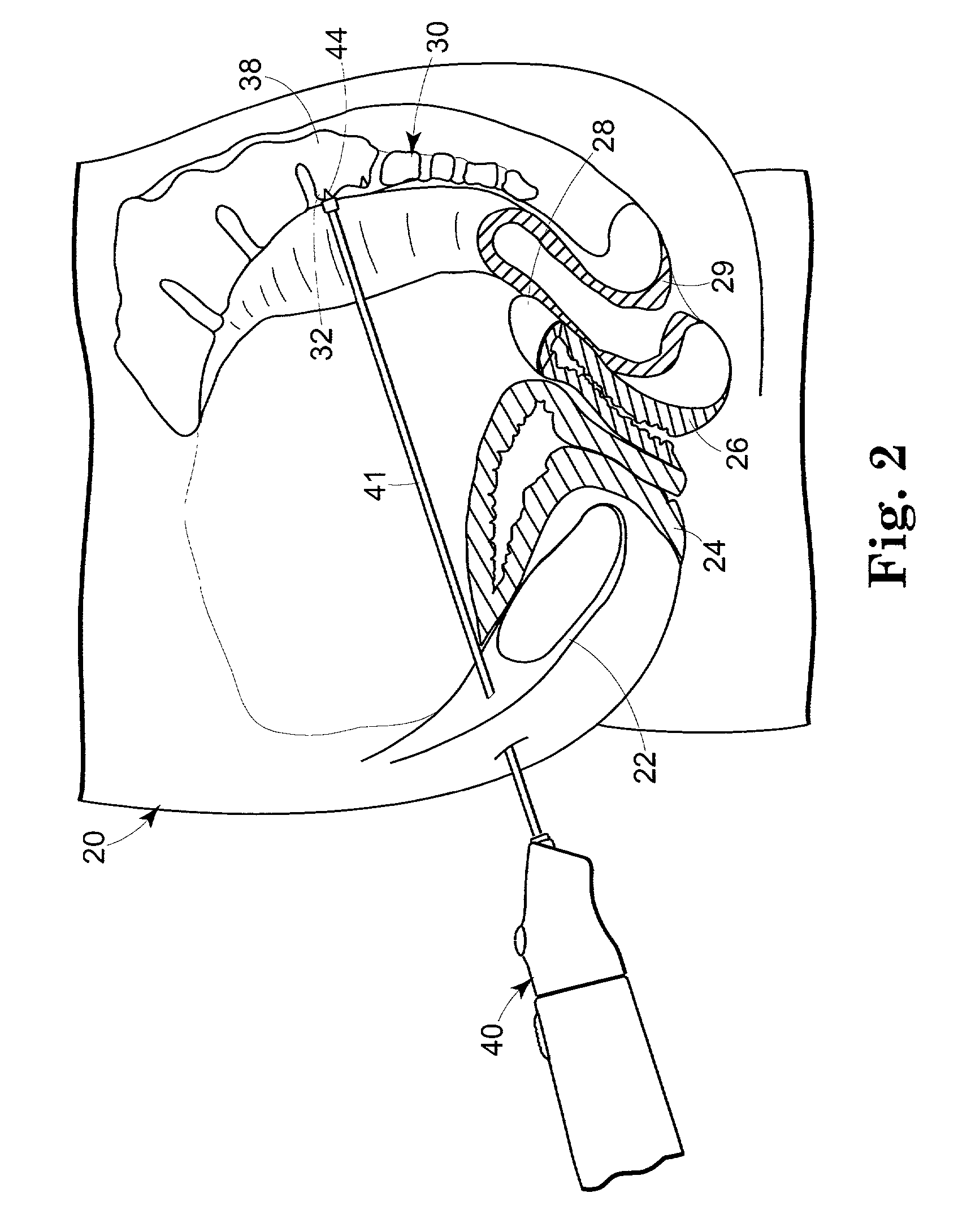
Described and depicted are pelvic sling implant and delivery systems. The sling implants can be used in treating stress incontinence and other pelvic floor disorders. The sling systems can include a mesh extension or support portion and one or more tip anchors. A delivery tool can include a handle (61), a needle (62) and a sheath (70), with the sheath having an elongate shaft portion slidable along at least a portion of the needle. The sheath can further include a distal hood portion having opposed flared portions (78) defining interior pleat channels adapted to selectively seat tine ends of the anchors.
Owner:BOSTON SCI SCIMED INC
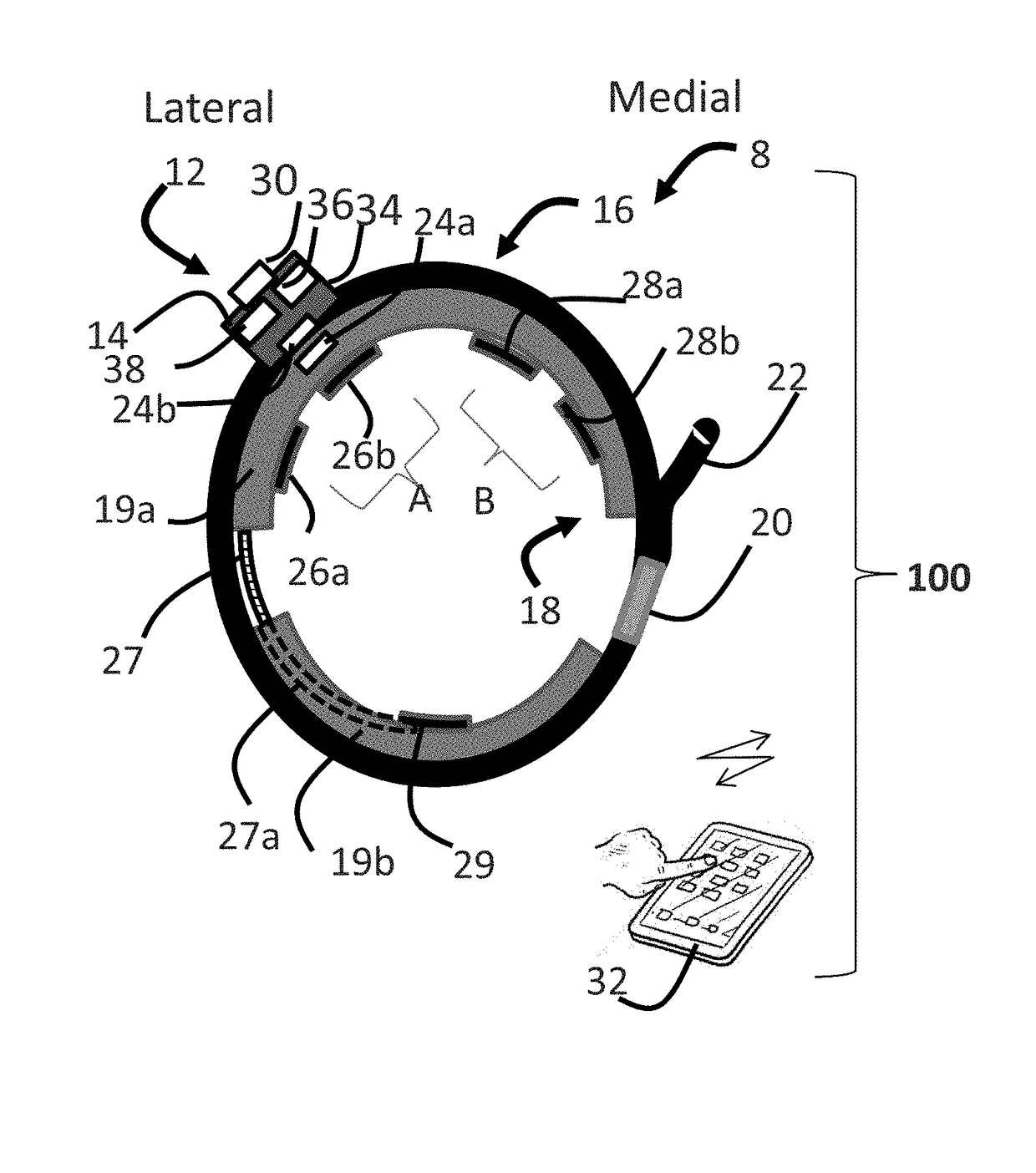
Systems and methods for assessing pelvic floor disorder therapy
ActiveUS20180296834A1Easy and rapid and clinically useful placementIncrease the number ofSensorsExternal electrodesDiseasePhysical therapy
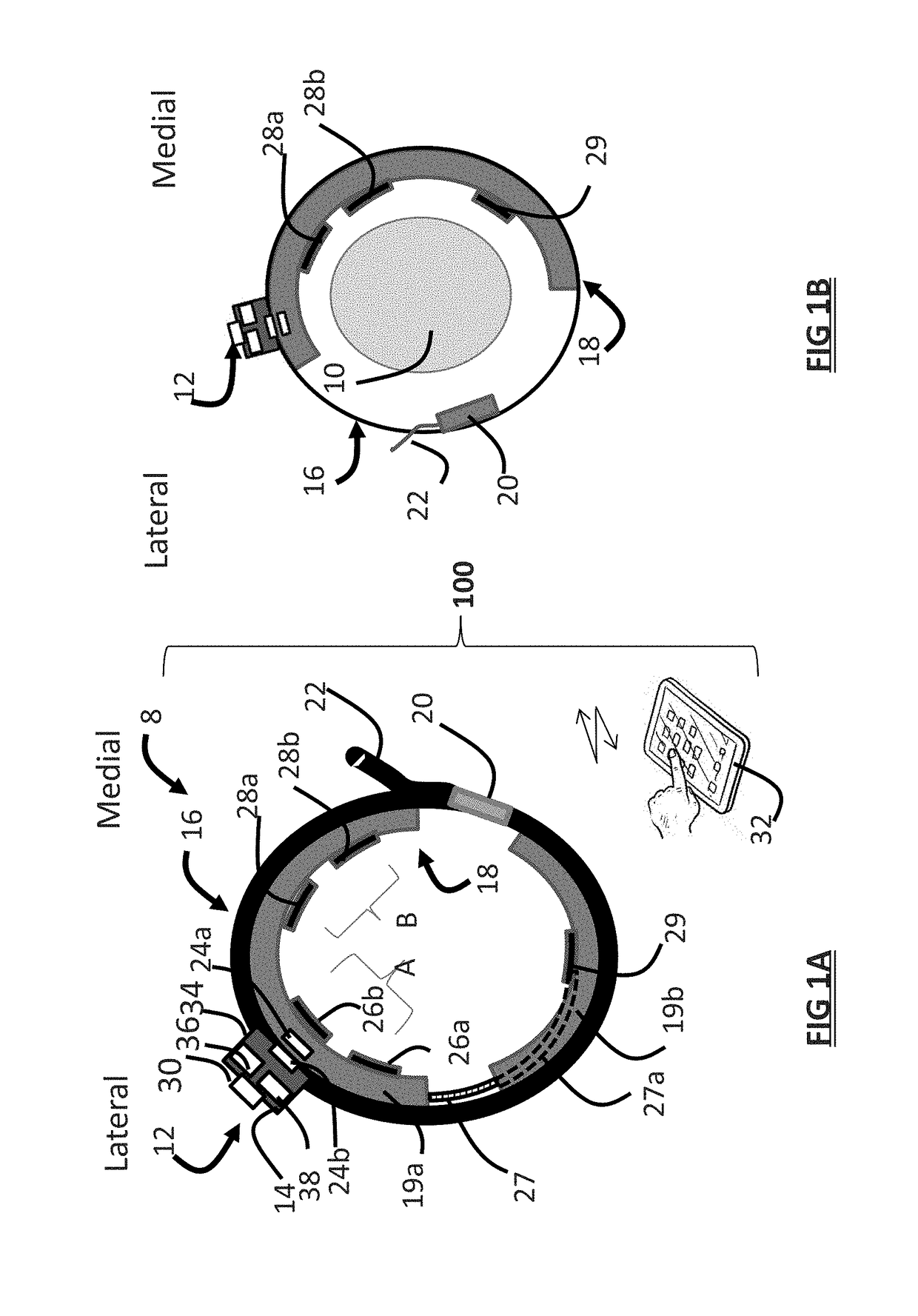
Systems and methods provide stimulation of peripheral targets such as targets in the lower limbs. Electrode arrays realized in circumferential or longitudinal embodiments have pads with horizontal and / or vertical offsets. Electrode array geometries are customizable and adaptable to individual users and treatment of different disorders. Novel systems of customization include software and hardware implemented solutions. A single device can provide treatment of two or more disorders or unwanted states using selected electrode geometries and stimulation protocols. Systems and methods for assessment of candidate stimulation sites and protocols use subjective or objective measures or both to determine which meet stimulation success criteria. Simulation is provided using transcutaneous, percutaneous, or implantable stimulators. A main advantage is the improved treatment of pelvic floor disorders, and especially overactive bladder (OAB).
Owner:EBT MEDICAL INC
Surgical implants, tools and methods for treating pelvic conditions
Described are pelvic implants (e.g., urinary incontinence sling, hammock, etc.) and method of implanting a pelvic implant that provide treatment for pelvic floor disorders such as incontinence, stress urinary incontinence, prolapse (e.g., cystocele, enterocele, rectocele, vault prolapse), fecal incontinence, and the like, wherein the implant and methods involve various features, such as the ability to adjust dimensions of an implant before, during, or after implantation.
Owner:AMS RES CORP
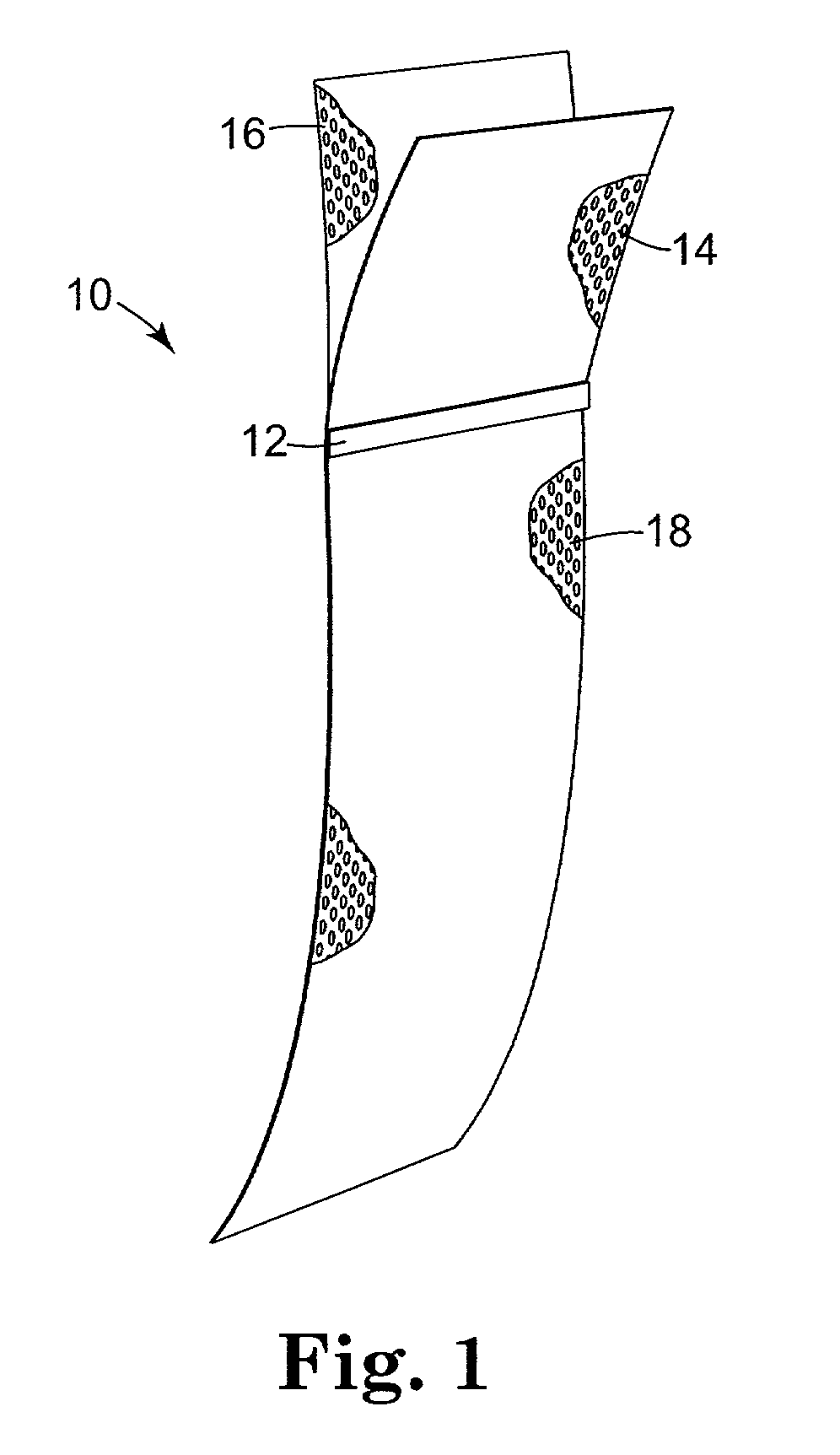
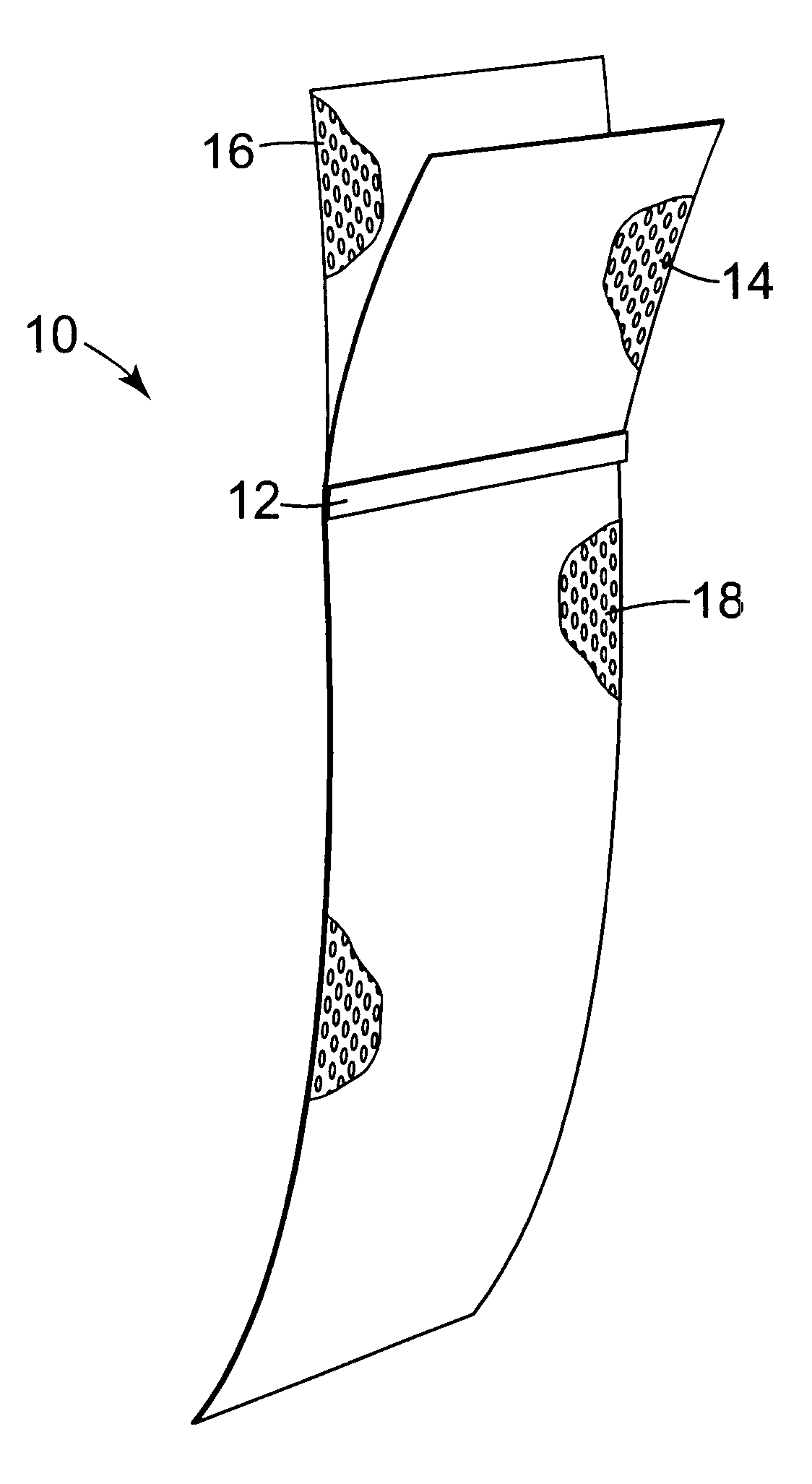
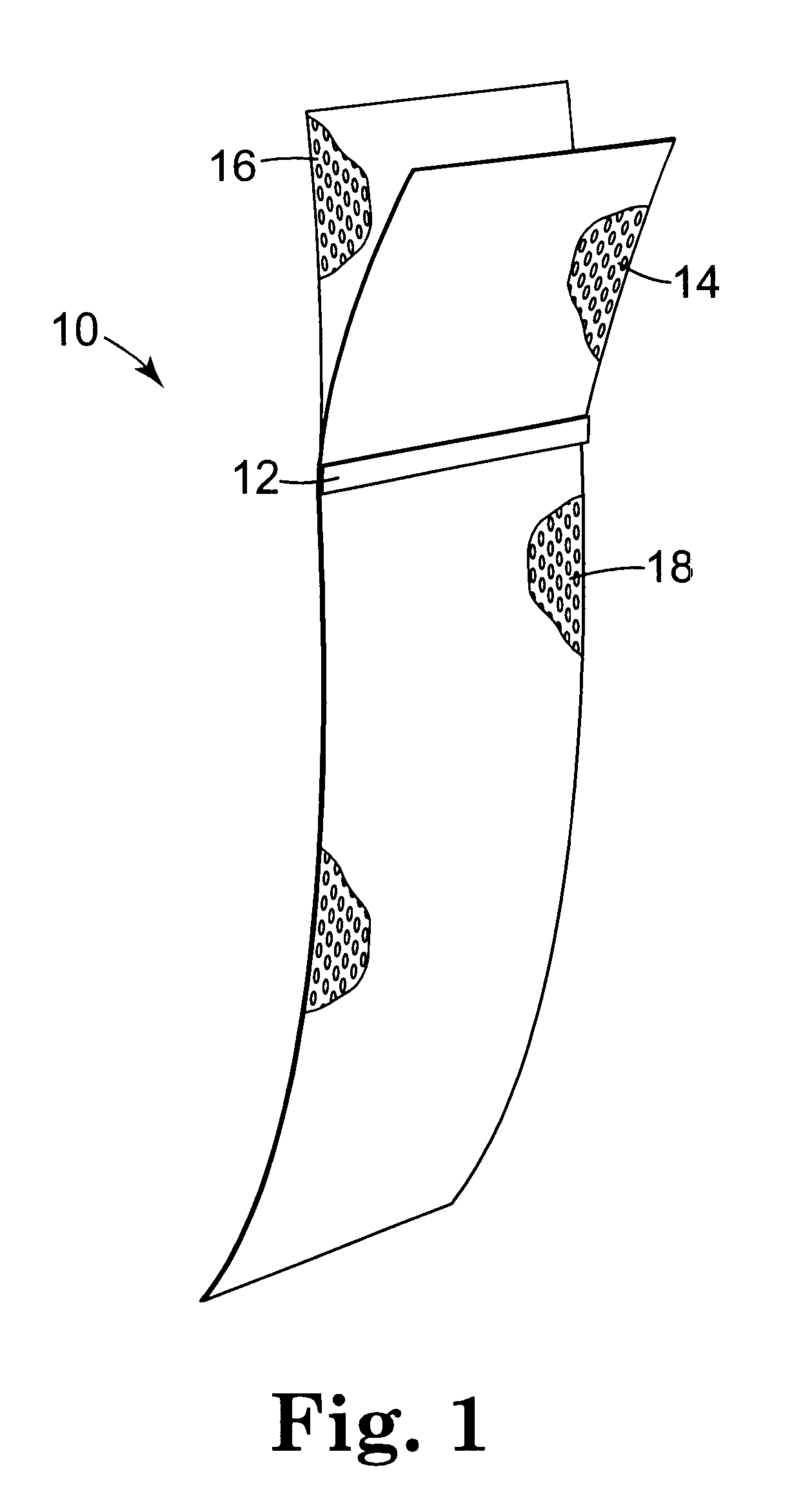
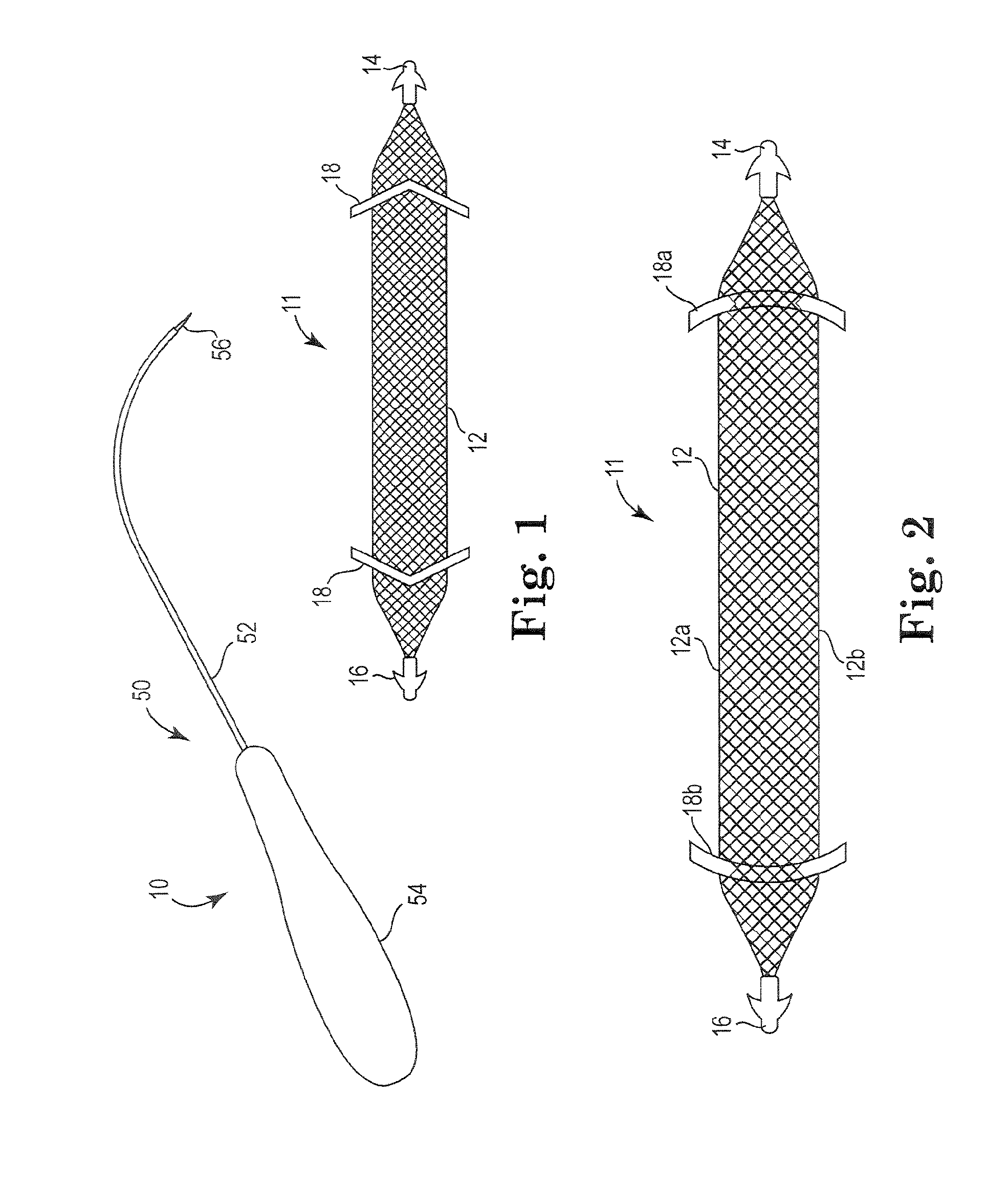
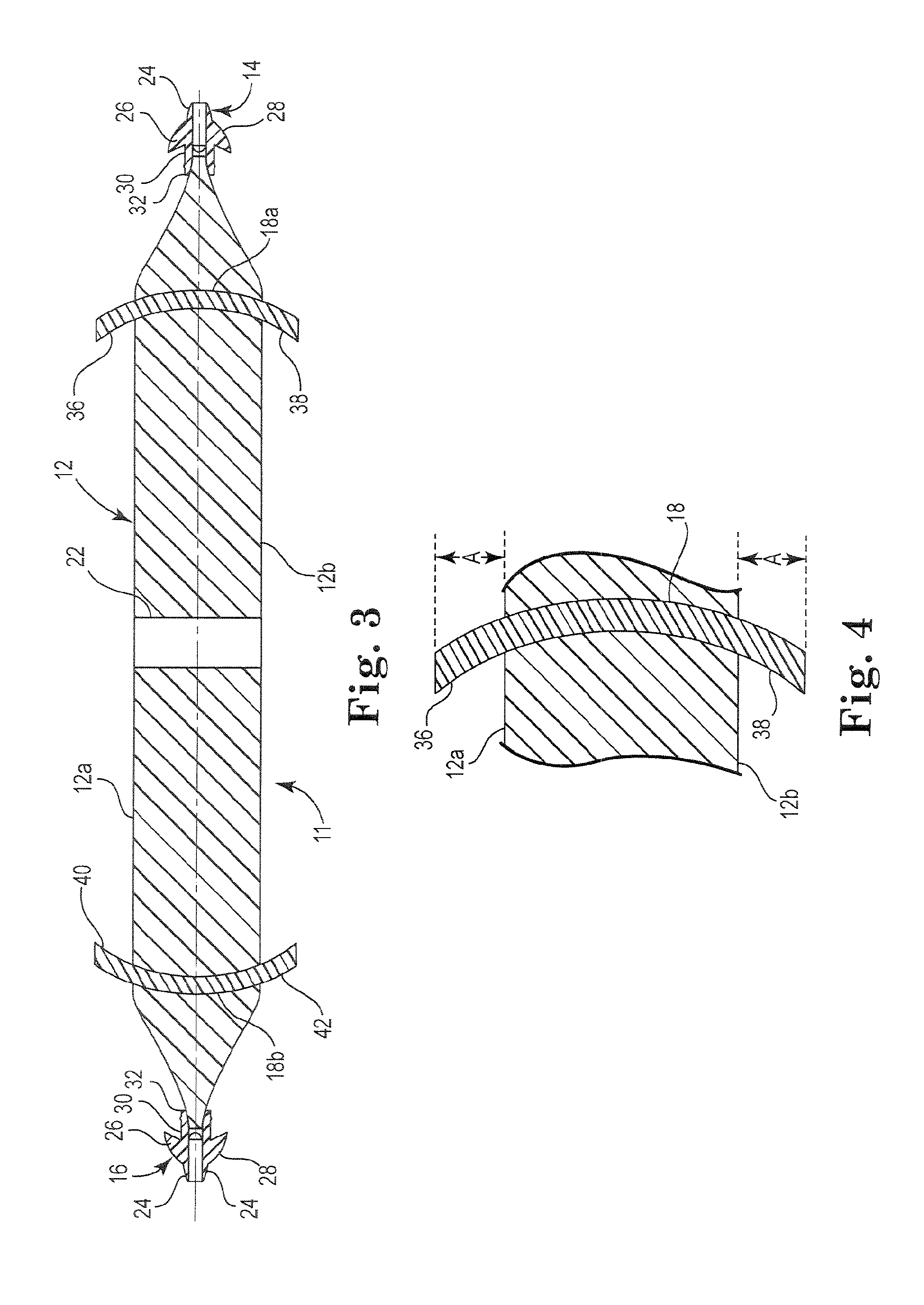
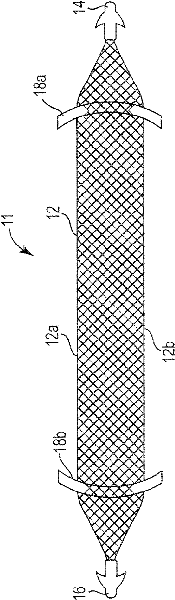
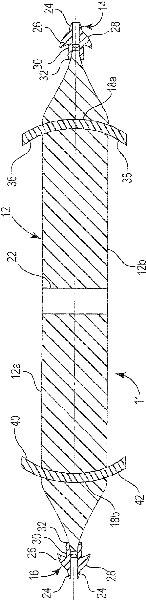
Pelvic implant system and method
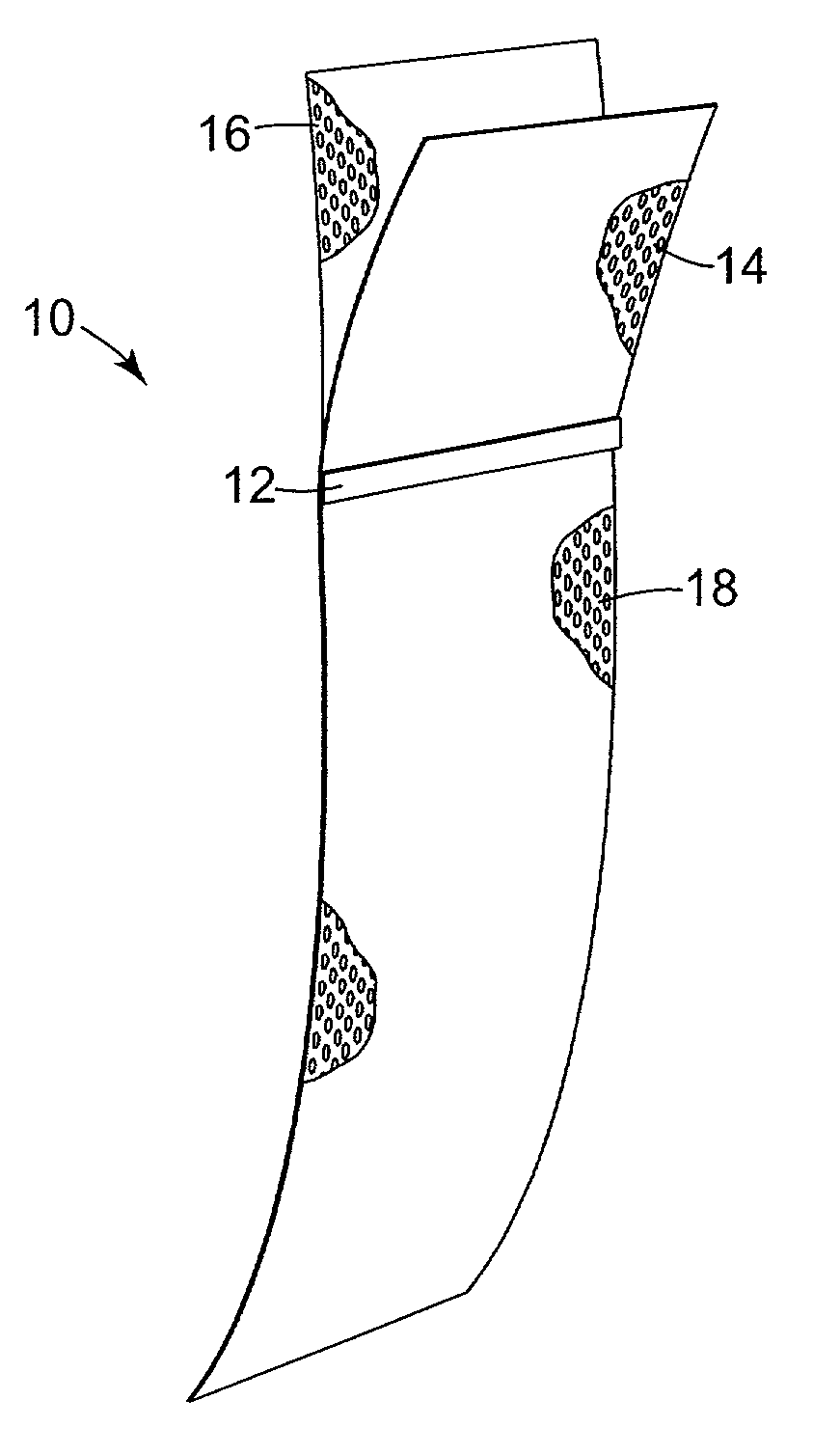
A pelvic Implant system is provided. The system can include one or more implant devices (11) to treat incontinence and other pelvic floor disorders or dysfunctions. The system can include one or more implant devices having an extension portion (12) (e.g., mesh), one or more tip portions (14,16) (e.g., self-fixating tips), and one or more intermediate anchors (18a, 18b) disposed along the extension portion, intermediate the one or more tip portions. The intermediate anchors are shaped and sized for engagement with the internal pelvic tissue, with the extension portion in turn providing support for the corresponding pelvic tissue.
Owner:AMS RES CORP
System and method for pelvic floor repair
ActiveUS20140324072A1Suture equipmentsUltrasonic/sonic/infrasonic diagnosticsTissue repairVaginal walls
A method of repairing a pelvic floor disorder and a system for carrying out the method are provided. The method is effected by positioning an imaging device in an abdominal, rectal, perianal or vaginal cavity, advancing a surgical instrument through a vaginal wall under guidance of the imaging device to thereby reach a target tissue and using the surgical instrument to attach the tissue repair device to the target tissue.
Owner:FEMSELECT LTD
System and method for pelvic floor repair
ActiveUS9517058B2Ultrasonic/sonic/infrasonic diagnosticsSuture equipmentsTissue repairPelvic diaphragm muscle
A method of repairing a pelvic floor disorder and a system for carrying out the method are provided. The method is effected by positioning an imaging device in an abdominal, rectal, perianal or vaginal cavity, advancing a surgical instrument through a vaginal wall under guidance of the imaging device to thereby reach a target tissue and using the surgical instrument to attach the tissue repair device to the target tissue.
Owner:FEMSELECT LTD
Minimally invasive levator avulsion repair
InactiveUS20110082331A1Easy to implantSuture equipmentsAnti-incontinence devicesPelvic Floor DiseasesPelvic diaphragm muscle
Described are pelvic implants and methods of surgically placing pelvic implants that provide treatment for pelvic floor disorders by support of the levator by creating an incision that allows access to a region of tissue of the pelvic floor and inserting a pelvic implant comprising a tissue support portion delivered by a delivery tool. A pelvic implant for supporting levator tissue comprising: a tissue support portion having a first end and a second; a tissue fastener disposed on the first end of the tissue support portion for fastening it to levator tissue; a guide extension portion disposed on the second end of the tissue support portion; and a support backer member attachable to the guide extension portion to adjust a tension of the tissue support portion and concomitantly the levator tissue.
Owner:BOSTON SCI SCIMED INC
Suturing device for treament of pelvic floor disorders
The invention relates to a suturing device used to place sutures inside the human body during various medical procedures. The suturing device includes an elongate body member, a needle deployment mechanism and a needle receiving portion. The elongate body member has a distal portion that defines an opening. The needle deployment mechanism is disposed at least partially within the elongate body member for moving a needle out of the opening at the distal portion of the elongate body member into a tissue; and the needle receiving portion is provided at the distal portion of the elongate body member to capture the needle. The needle receiving portion has a non-planar surface with walls that are non-parallel with respect to a longitudinal direction of the needle receiving portion such that the walls converge to form an aperture formed in the needle receiving portion to receive the needle.
Owner:BOSTON SCI SCIMED INC
Implants And Procedures For Treatment Of Pelvic Floor Disorders
InactiveUS20130281770A1Preserve natural lengthNot to damageSuture equipmentsAnti-incontinence devicesDiseasePelvic diaphragm muscle
Implants for the treatment of pelvic support conditions and methods of implementing the same. The implants comprise relatively soft, flexible bodies and relatively strong arms extending in predetermined orientation therefrom. Methods and devices for placing the implants minimize trauma to the pelvic floor and provide well-anchored support to pelvic organs without interfering with sexual or other bodily functions.
Owner:CALDERA MEDICAL
Implants and procedures for treatment of pelvic floor disorders
InactiveUS8480558B2Preserve natural lengthNot to damageSuture equipmentsAnti-incontinence devicesDiseasePelvic diaphragm muscle
Implants for the treatment of pelvic support conditions and methods of implementing the same. The implants comprise relatively soft, flexible bodies and relatively strong arms extending in predetermined orientation therefrom. Methods and devices for placing the implants minimize trauma to the pelvic floor and provide well-anchored support to pelvic organs without interfering with sexual or other bodily functions.
Owner:CALDERA MEDICAL
Treatment of pelvic floor disorders using targeted lower limb nerve stimulation
ActiveUS20200139118A1Increase the number ofReduce frequencyUltrasound therapyExternal electrodesNervous systemTherapy related
Methods and systems for improving nerve stimulation are disclosed which relate to shaping characteristics of the stimulation field such as by using different geometries and locations of stimulation. In embodiments, systems and methods are provided to improve selective modulation of specific targeted neural substrate, while minimizing the activation of adjacent non-targeted nervous tissue. While aspects of the disclosed technologies can be applied to any part of the central and peripheral nervous systems for treatment various disorders and providing symptom relief to provide for therapy related to pelvic floor disorders such as overactive bladder
Owner:EBT MEDICAL INC
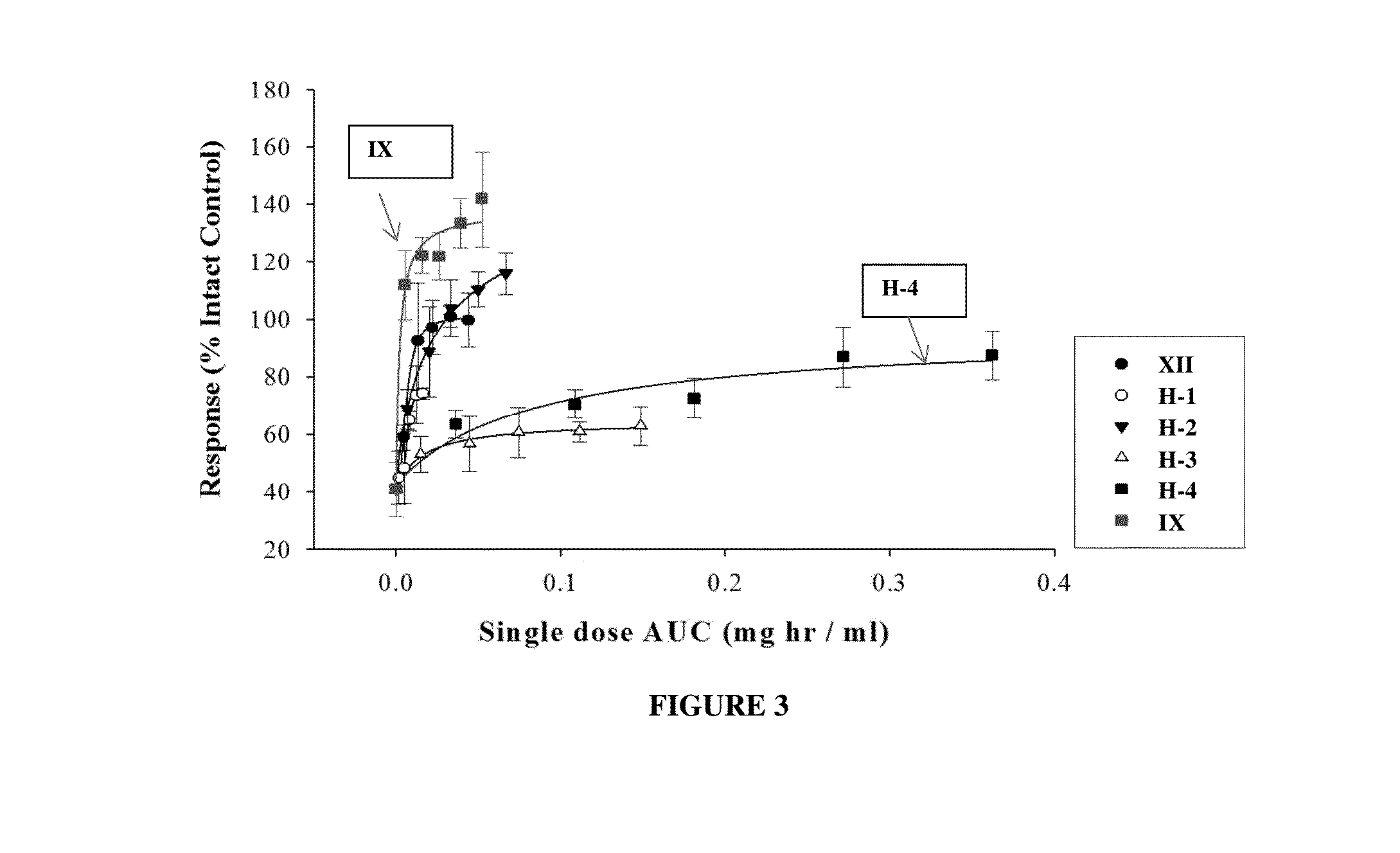
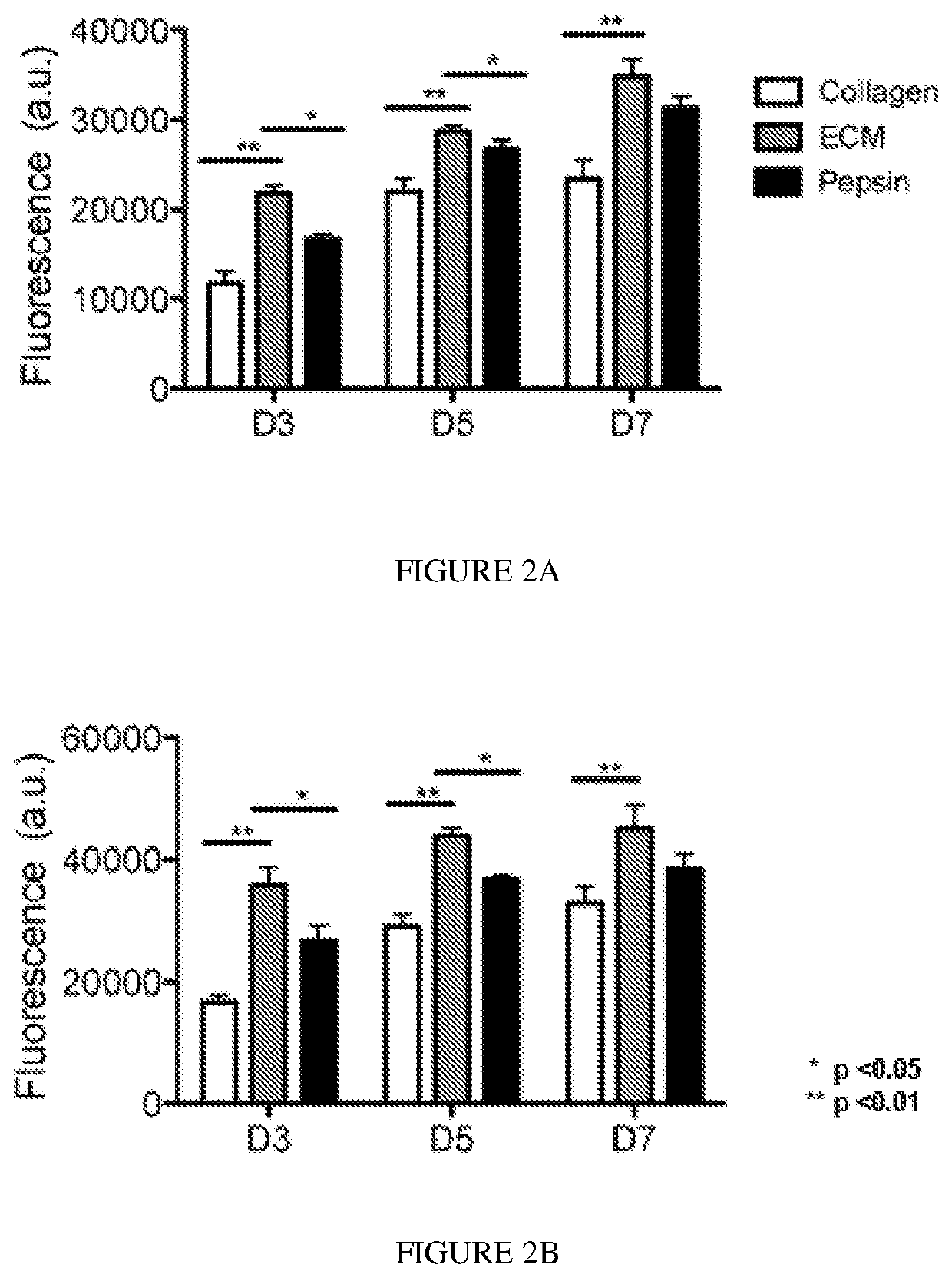
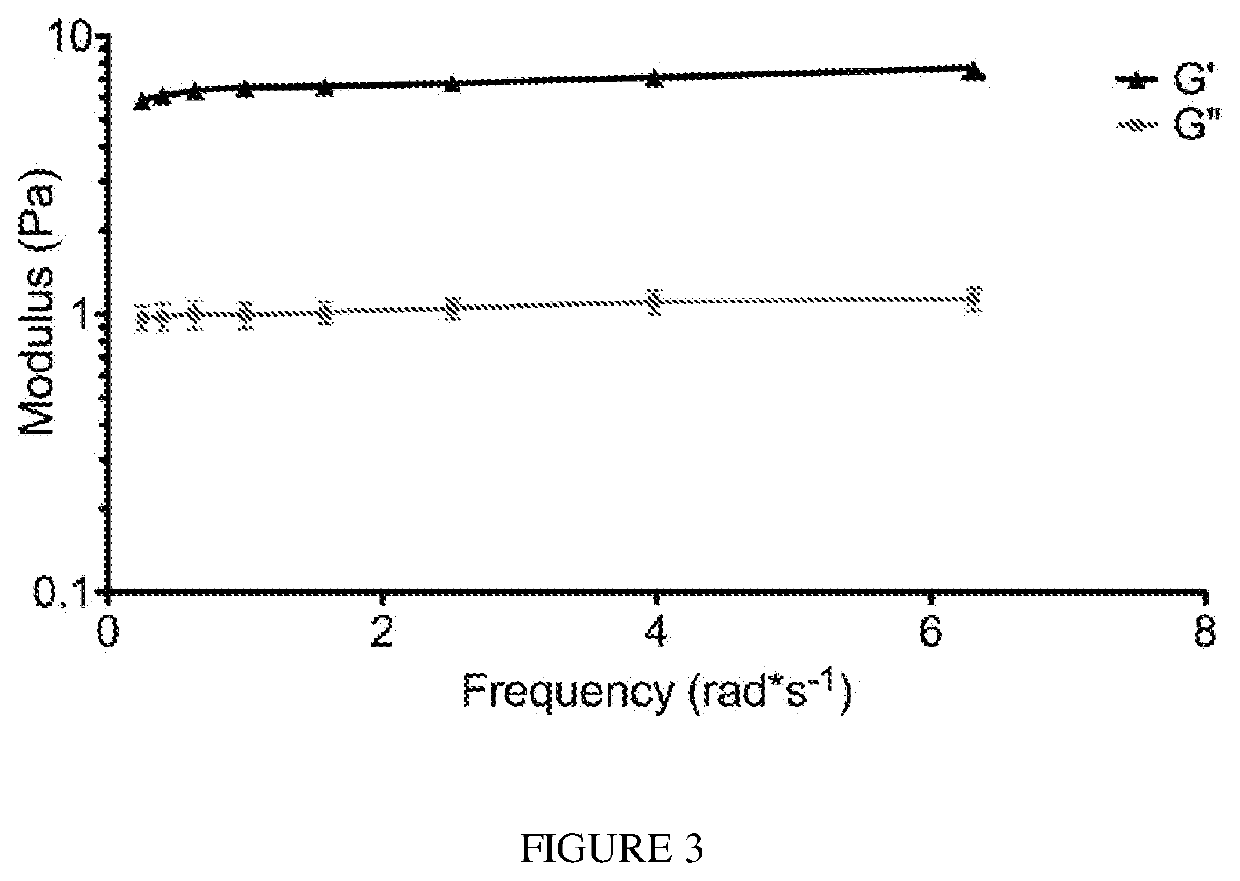
Biodegradable articles and methods for treatment of pelvic floor disorders including extracellular matrix material
InactiveUS20160213815A1Promote tissue regenerationIncreased formationAnti-incontinence devicesSurgeryDiseaseUrethral stents
Biodegradable implants including an ECM material and methods for treating a pelvic floor condition are described. ECM particles can be present within or on the surface of a pelvic implant, such as a biodegradable mesh, a pouch, or a urethral stent. After implantation the implant can provide tissue support, degrade over a period of time, and deposit the ECM material in the implantation area for regeneration of tissue and long term benefit.
Owner:BOSTON SCI SCIMED INC
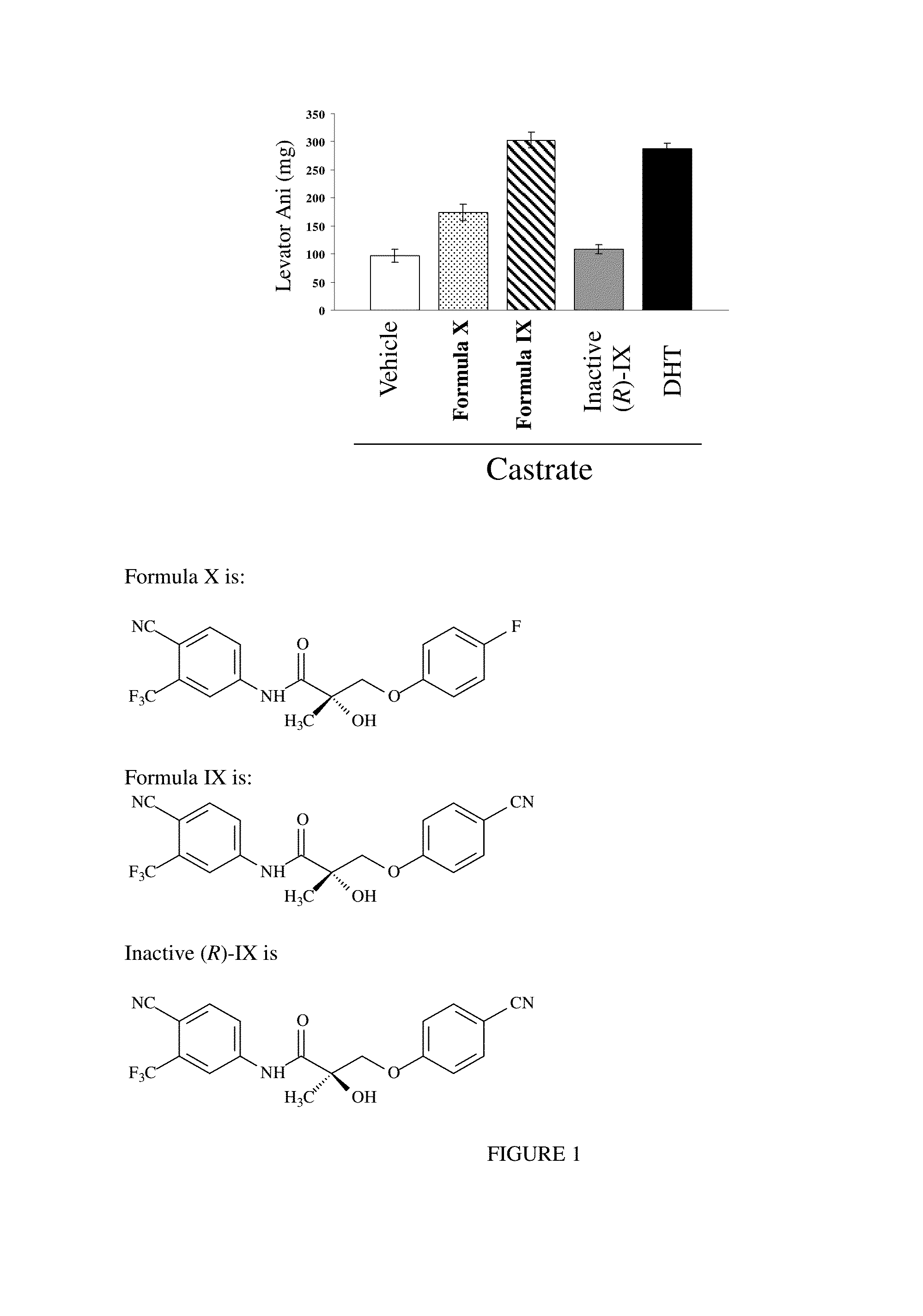
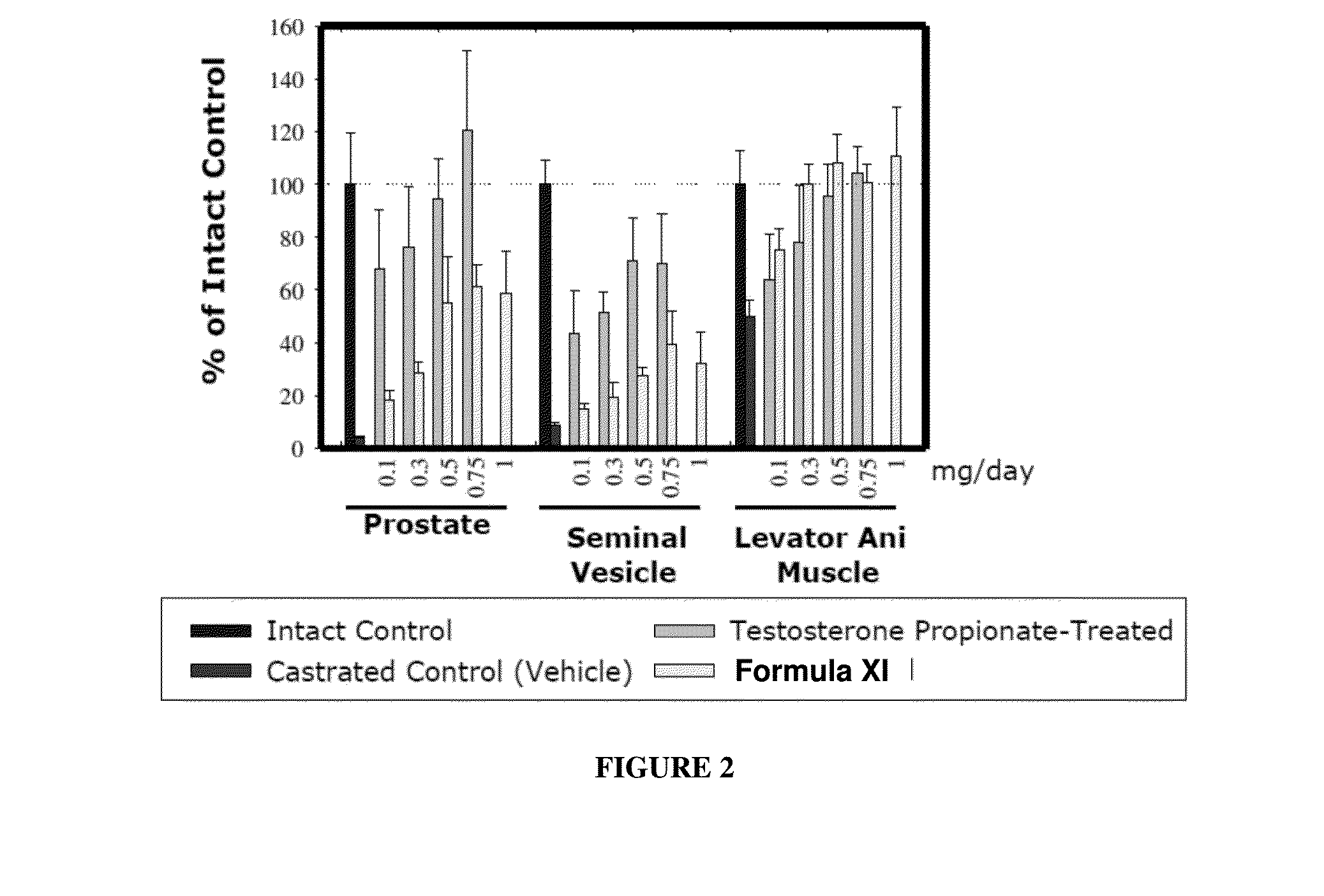
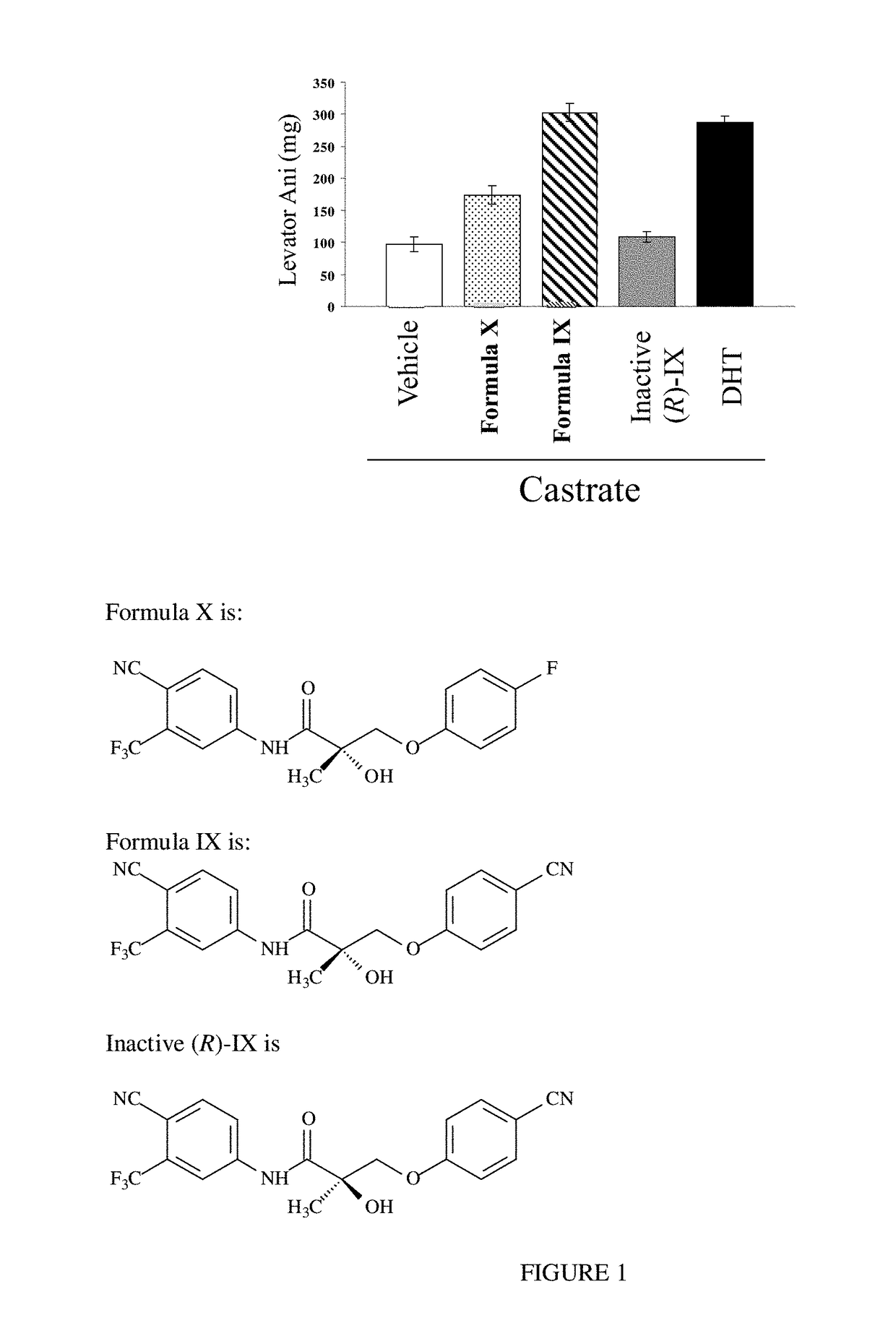
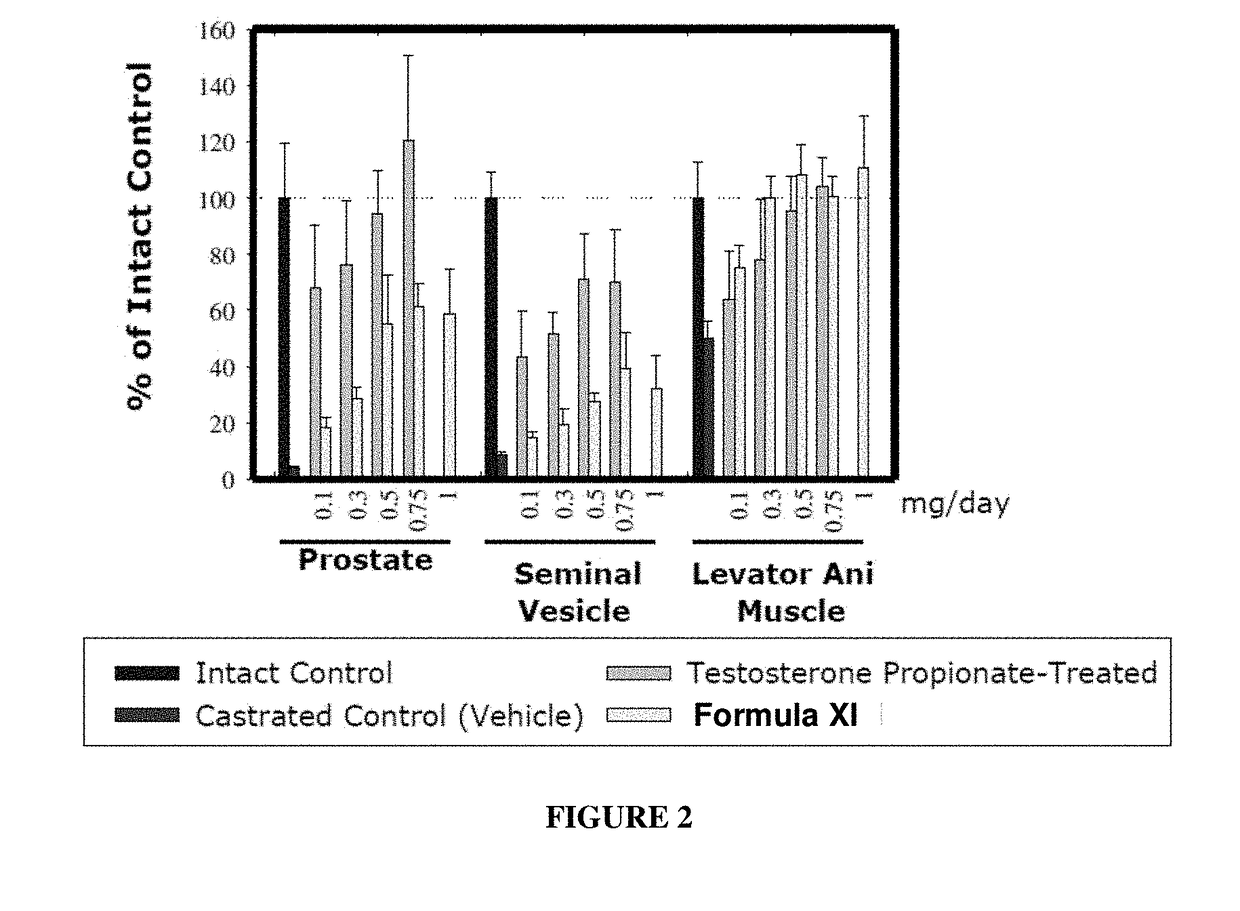
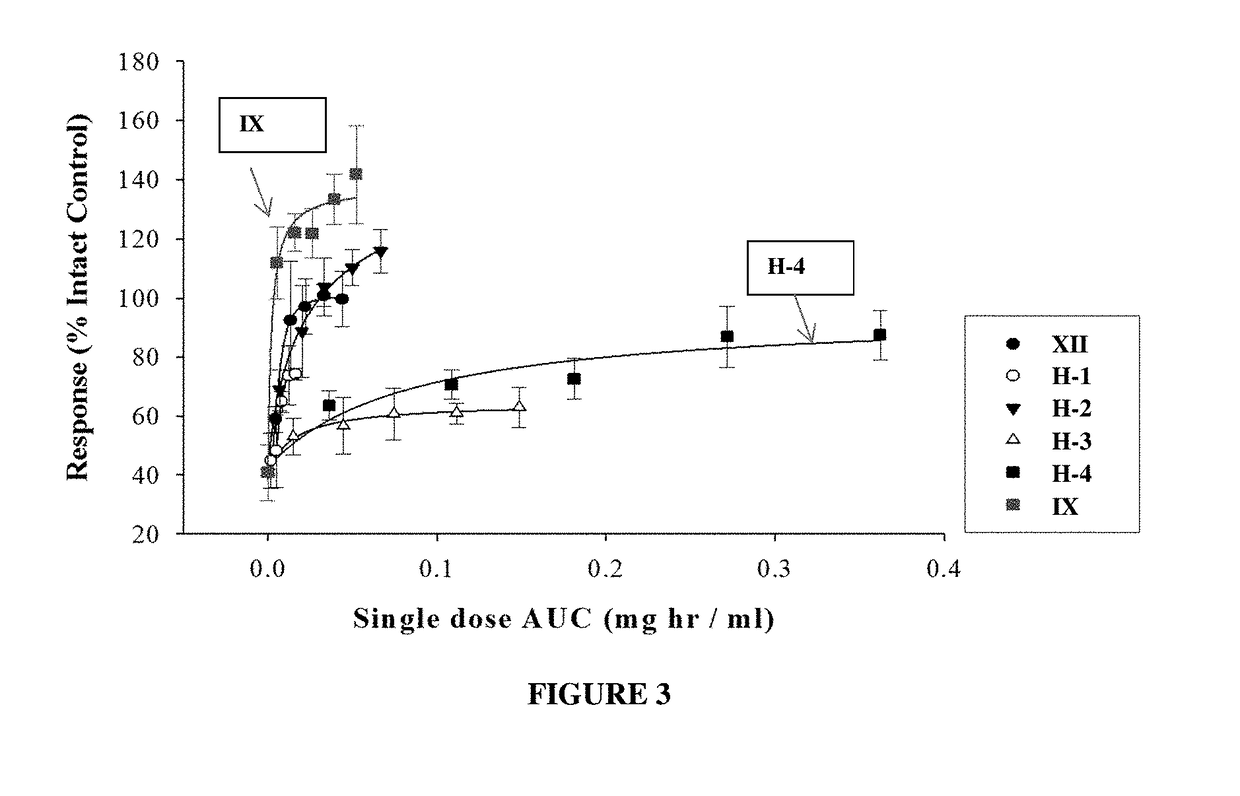
METHODS OF TREATING UROLOGICAL DISORDERS USING SARMs
InactiveUS20160106702A1Preventing and suppressing and inhibiting urinary incontinenceBiocideDigestive systemStress incontinenceUrological Disorders
The present invention is directed to methods of treating, preventing, suppressing and / or inhibiting urological disorders such as urinary incontinence including stress urinary incontinence and pelvic-floor disorders by administering a SARM compound of the invention.
Owner:ONCTERNAL THERAPEUTICS INC
Extracellular matrix for treating pelvic floor disorders and skeletal muscle degeneration
ActiveUS11376346B2Promote differentiationReduce manufacturing costPharmaceutical delivery mechanismUnknown materialsDiseaseCell-Extracellular Matrix
Described herein are compositions comprising decellularized extracellular matrix derived from skeletal muscle or other suitable tissue, and therapeutic uses thereof. Methods for treating, repairing or regenerating defective, diseased, damage, ischemic, ulcer cells, tissues or organs in a subject preferably a human, with diseases associated with muscular degeneration, using a decellularized extracellular matrix of the invention are provided. Methods of preparing culture surfaces and culturing cells with absorbed decellularized extracellular matrix are provided.
Owner:RGT UNIV OF CALIFORNIA
Pelvic implant system and method
A pelvic Implant system is provided. The system can include one or more implant devices (11) to treat incontinence and other pelvic floor disorders or dysfunctions. The system can include one or more implant devices having an extension portion (12) (e.g., mesh), one or more tip portions (14,16) (e.g., self-fixating tips), and one or more intermediate anchors (18a, 18b) disposed along the extension portion, intermediate the one or more tip portions. The intermediate anchors are shaped and sized for engagement with the internal pelvic tissue, with the extension portion in turn providing support for the corresponding pelvic tissue.
Owner:AMS RES CORP
METHODS OF TREATING UROLOGICAL DISORDERS USING SARMs
The present invention is directed to methods of treating, preventing, suppressing and / or inhibiting urological disorders such as urinary incontinence including stress urinary incontinence, urge urinary incontinence, mixed incontinence, and pelvic floor disorders by administering a SARM compound of the invention.
Owner:ONCTERNAL THERAPEUTICS INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com