Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
31 results about "Implantable infusion pumps" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
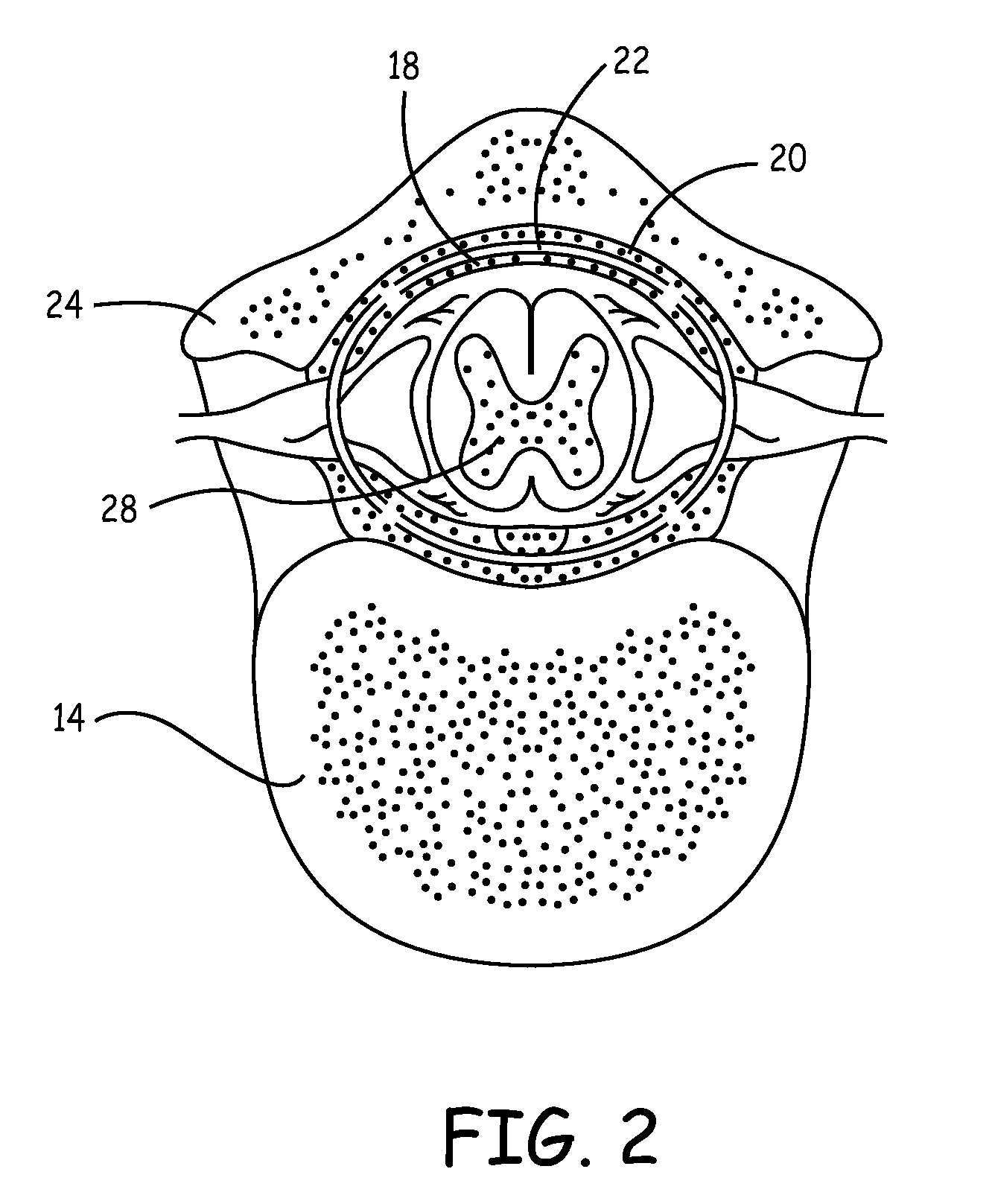
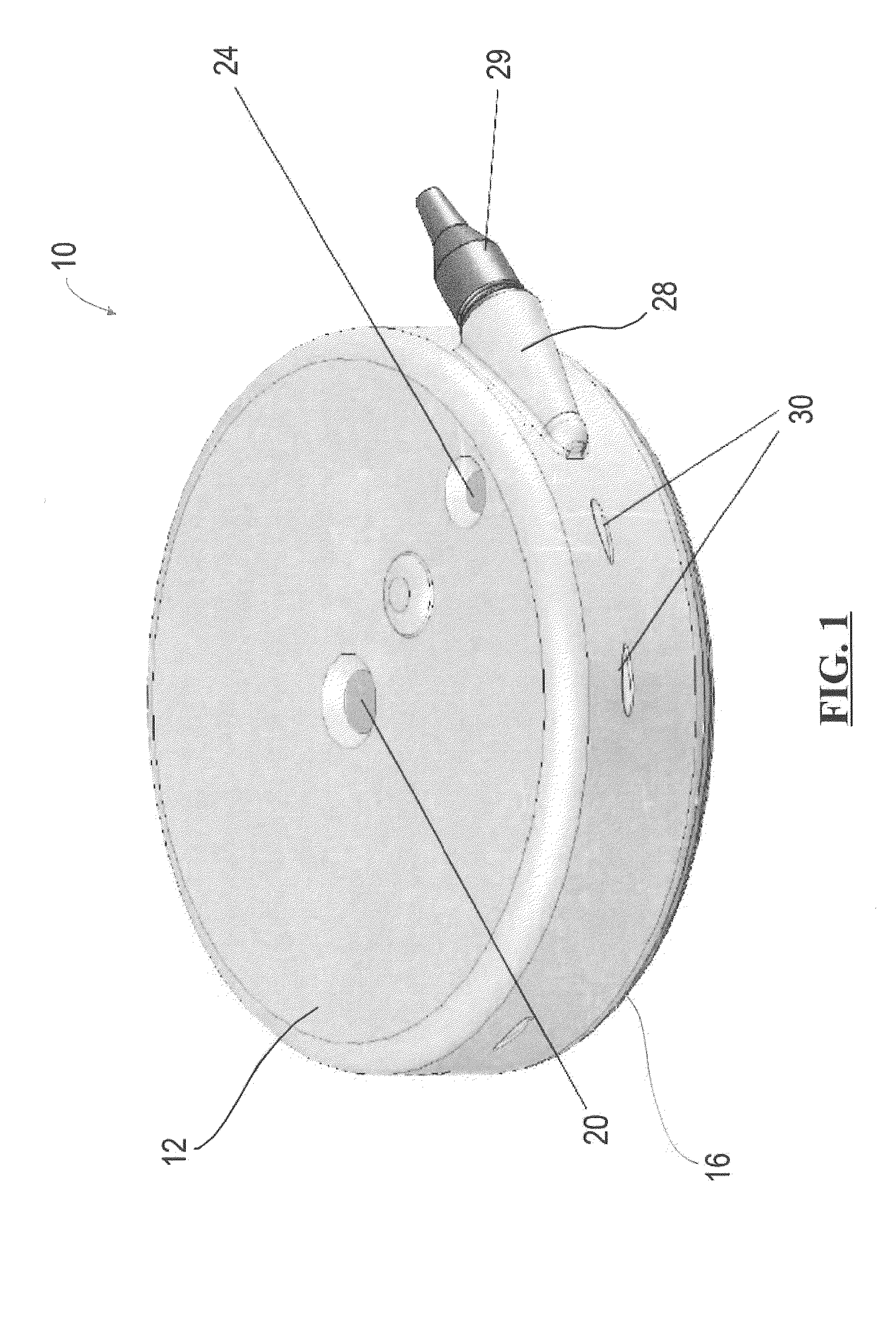
Implantable infusion pumps are devices that are surgically implanted under the skin, typically in the abdominal region. They are connected to an implanted catheter and are used to deliver medications and fluids within the body. Implantable infusion pumps are periodically refilled with medications or fluids by a health care provider.
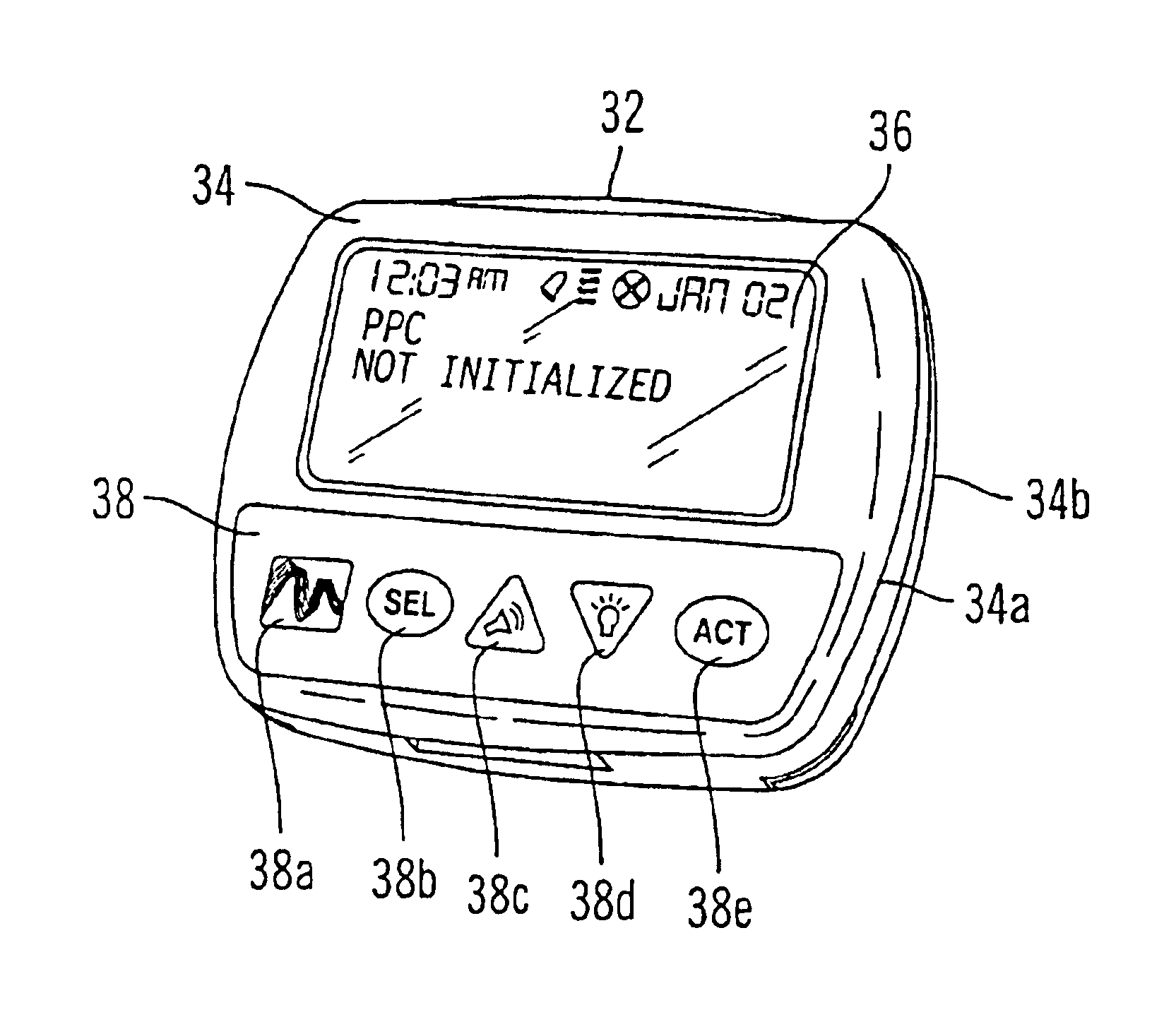
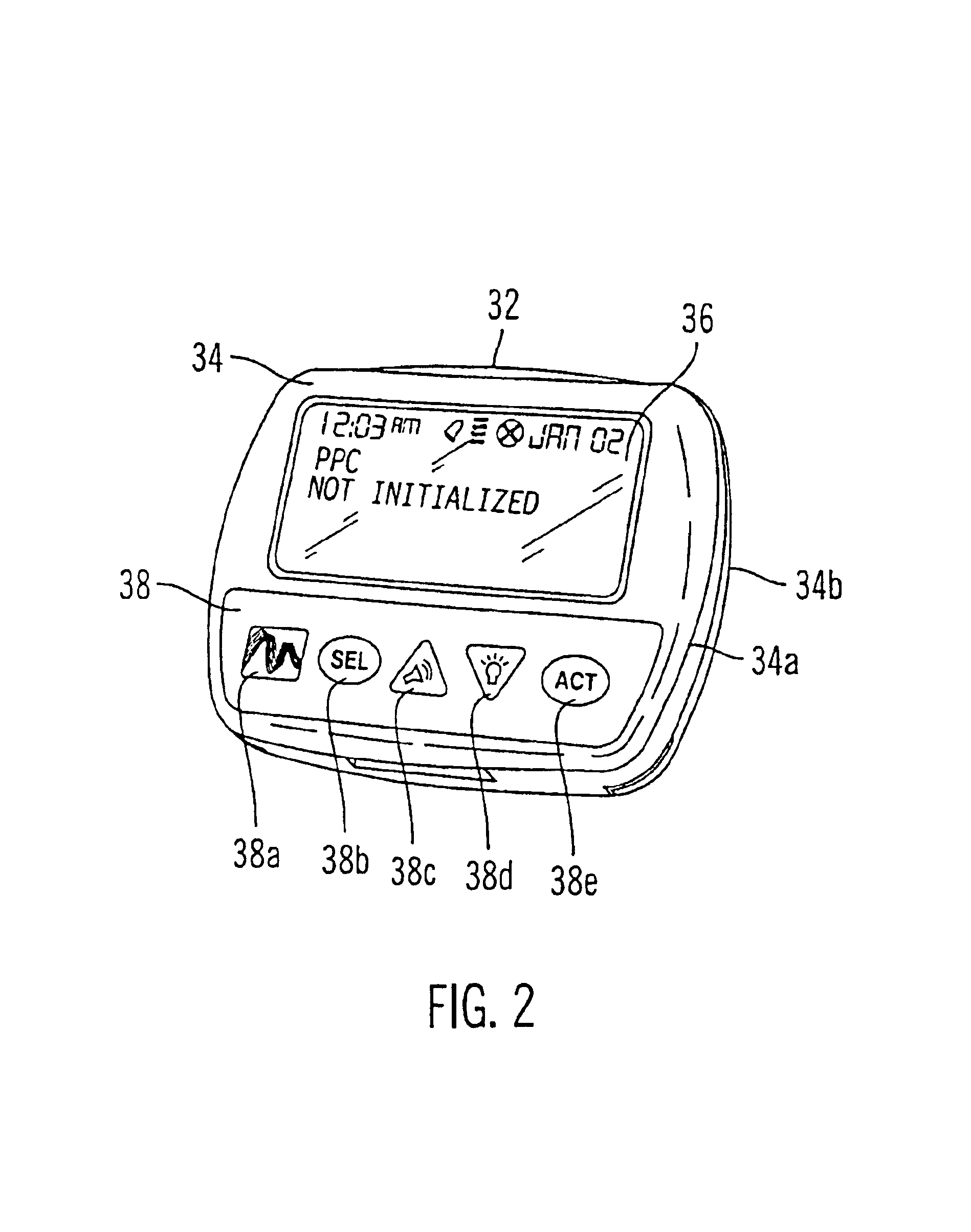
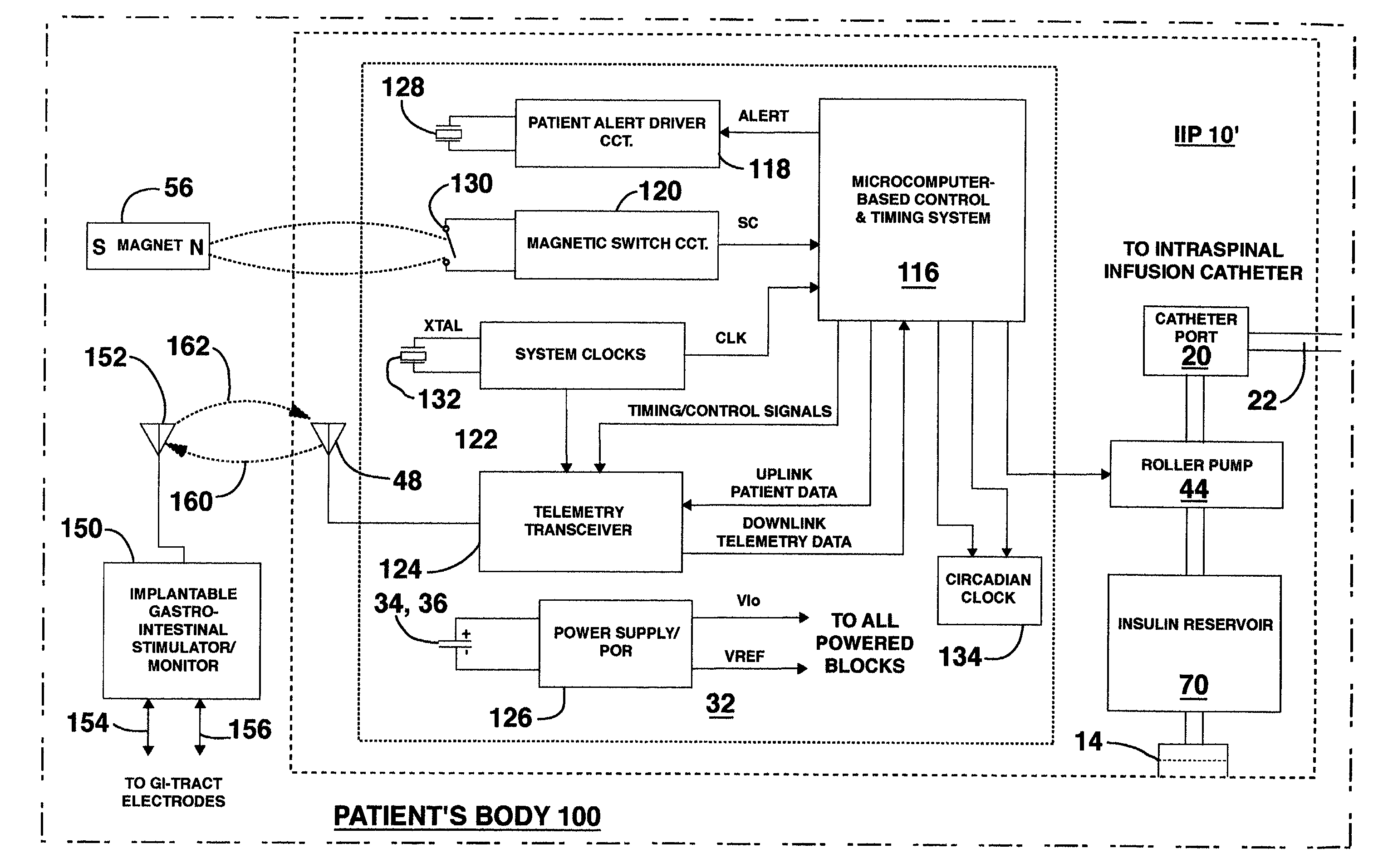
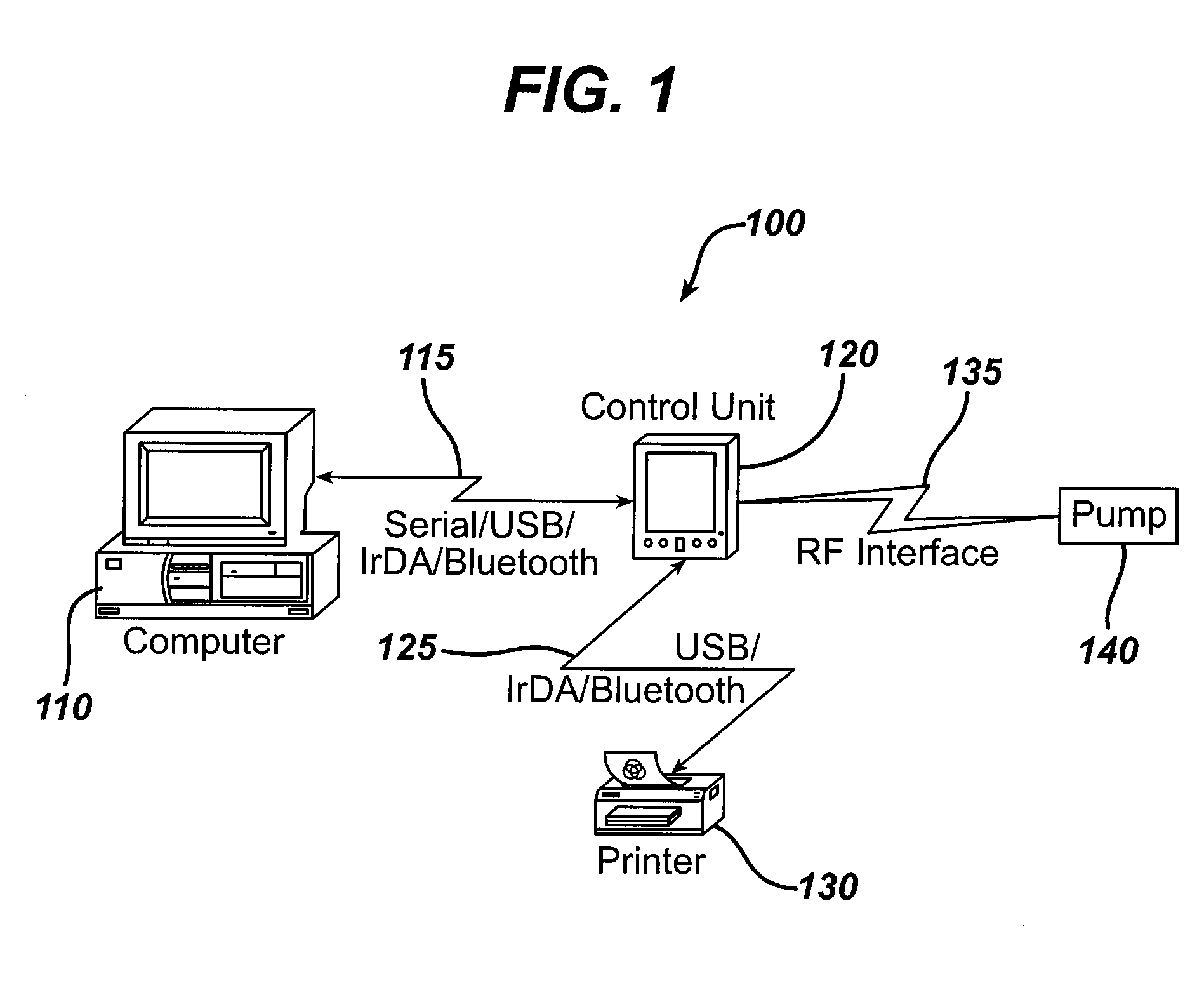
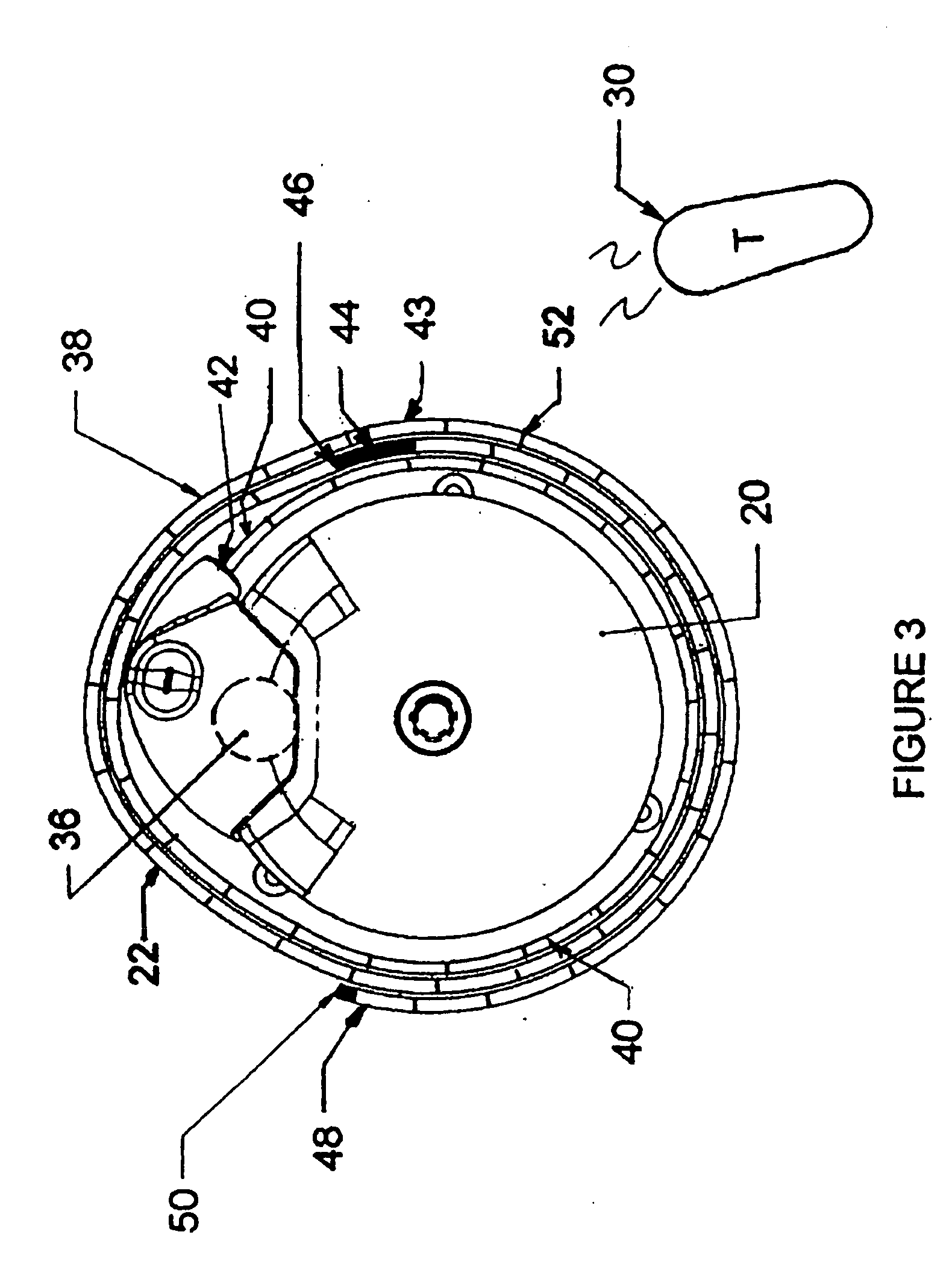
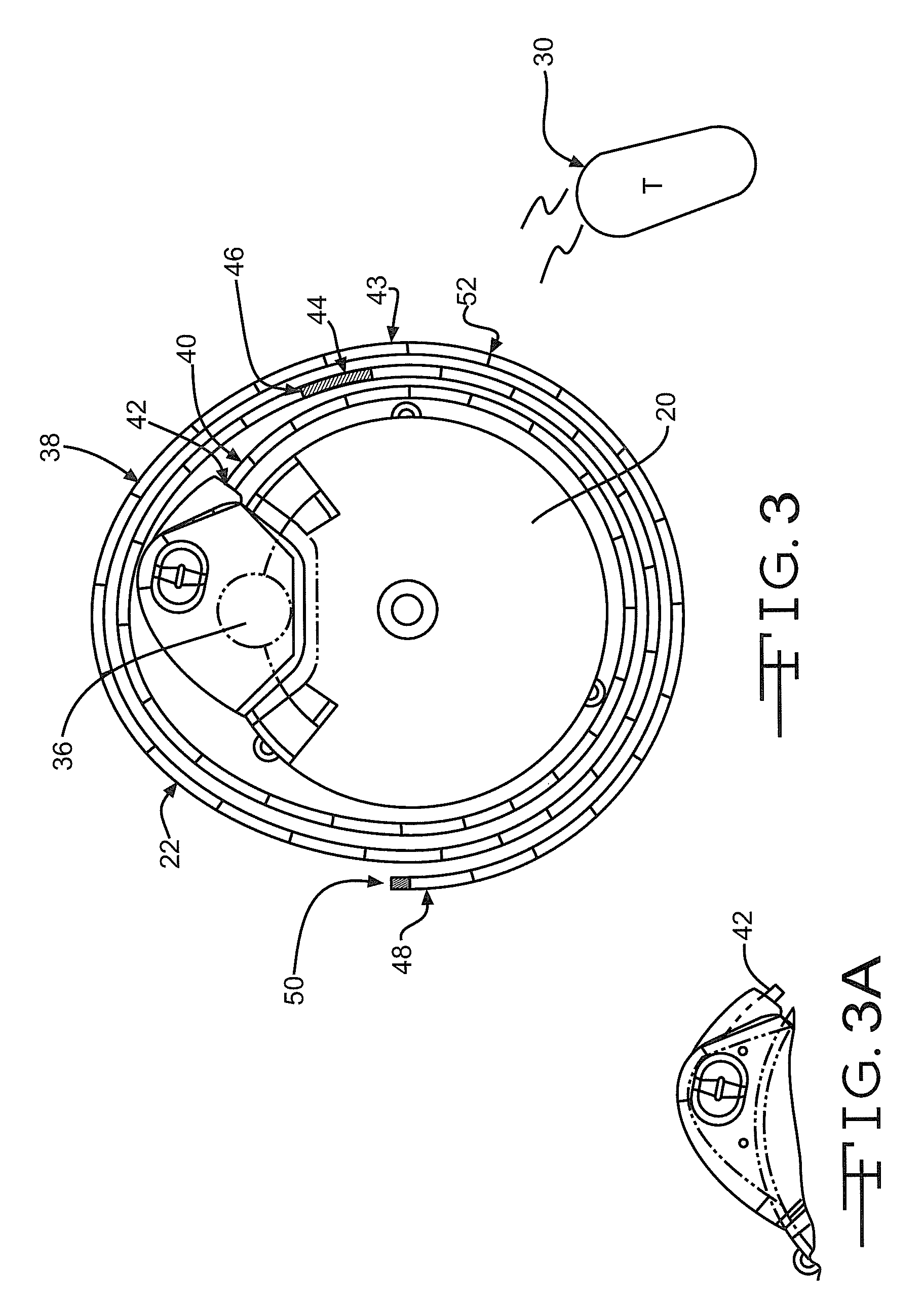
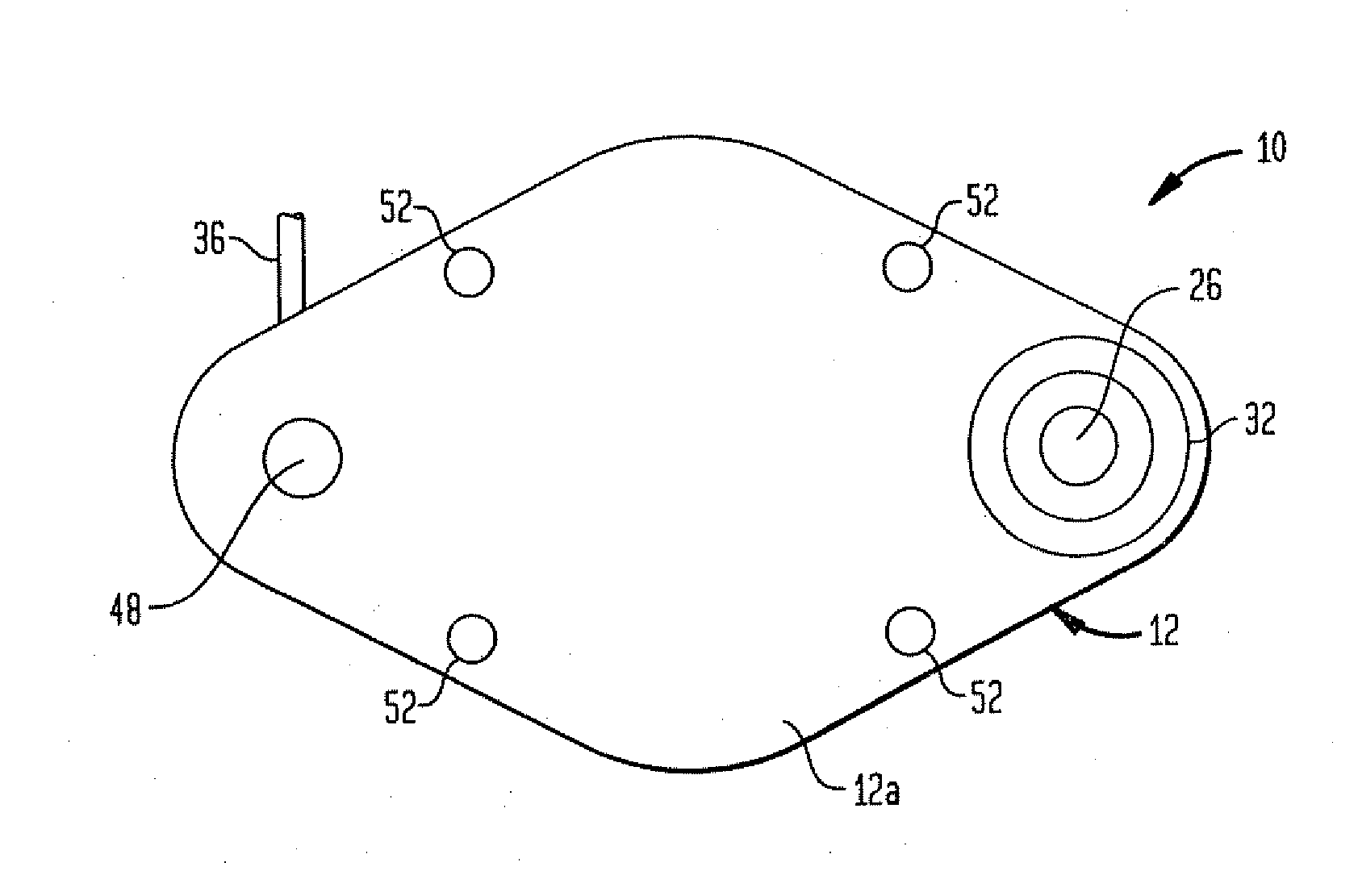
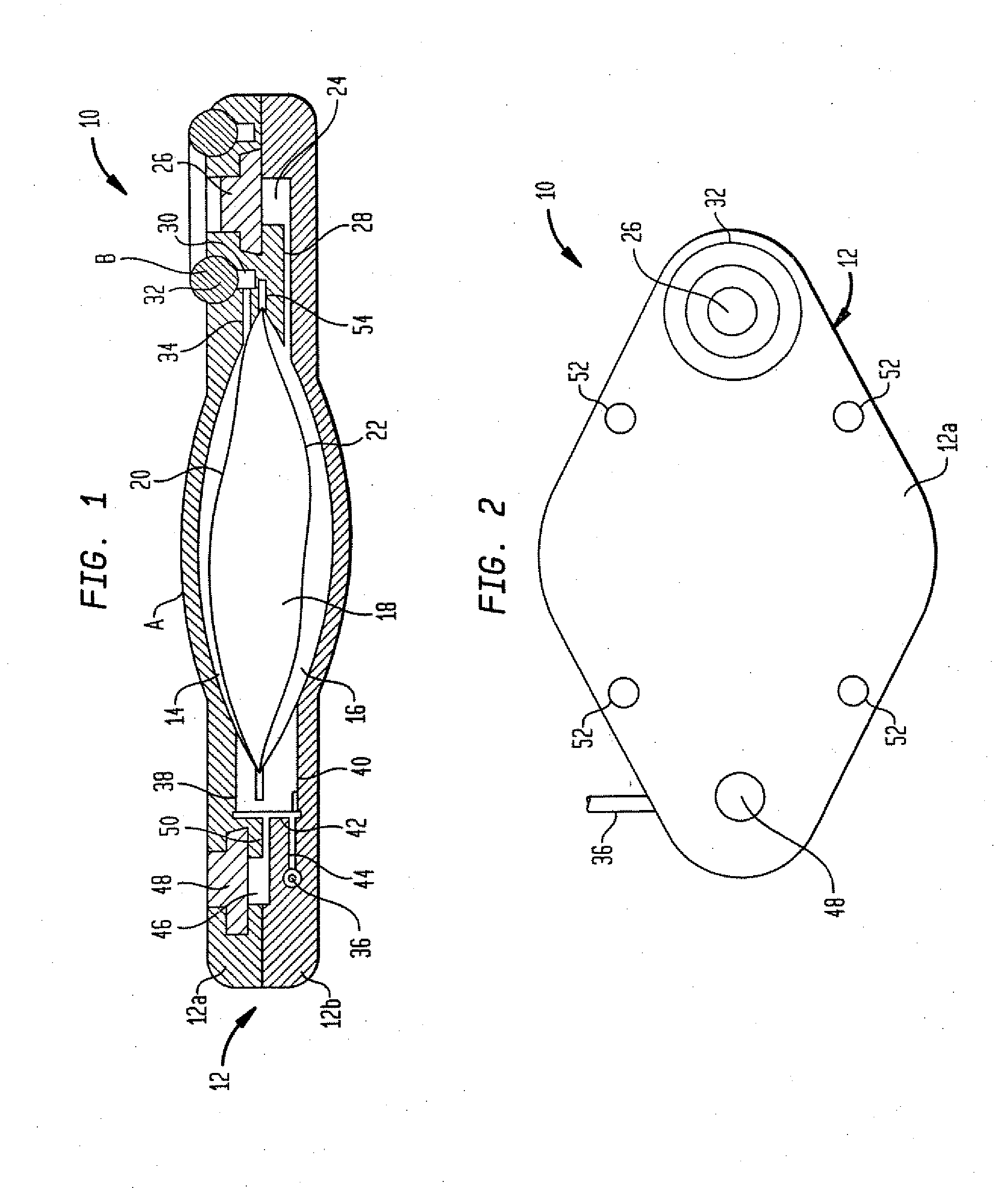
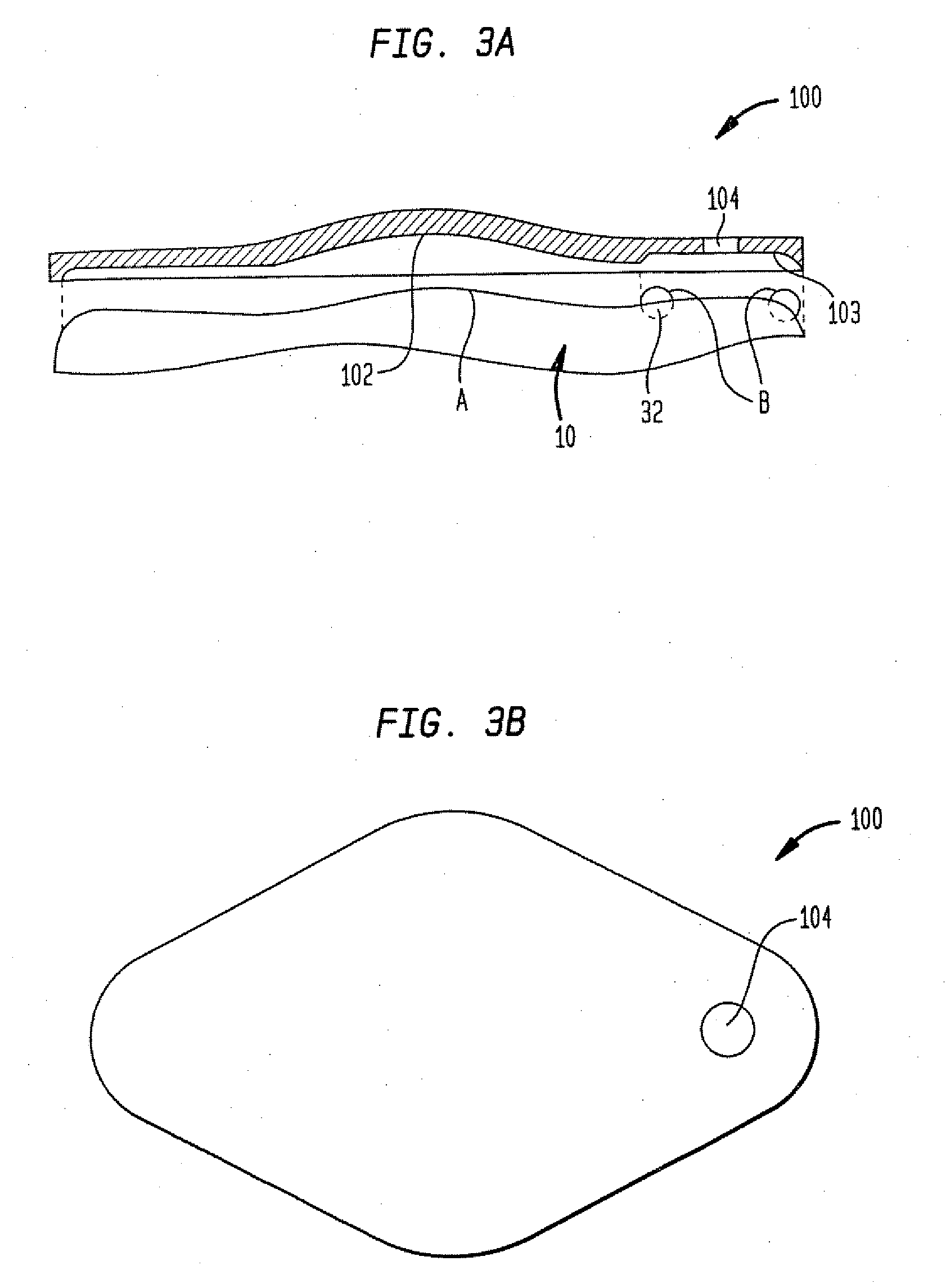
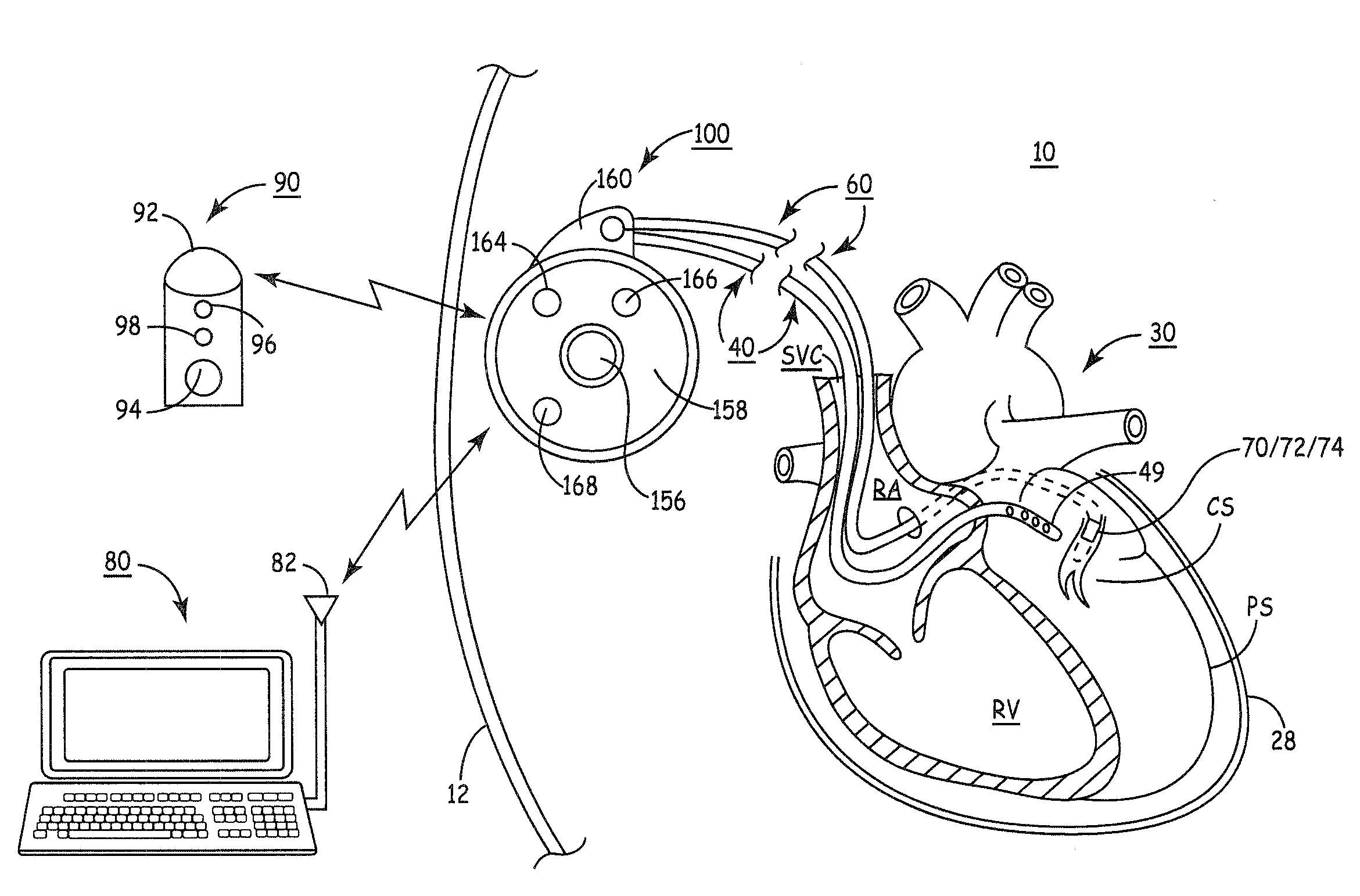
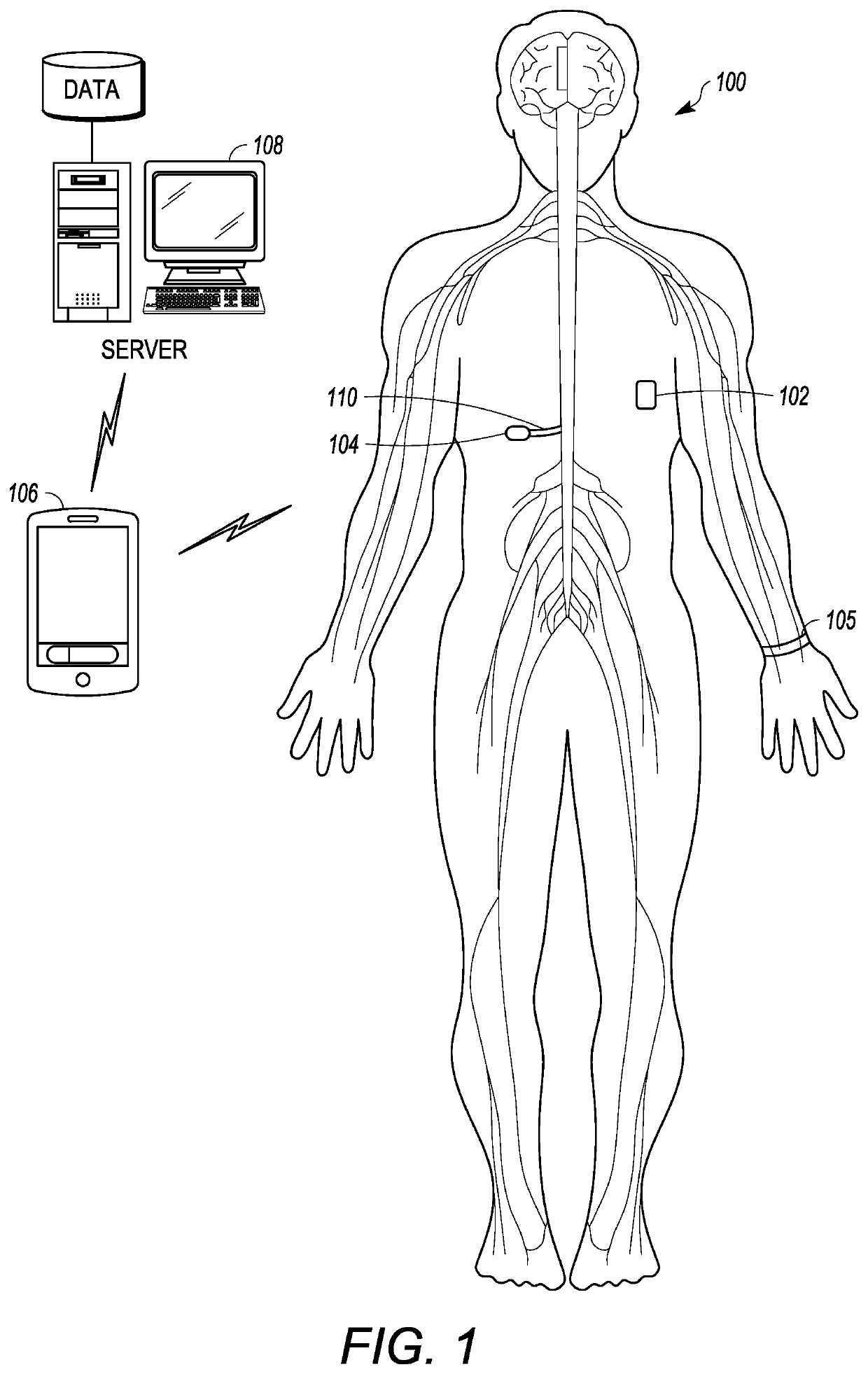
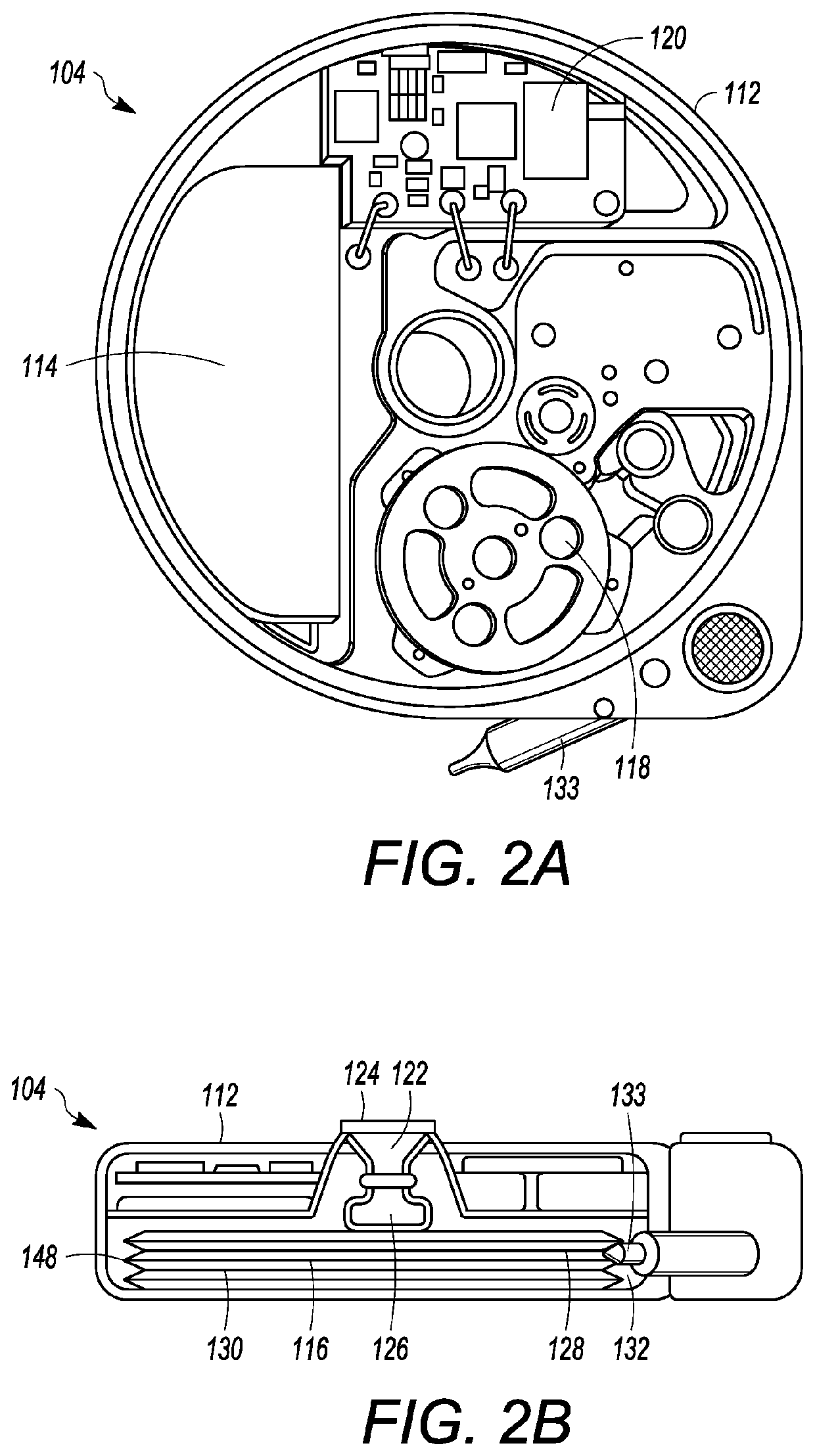
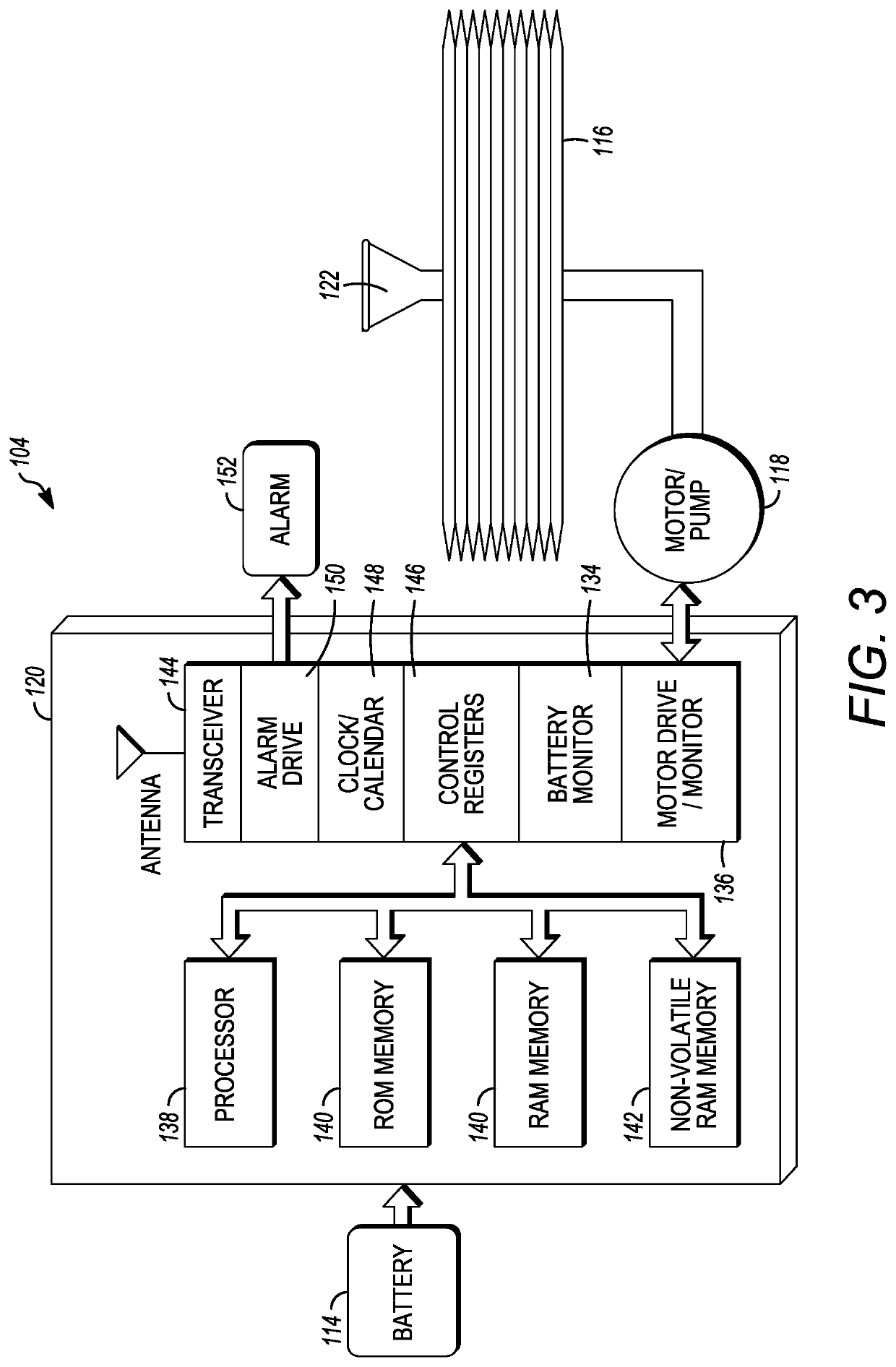
Microprocessor controlled ambulatory medical apparatus with hand held communication device
InactiveUS6873268B2Enhance user interfaceReduce system sizeEnergy efficient ICTElectrotherapyDrugs infusionHand held
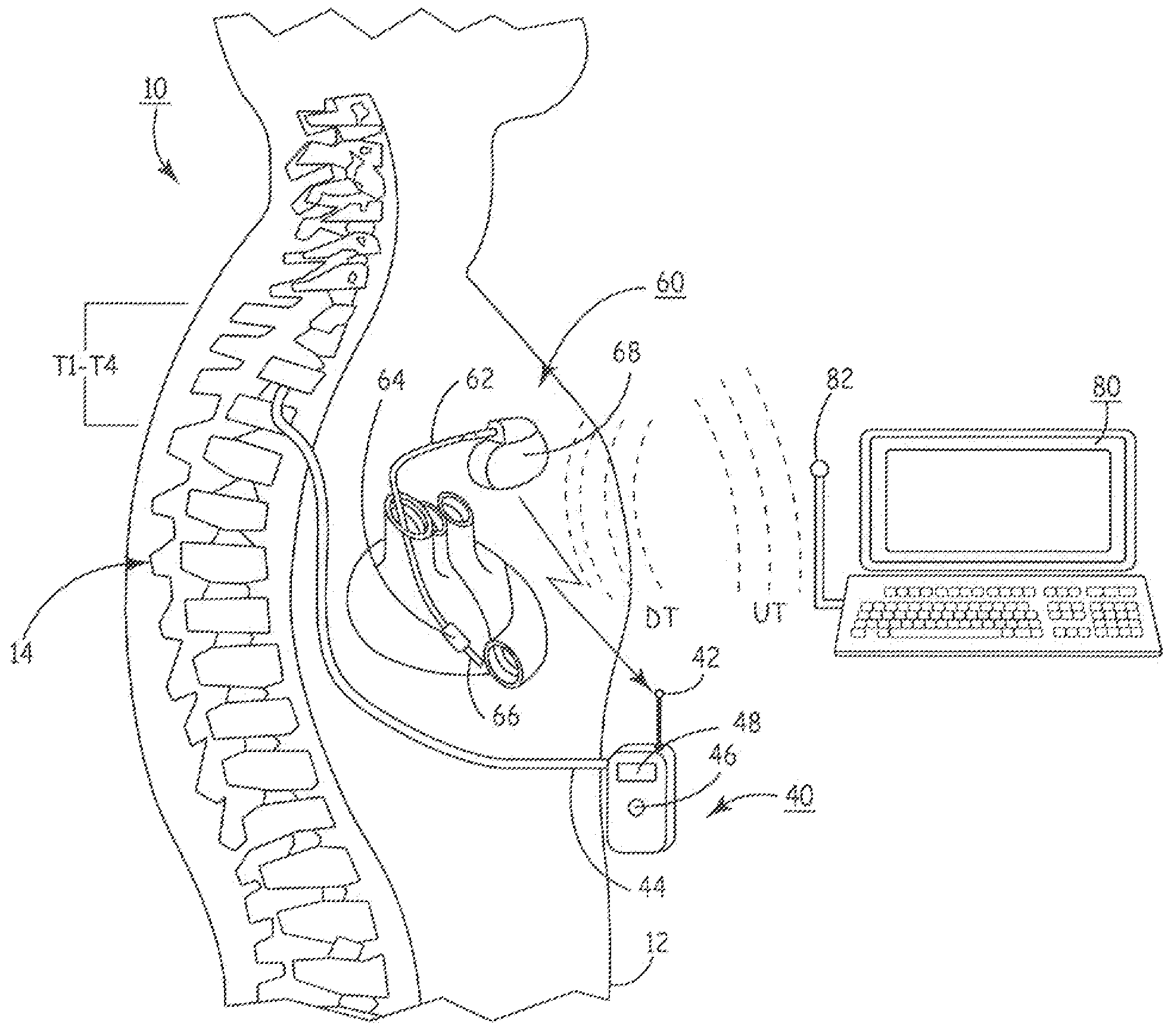
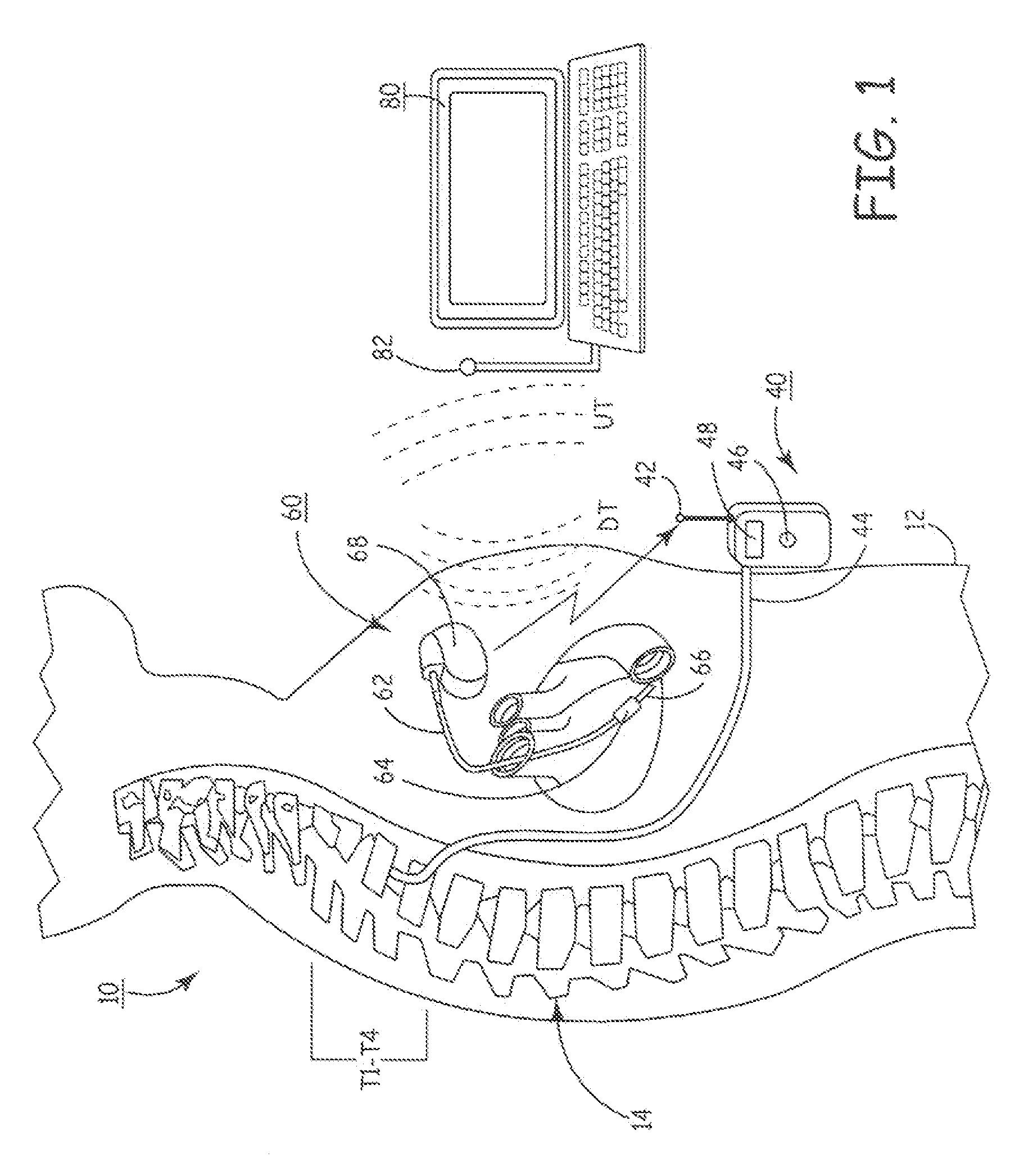
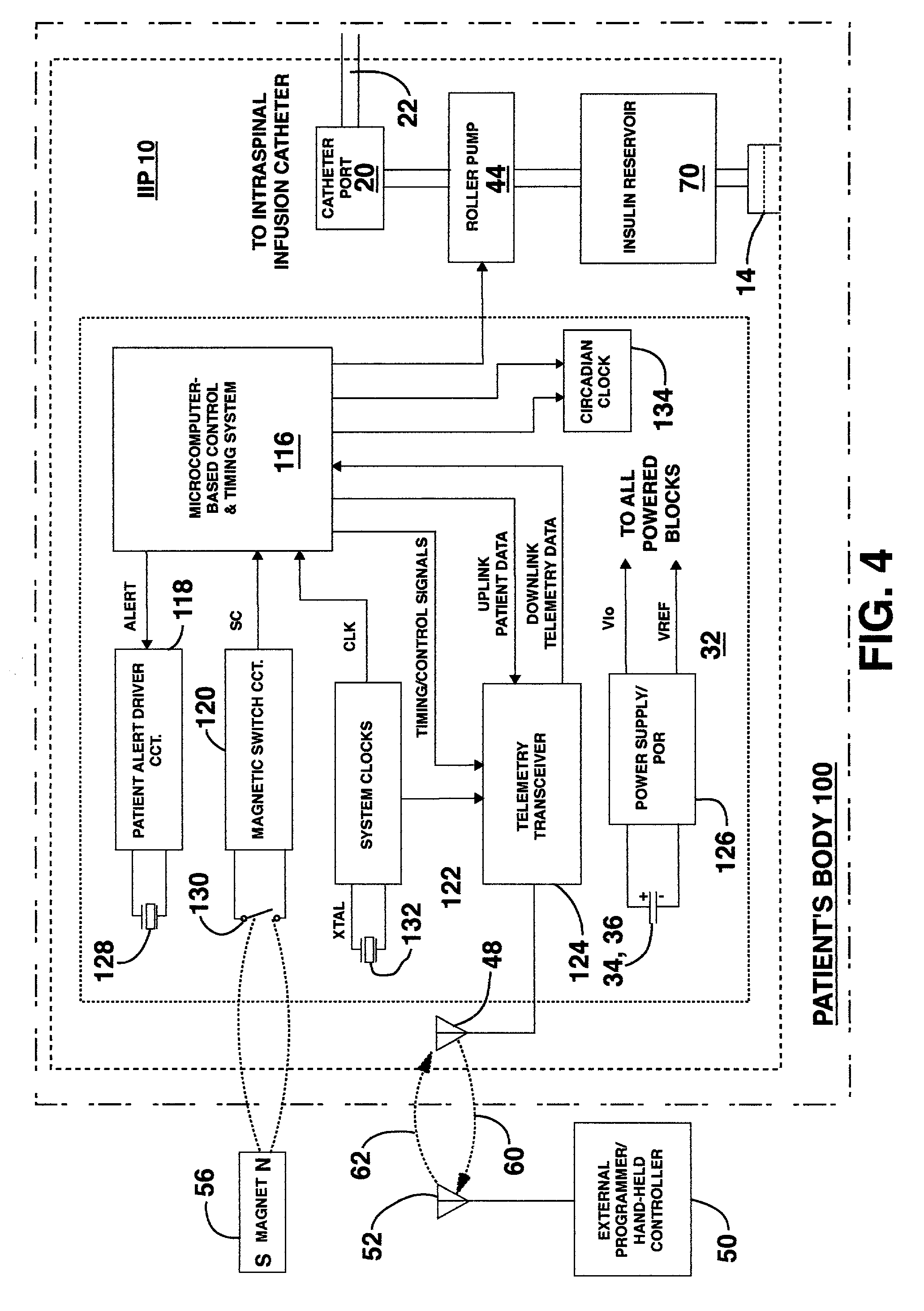
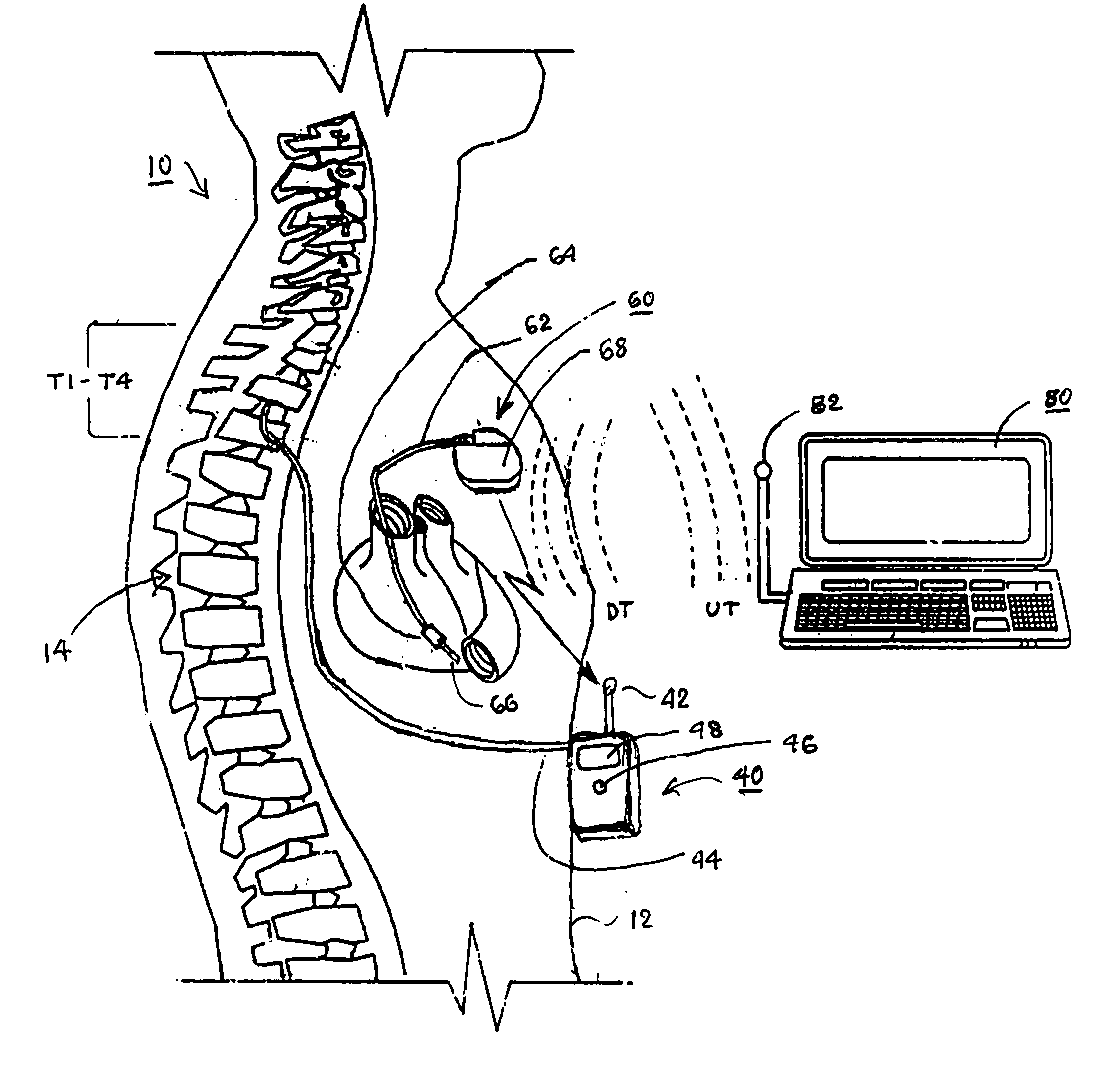
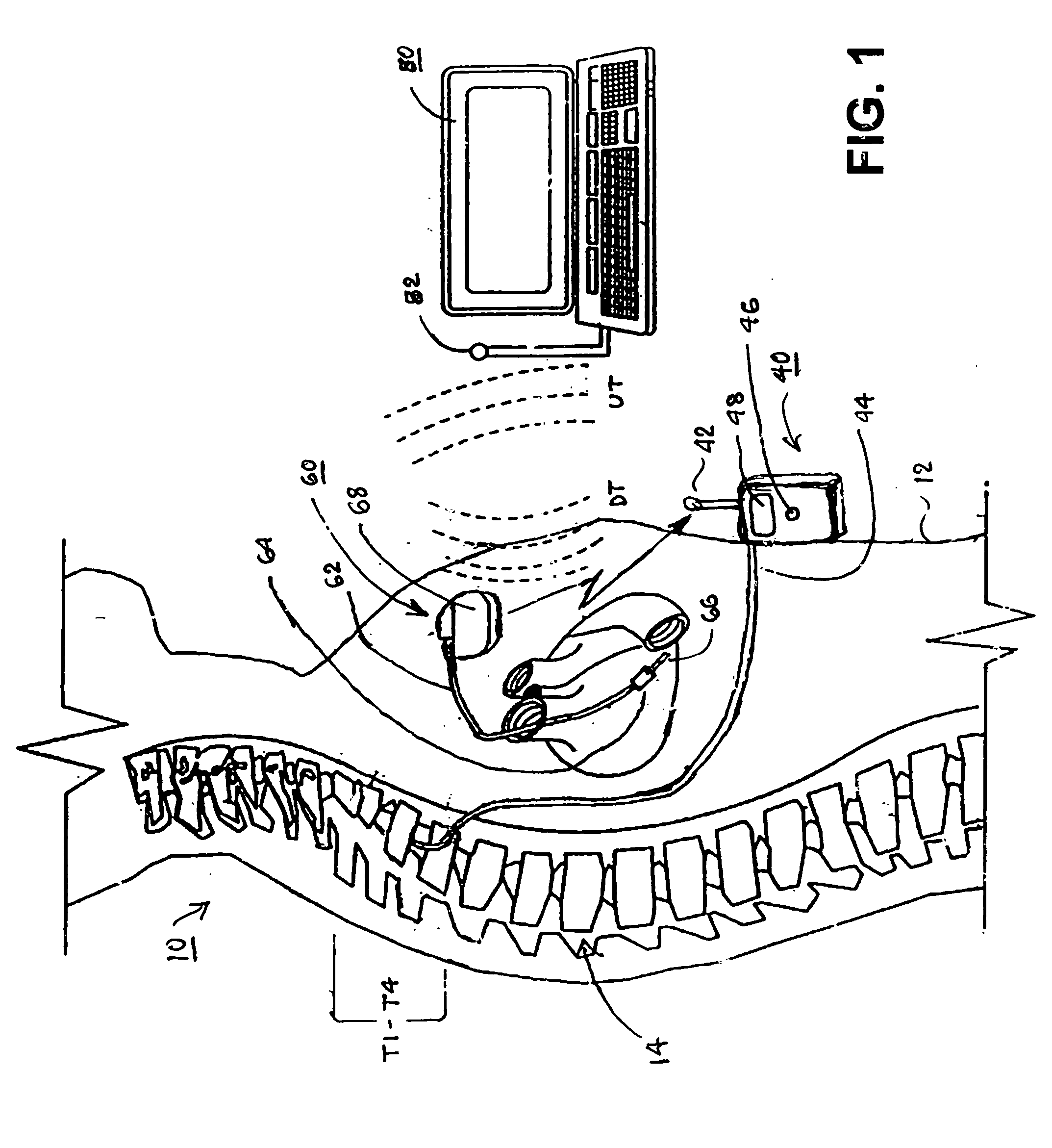
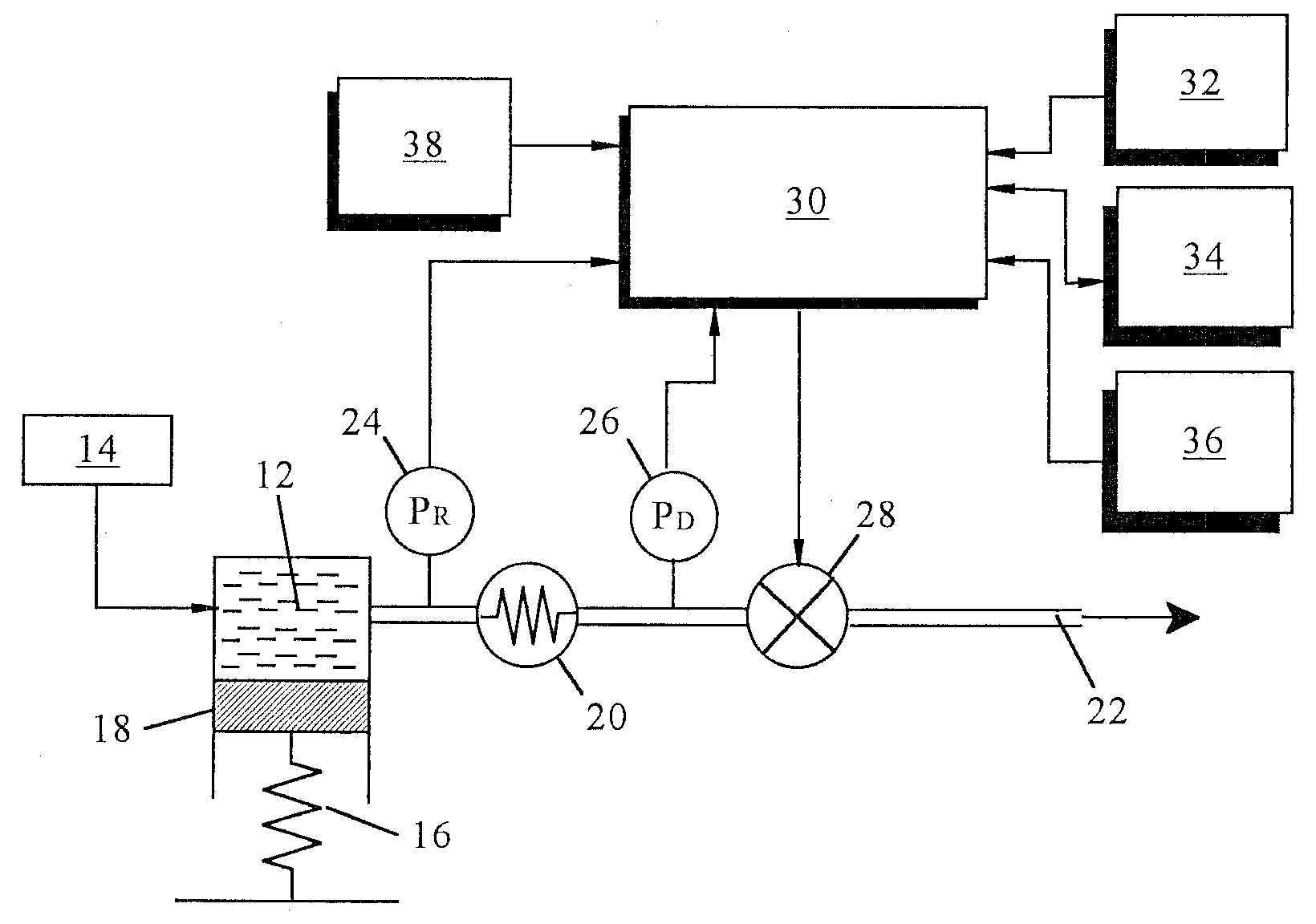
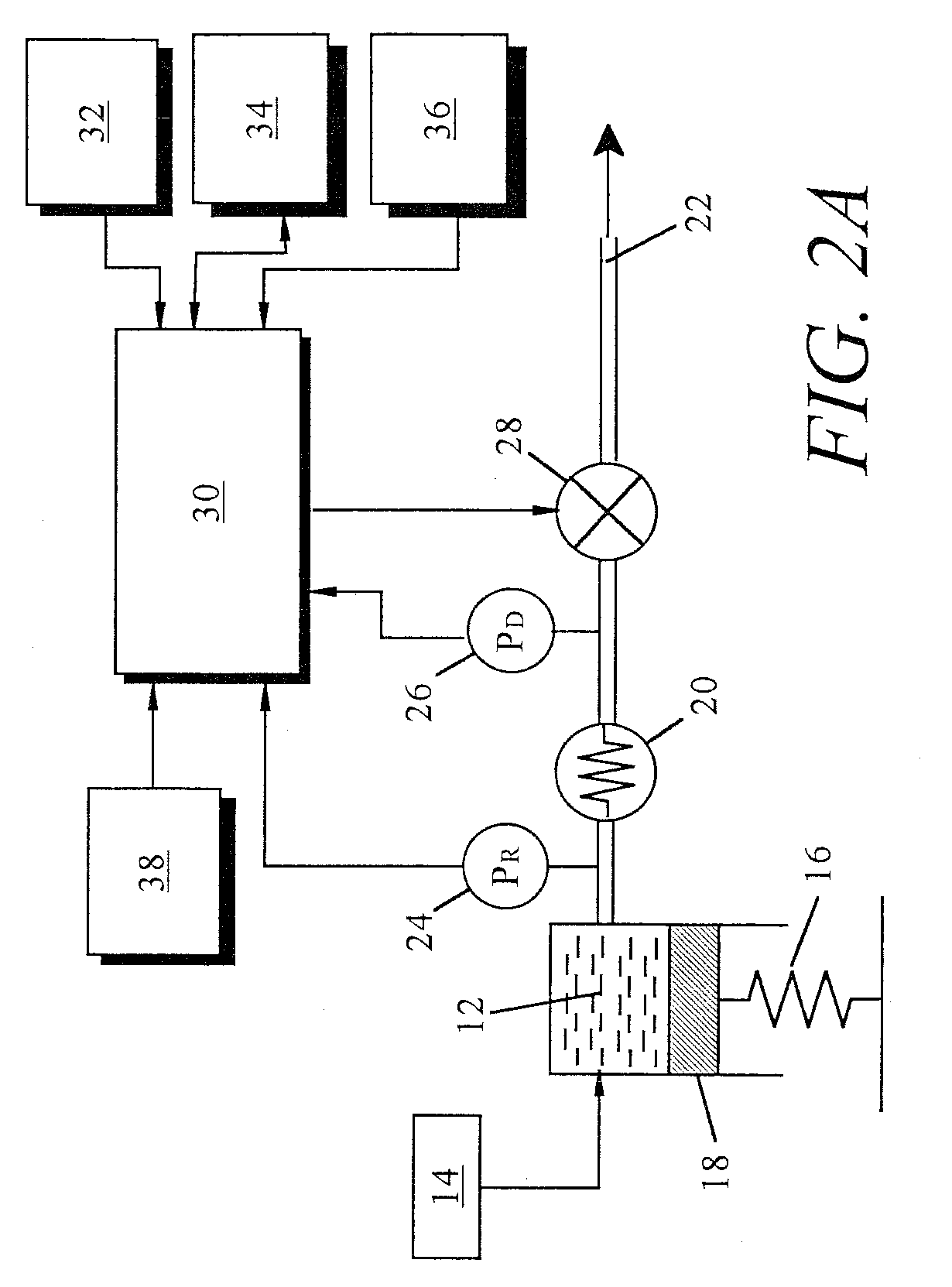
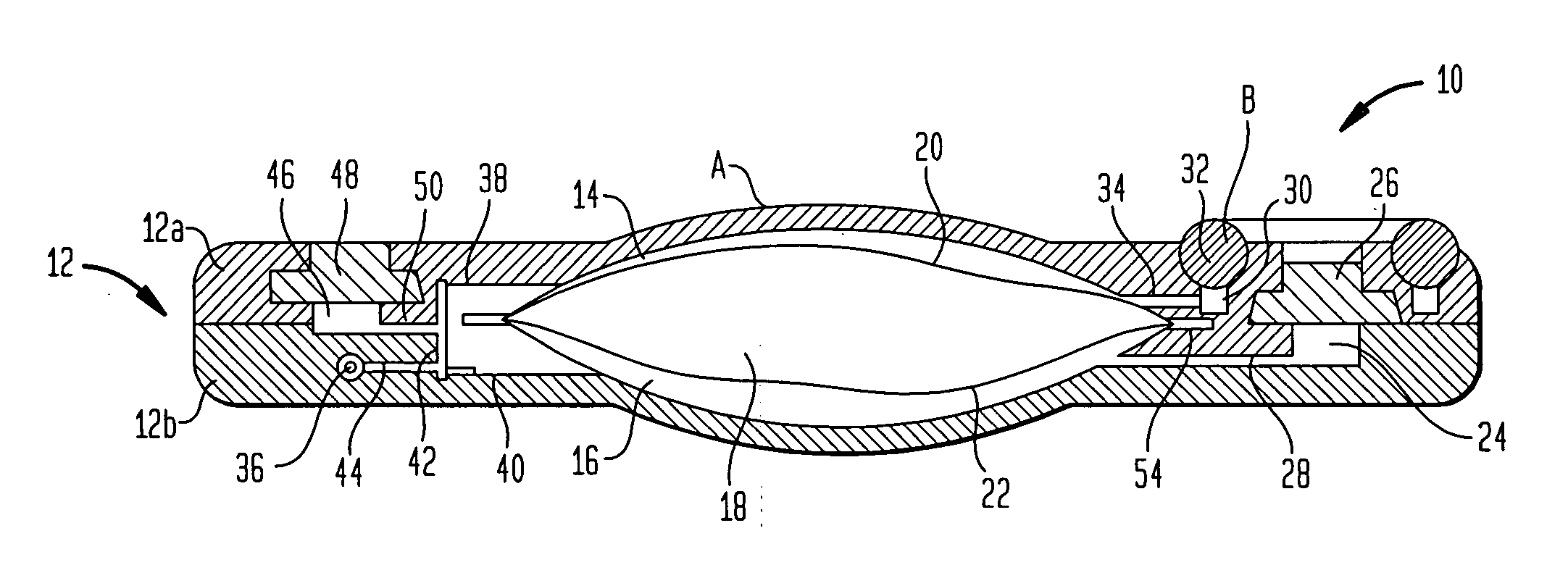
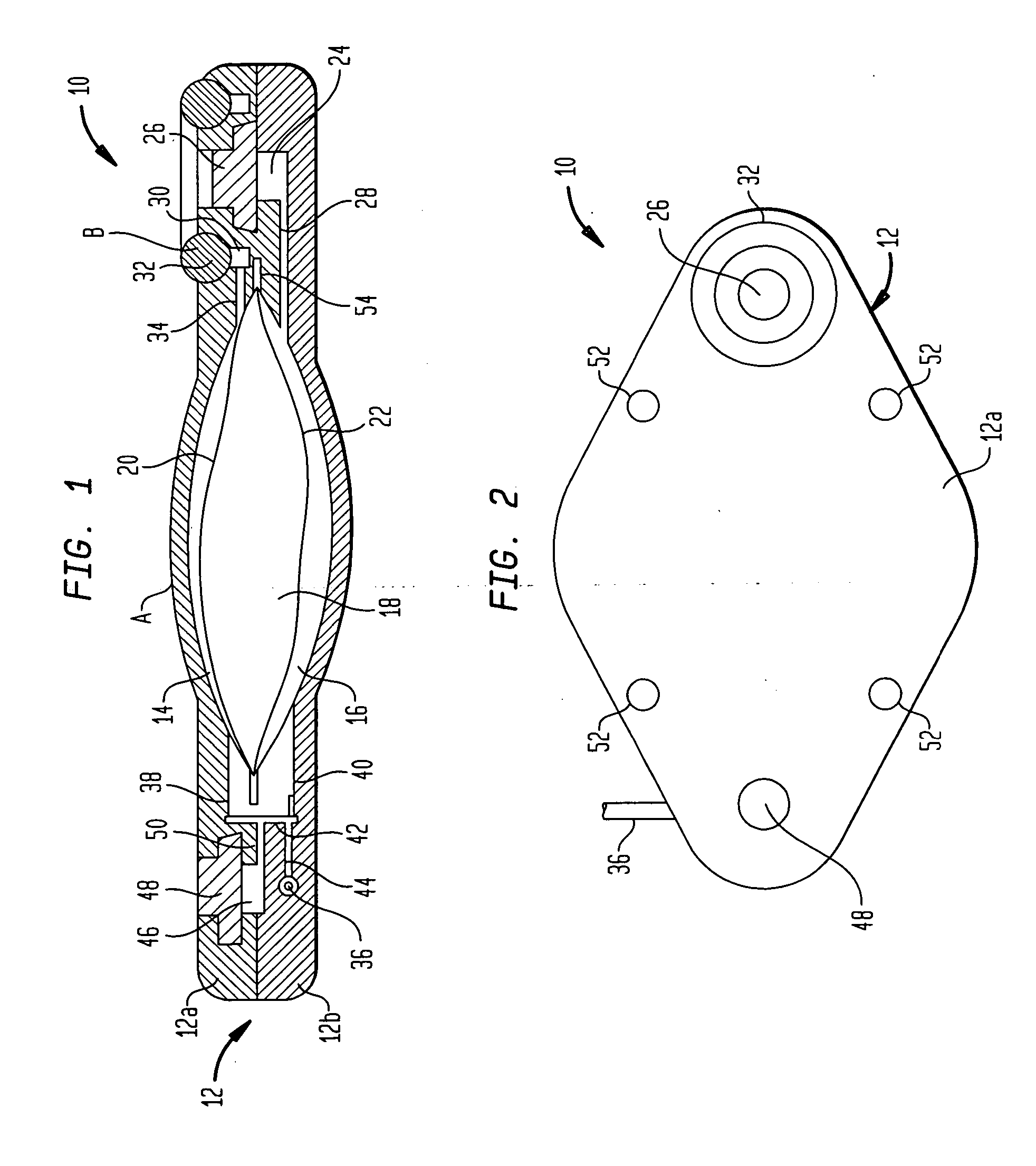
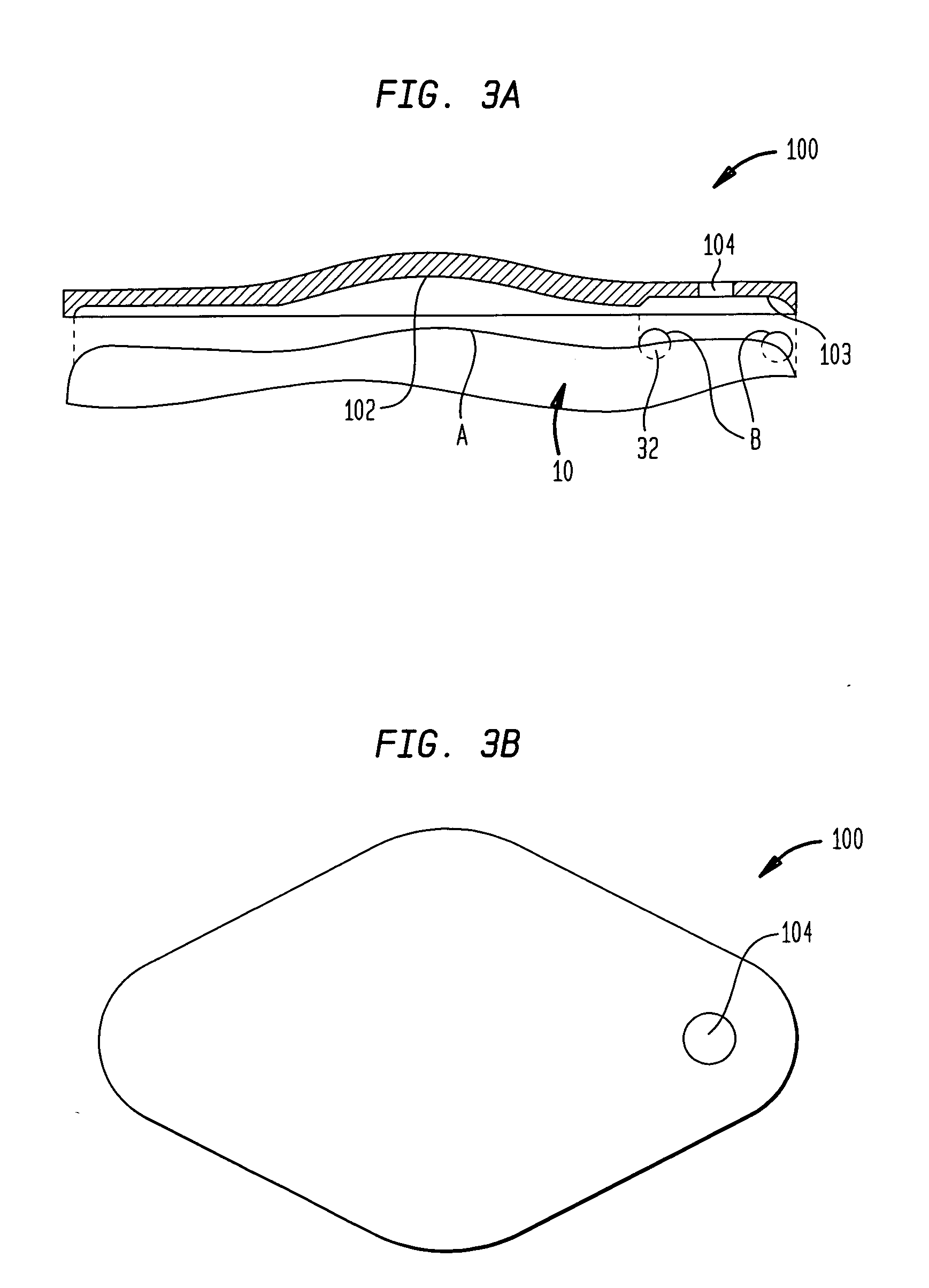
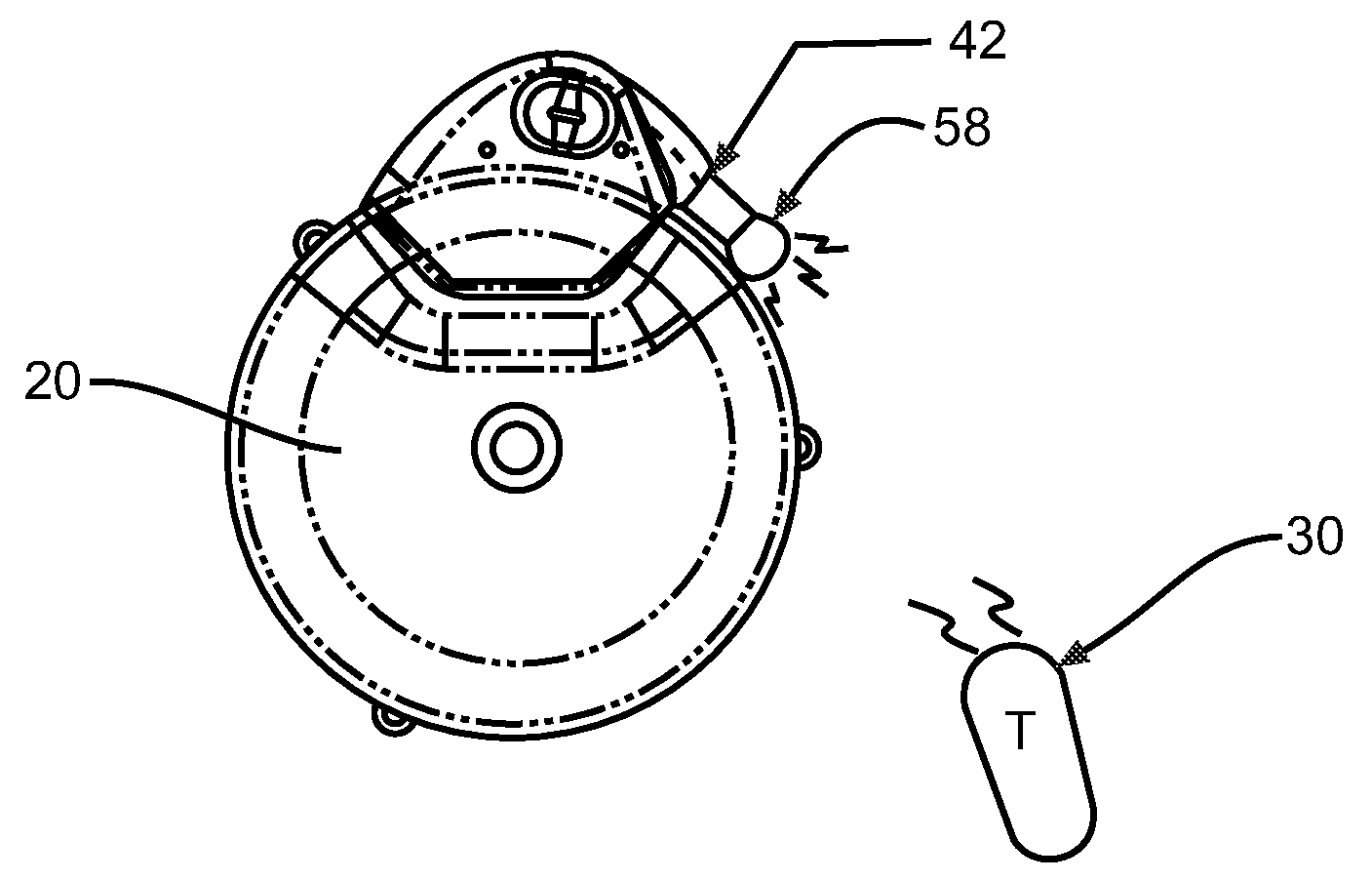
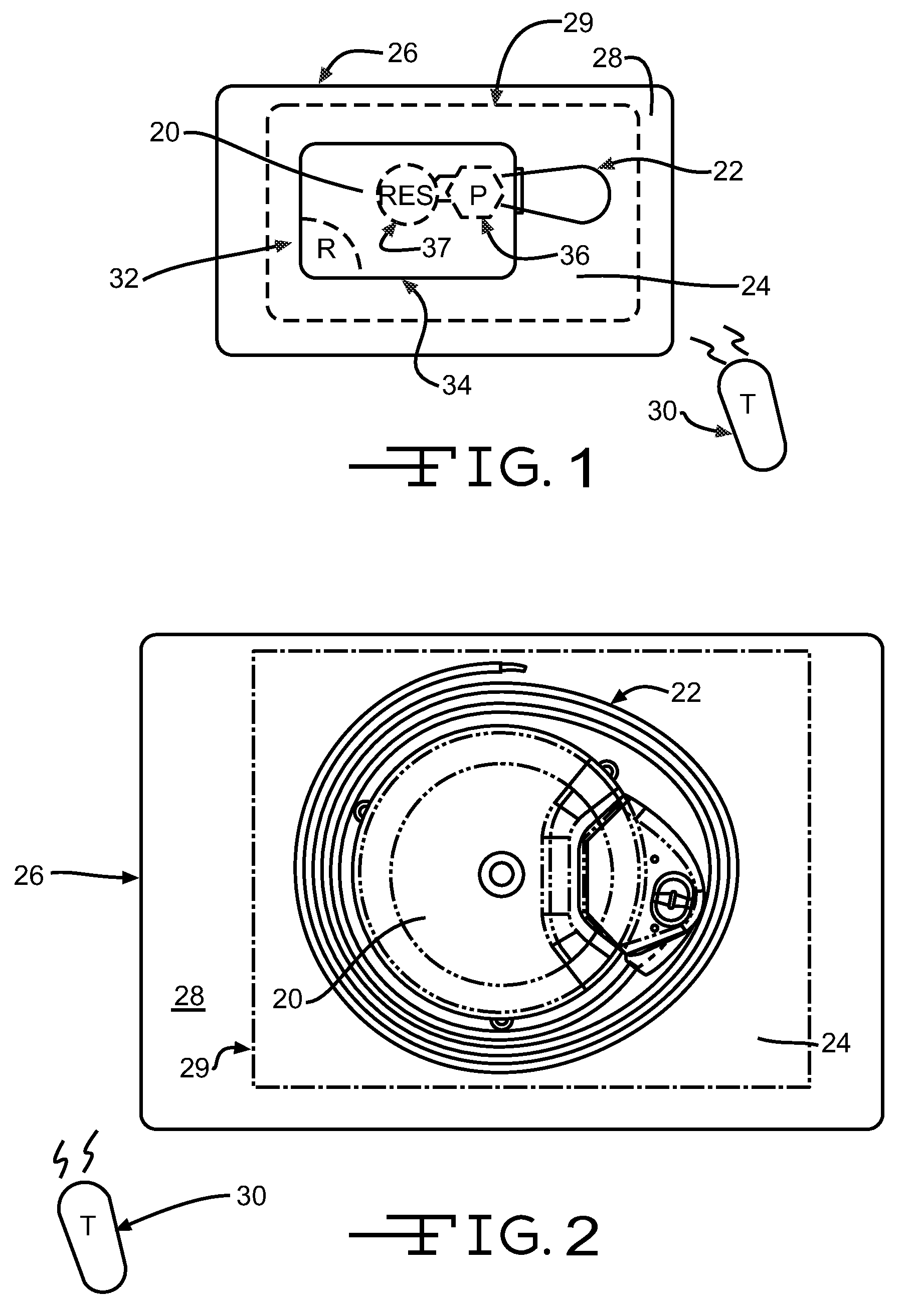
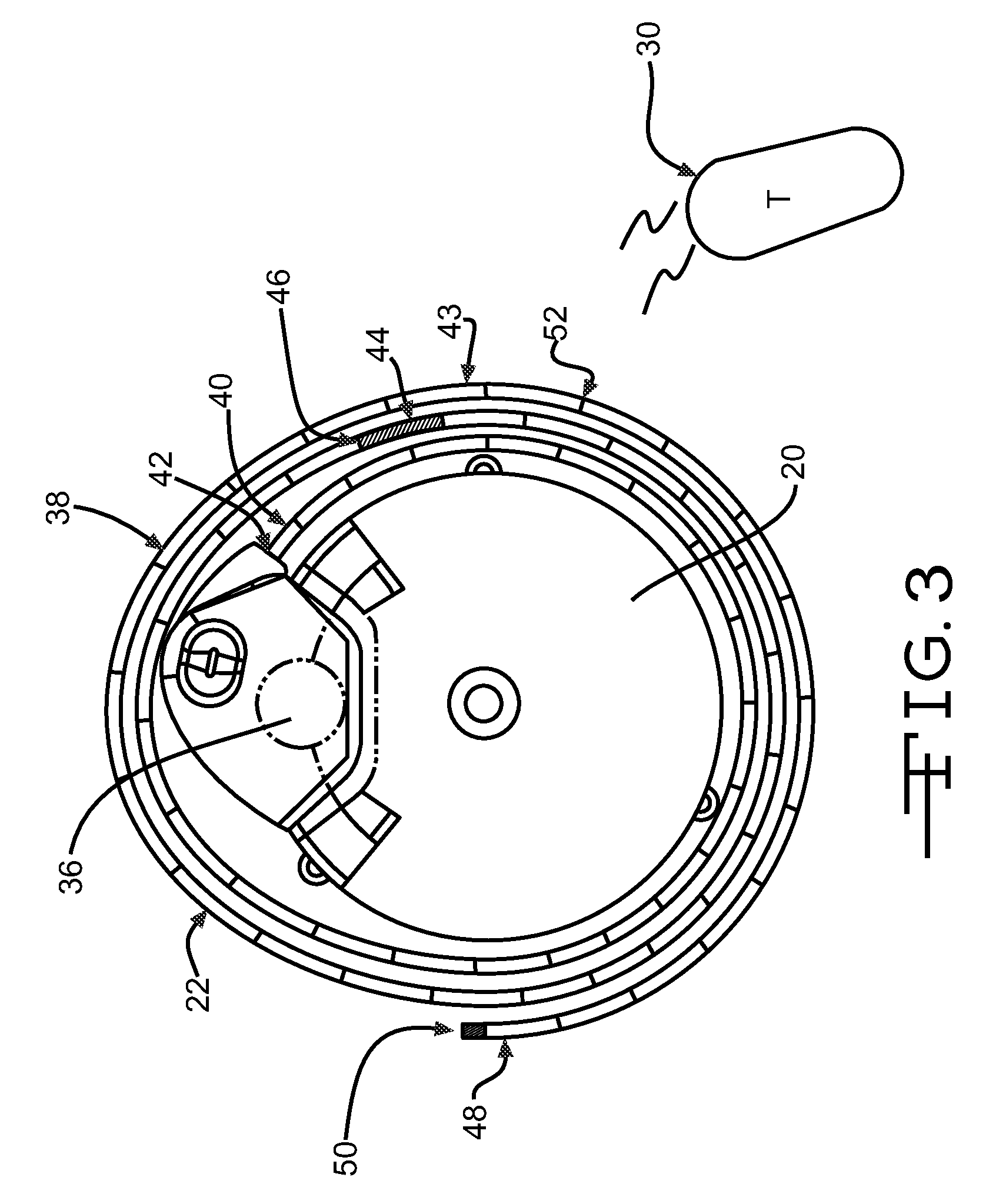
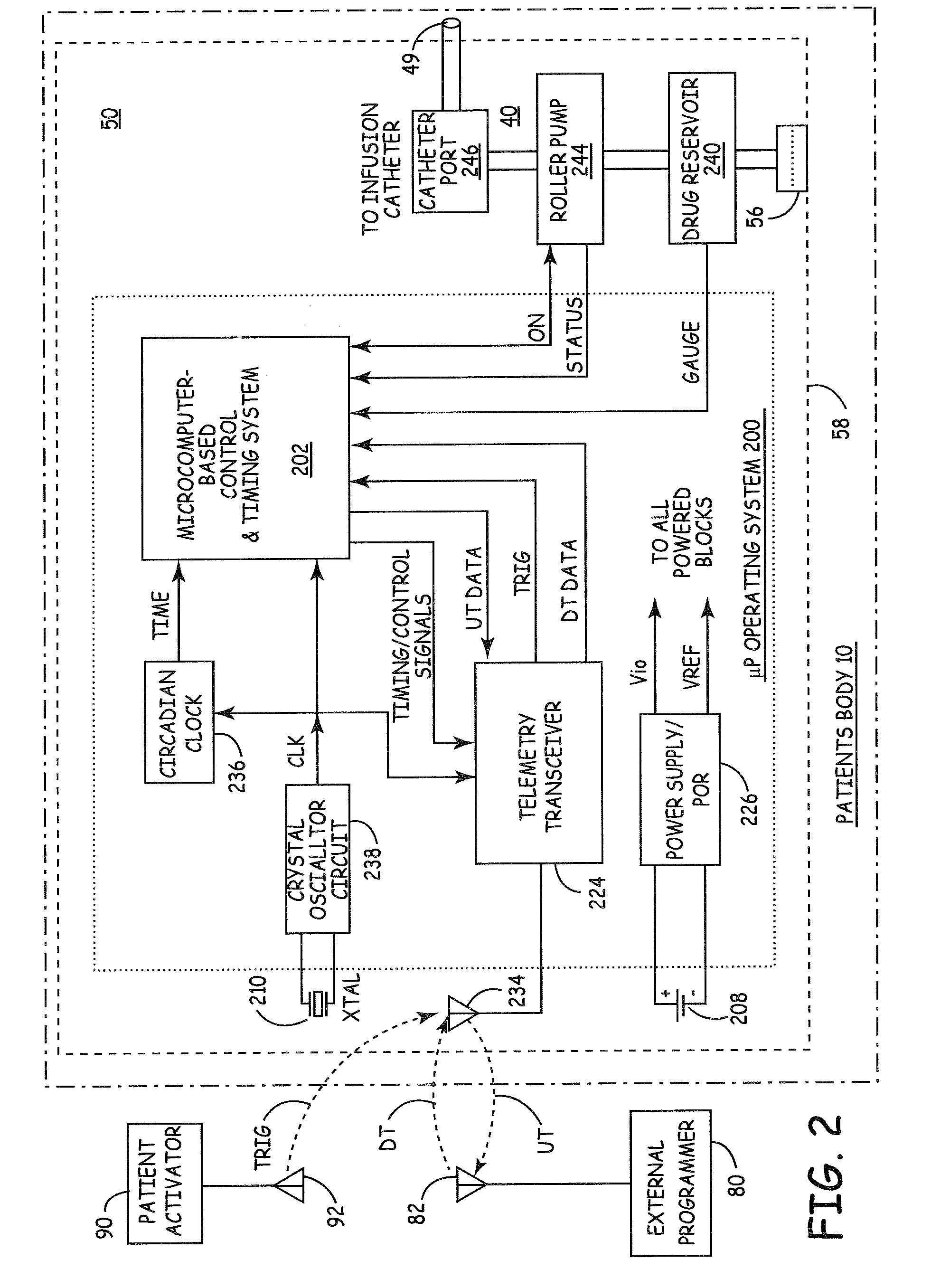
An implantable infusion pump possesses operational functionality that is, at least in part, controlled by software operating in two processor ICs which are configured to perform some different and some duplicate functions. The pump exchanges messages with an external device via telemetry. Each processor controls a different part of the drug infusion mechanism such that both processors must agree on the appropriateness of drug delivery for infusion to occur. Delivery accumulators are incremented and decremented with delivery requests and with deliveries made. When accumulated amounts reach or exceed, quantized deliverable amounts, infusion is made to occur. The accumulators are capable of being incremented by two or more independent types of delivery requests. Operational modes of the infusion device are changed automatically in view of various system errors that are trapped, various system alarm conditions that are detected, and when excess periods of time lapse between pump and external device interactions.
Owner:MEDTRONIC MIMIMED INC
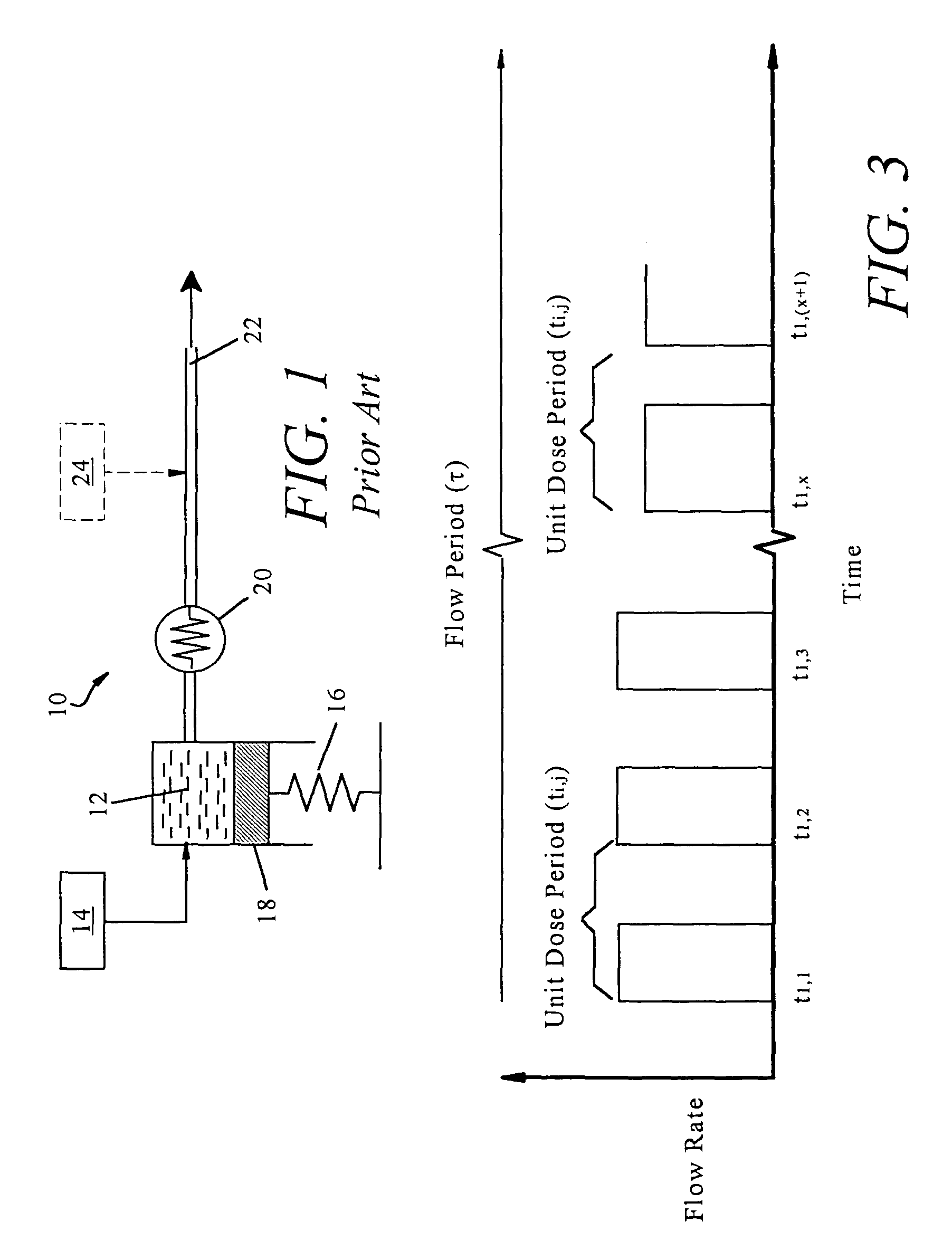
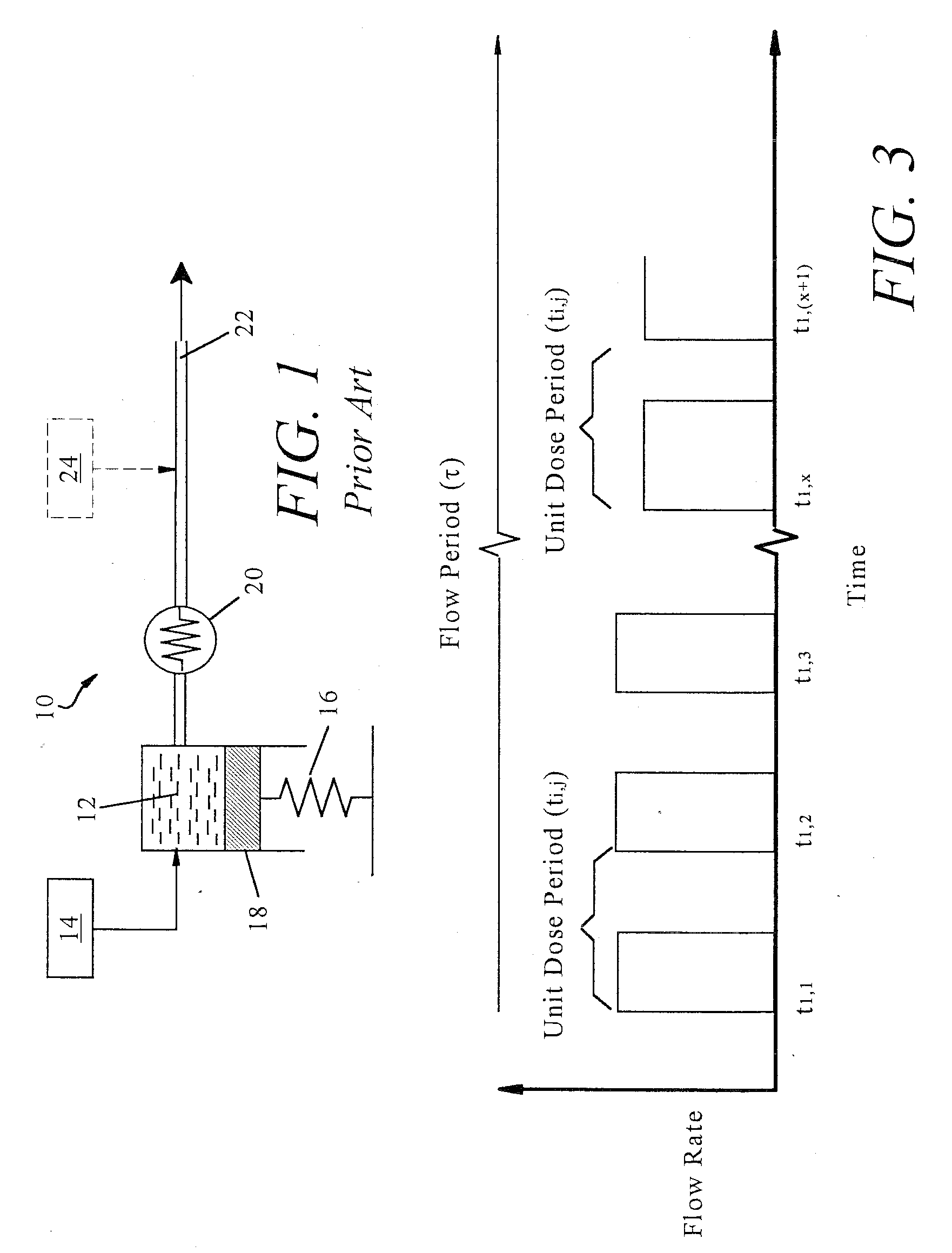
Non-constant pressure infusion pump
InactiveUS7338464B2Easy constructionPharmaceutical delivery mechanismMedical devicesPeriod effectsBiomedical engineering
The present invention relates to an implantable infusion pump having a refillable infusate reservoir in fluid communication with a delivery site via a flow path. This flow path includes a flow resistance. The infusion pump includes a sensing device(s), positioned relative to the flow path, to provide data regarding a flow rate along the flow path. The infusion pump effects a division of a total flow period into at least a plurality of unit dose periods, each unit dose period effecting delivery of a unit dose of infusate. The cumulative effect of delivering the total number of unit dose periods is the delivery of a desired dose over the total flow period. The present invention permits a reservoir pressure to vary over any portion of total flow period but effects a constant-pressure state over each unit dose cycle.
Owner:ADVANCED NEUROMODULATION SYST INC
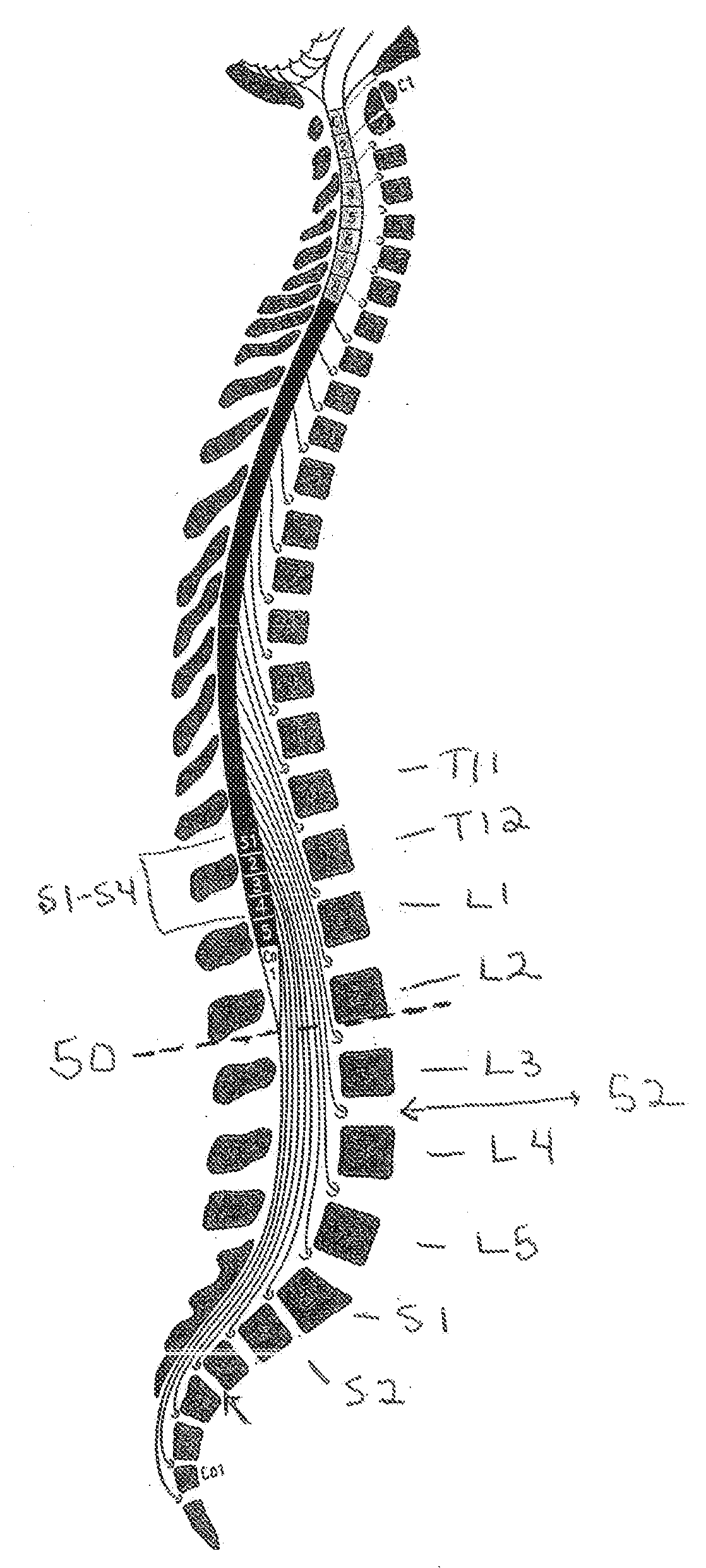
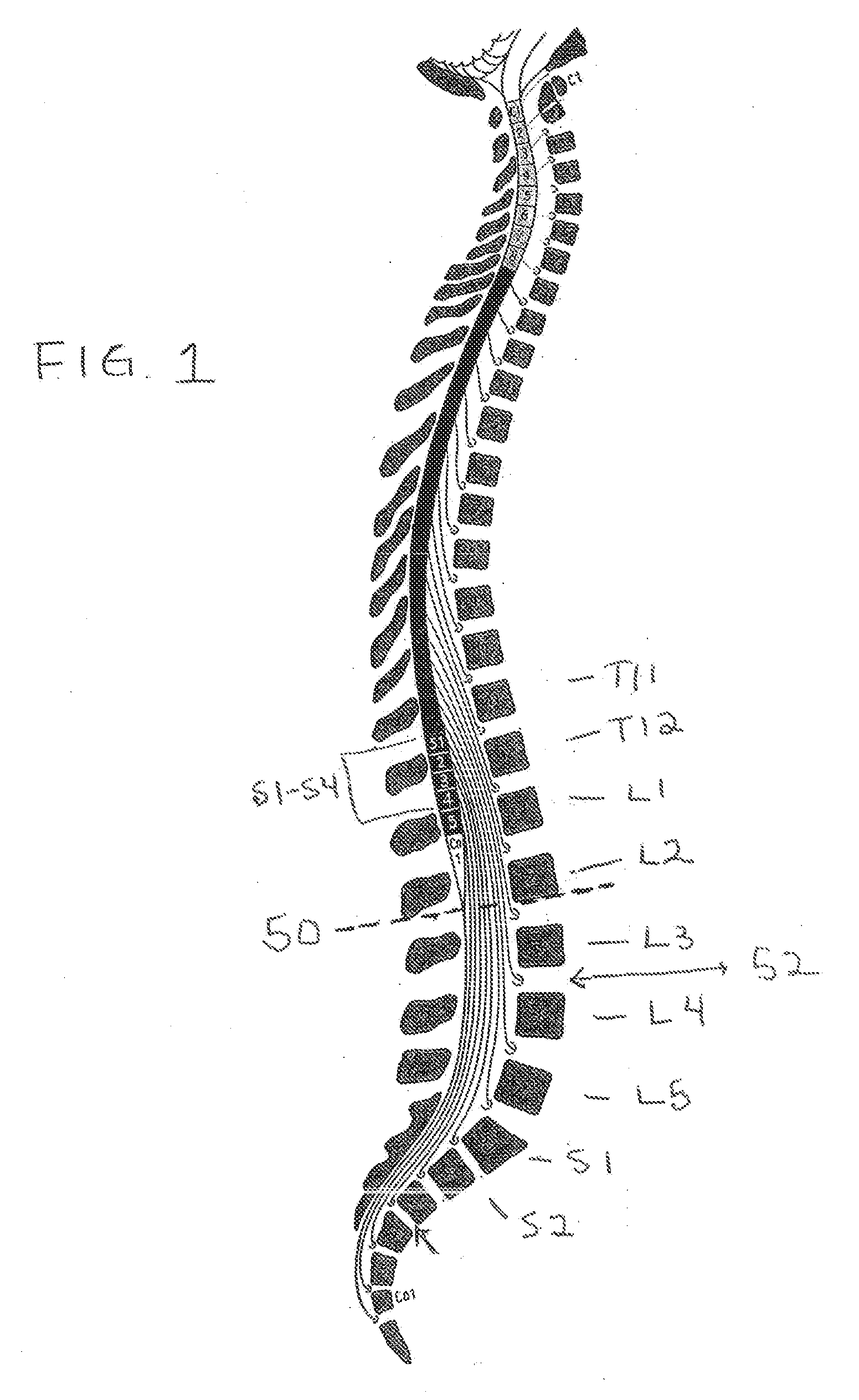
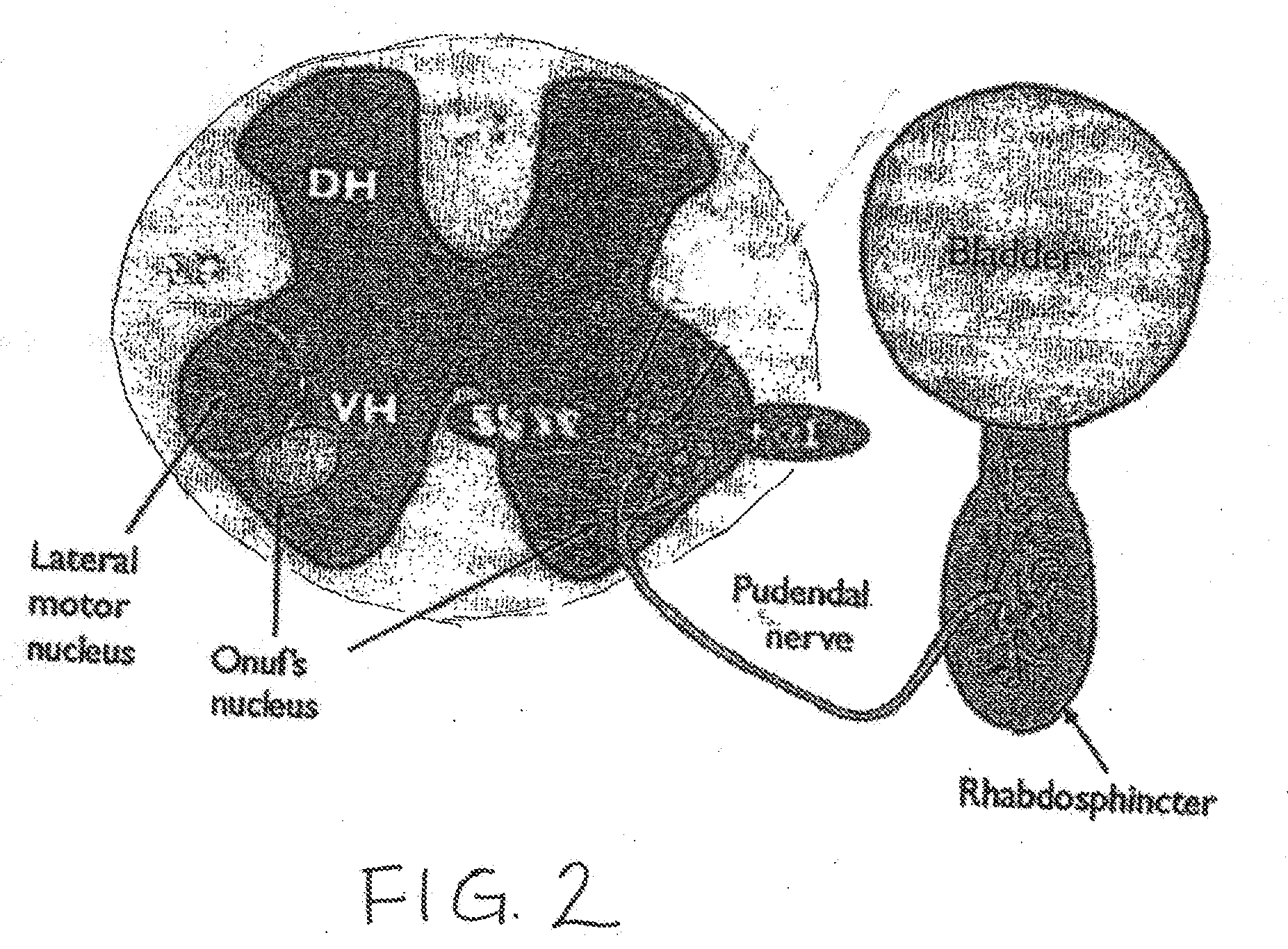
Drug Delivery Methods and Devices for Treating Stress Urinary Incontinence
InactiveUS20070253995A1Chronic pelvic painBiocidePharmaceutical delivery mechanismStress incontinenceOnuf's nucleus
The disclosure describes a method and system for delivering a drug to the Onuf's nucleus of a patient for the treatment or prevention of stress urinary incontinence. The system includes drug delivery devices that deliver one or more drugs to a site located adjacent to, around or within the Onuf's nucleus stress incontinence alleviation. A depot configured to release a therapeutically effective amount of a stress incontinence-reducing drug is one drug delivery device disclosed. Other drug delivery devices include implantable infusion pumps.
Owner:MEDTRONIC INC
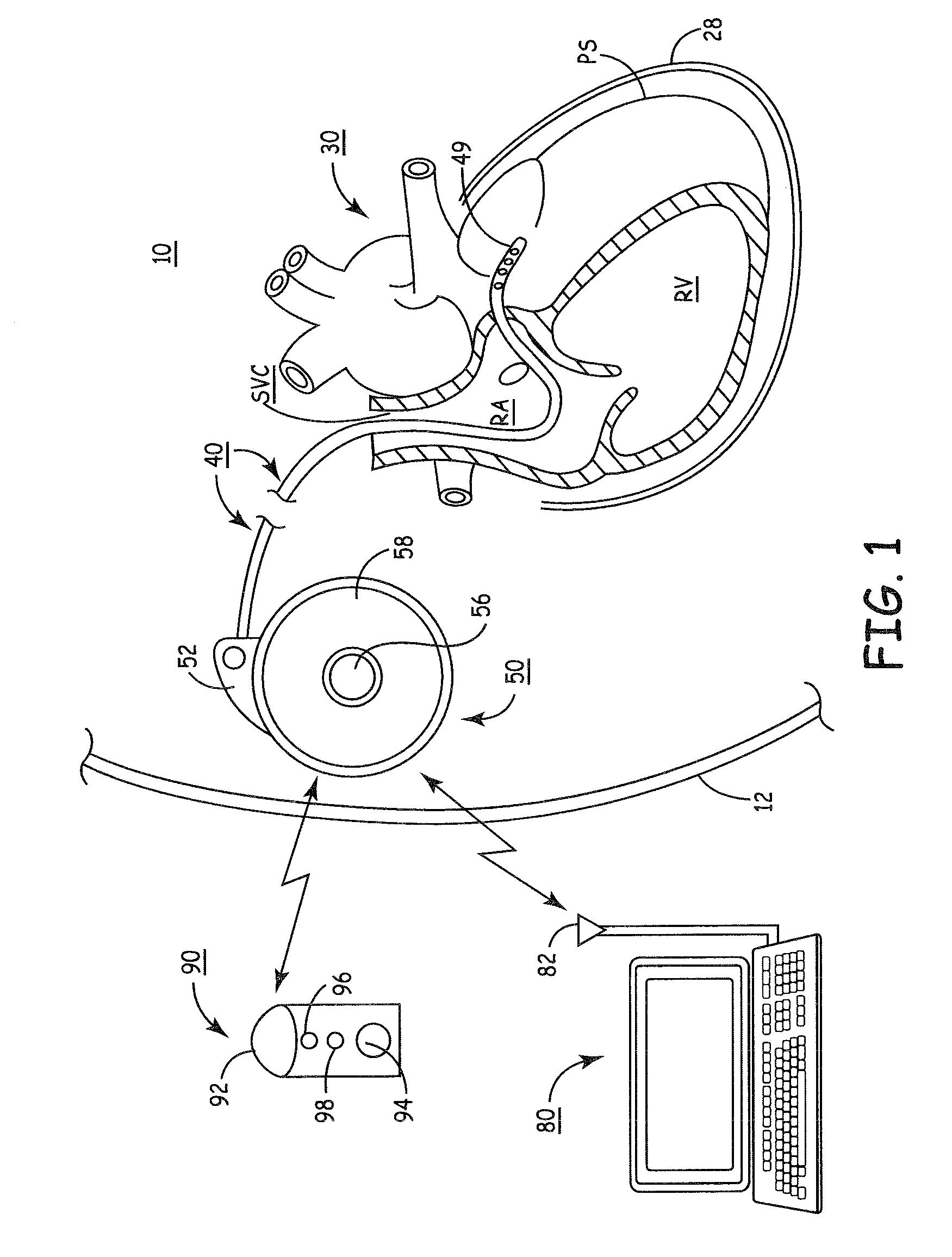
Delivery of a sympatholytic cardiovascular agent to the central nervous system
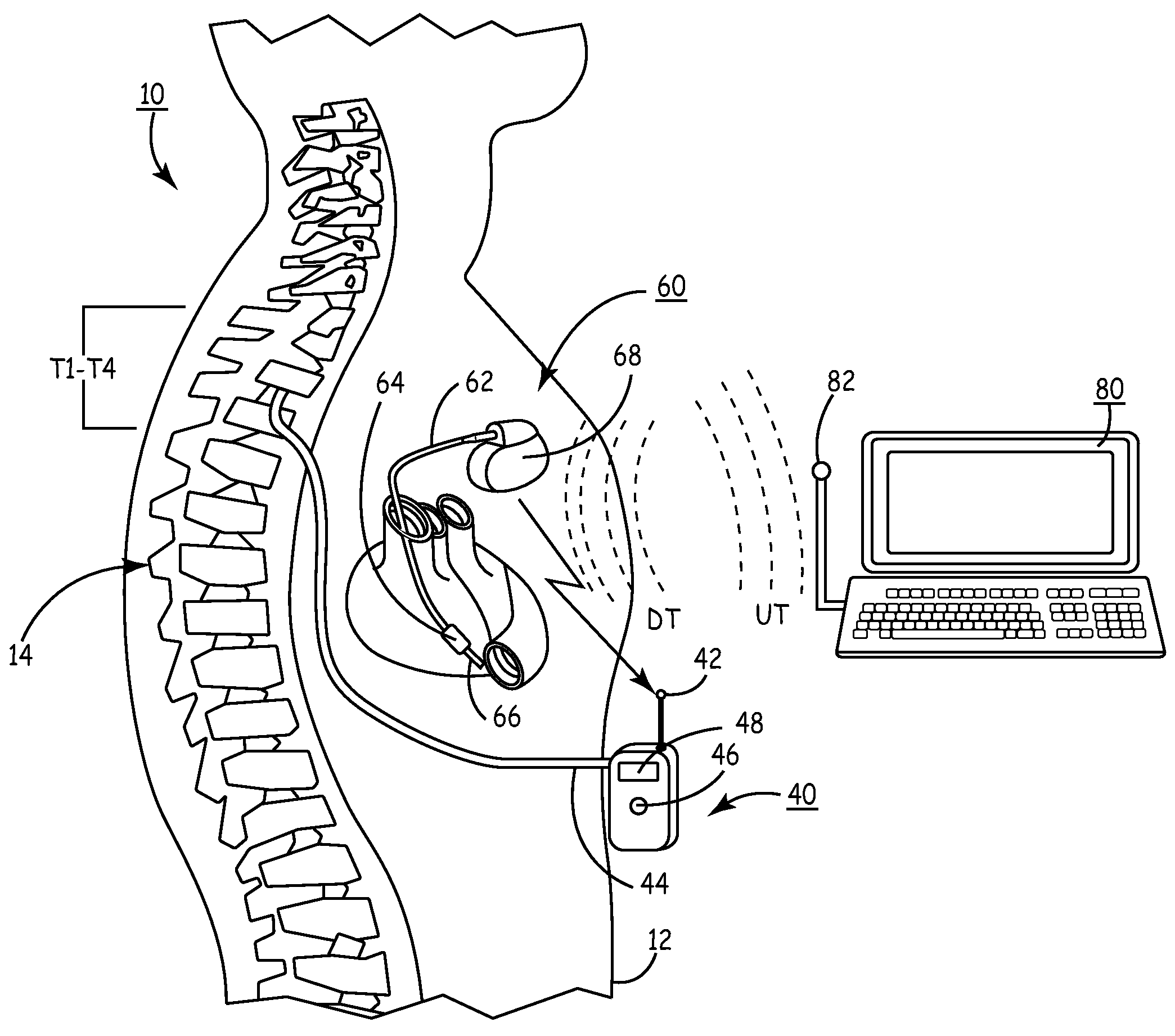
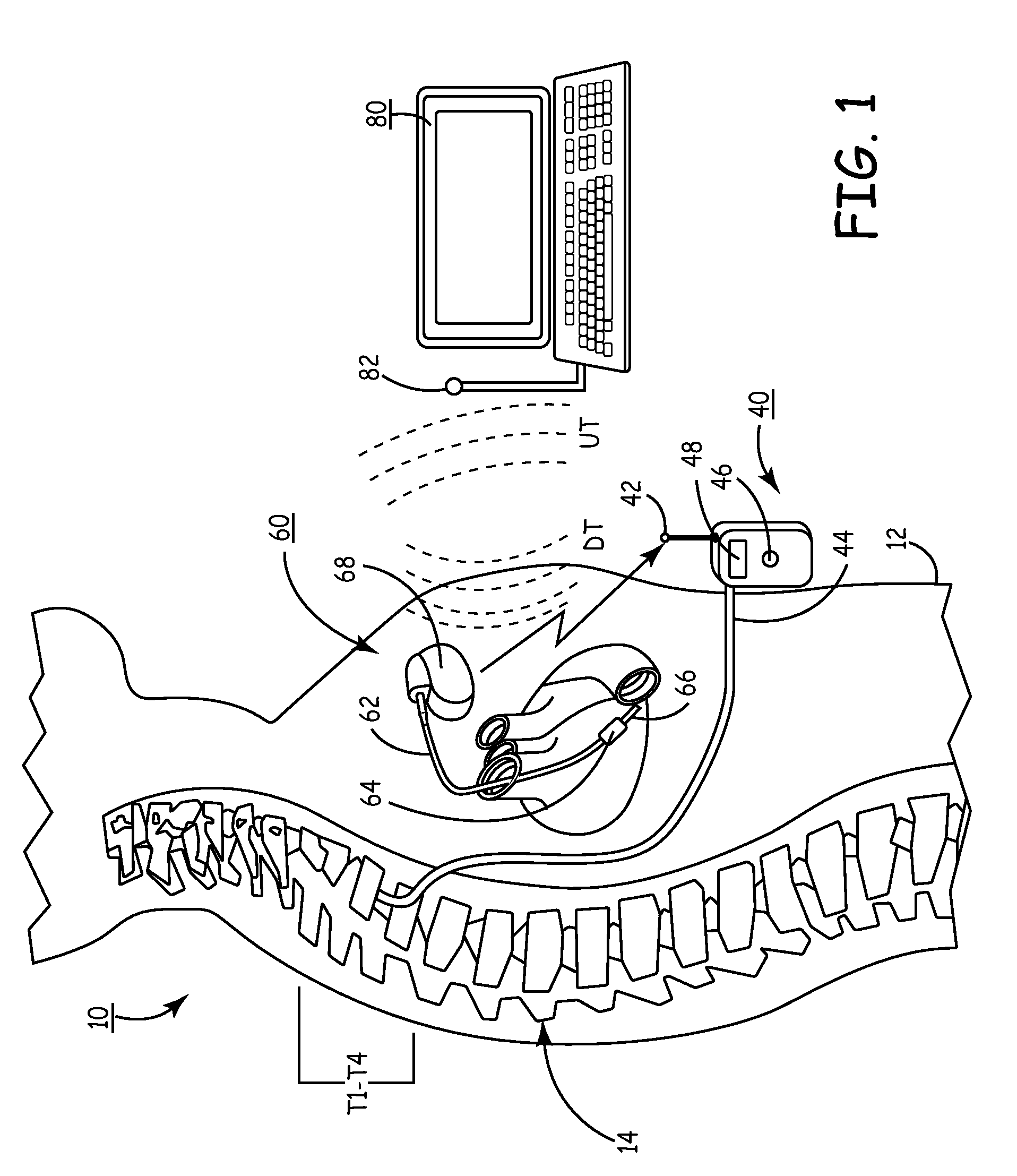
A sympatholytic cardiovascular agent delivered by a drug delivery pump to a central nervous system site to alleviate symptoms of acute or chronic cardiac insult or impaired cardiac performance. The drug delivery pump can be external or implantable infusion pump (IIP) coupled with a drug infusion catheter extending to the site. A patient activator can command delivery of a dosage and / or an implantable heart monitor (IHM) coupled with a sensor can detect physiologic parameters associated with cardiac insult or impaired cardiac performance and trigger dosage delivery. The IIP and IHM can be combined into a single implantable medical device (IMD) or can constitute separate IMDs that communicate by any of known communication mechanisms. The sympatholytic cardiovascular agent is one of the group consisting of an alpha-adrenergic agonist and an alpha2-adrenergic agonist (e.g., clonidine, p-aminoclonidine, guanabenz, lidamidine, tizanidine, moxonidine, methyldopa, xylazine, guanfacine, detomidine, medetomidine, and dexmedetomidine).
Owner:MEDTRONIC INC
Variable flow infusion pump system
InactiveUS20070112328A1Increase and decrease flow rateAlter shapeInfusion devicesPharmaceutical delivery mechanismEngineeringElectronics
Owner:AQULINX MEDICAL
Methods and apparatus for delivering a drug influencing appetite for treatment of eating disorders
Methods and systems for treating patients suffering from eating disorders, e.g. obesity, through the dispensation of a drug by an implantable infusion pump (IIP) delivering drug into the cerebral spinal fluid (CSF) at a site of the intrathecal space in amounts and at times effective to suppress the patient's appetite through interaction of the drug transported through the CSF with receptors in the brain. Delivery of a programmed drug dosage is preferably by one of time-out of programmed time(s) of day, a command received from the patient, or a trigger signal developed from a sensed GI tract signal accompanying peristalsis.
Owner:MEDTRONIC INC
Delivery of a sympatholytic cardiovascular agent to the central nervous system to counter heart failure and pathologies associated with heart failure
A sympatholytic cardiovascular agent delivered by a drug delivery pump to a central nervous system site to alleviate symptoms and otherwise treat heart failure (HF) and pathologies associated with HF. The drug delivery pump can be external or implantable infusion pump (IIP) coupled with a drug infusion catheter extending to the site. A patient activator can command delivery of a dosage and / or an implantable heart monitor (IHM) coupled with a sensor can detect physiologic parameters associated with HF (or pathologies associated with HF) and trigger dosage delivery. The IIP and IHM can be combined into a single implantable medical device (IMD) or can constitute separate IMDs that communicate by any of known communication mechanisms. The sympatholytic cardiovascular agent is one of the group consisting of an alpha-adrenergic agonist and an alpha2-adrenergic agonist, e.g., clonidine, p-aminoclonidine, guanabenz, lidamidine, tizanidine, moxonidine, methyldopa, xylazine, guanfacine, detomidine, medetomidine, and dexmedetomidine.
Owner:MEDTRONIC INC
Delivery of a sympatholytic cardiovascular agent to the central nervous system to counter heart failure and pathologies associated with heart failure
A sympatholytic cardiovascular agent delivered by a drug delivery pump to a central nervous system site to alleviate symptoms and otherwise treat heart failure (HF) and pathologies associated with HF. The drug delivery pump can be external or implantable infusion pump (IIP) coupled with a drug infusion catheter extending to the site. A patient activator can command delivery of a dosage and / or an implantable heart monitor (IHM) coupled with a sensor can detect physiologic parameters associated with HF (or pathologies associated with HF) and trigger dosage delivery. The IIP and IHM can be combined into a single implantable medical device (IMD) or can constitute separate IMDs that communicate by any of known communication mechanisms. The sympatholytic cardiovascular agent is one of the group consisting of an alpha-adrenergic agonist and an alpha2-adrenergic agonist, e.g., clonidine, p-aminoclonidine, guanabenz, lidamidine, tizanidine, moxonidine, methyldopa, xylazine, guanfacine, detomidine, medetomidine, and dexmedetomidine.
Owner:MEDTRONIC INC
Variable flow infusion pump system
InactiveUS20100069892A1Increase and decrease flow rateFacilitate communicationInfusion devicesMedical devicesElectronicsElectronic equipment
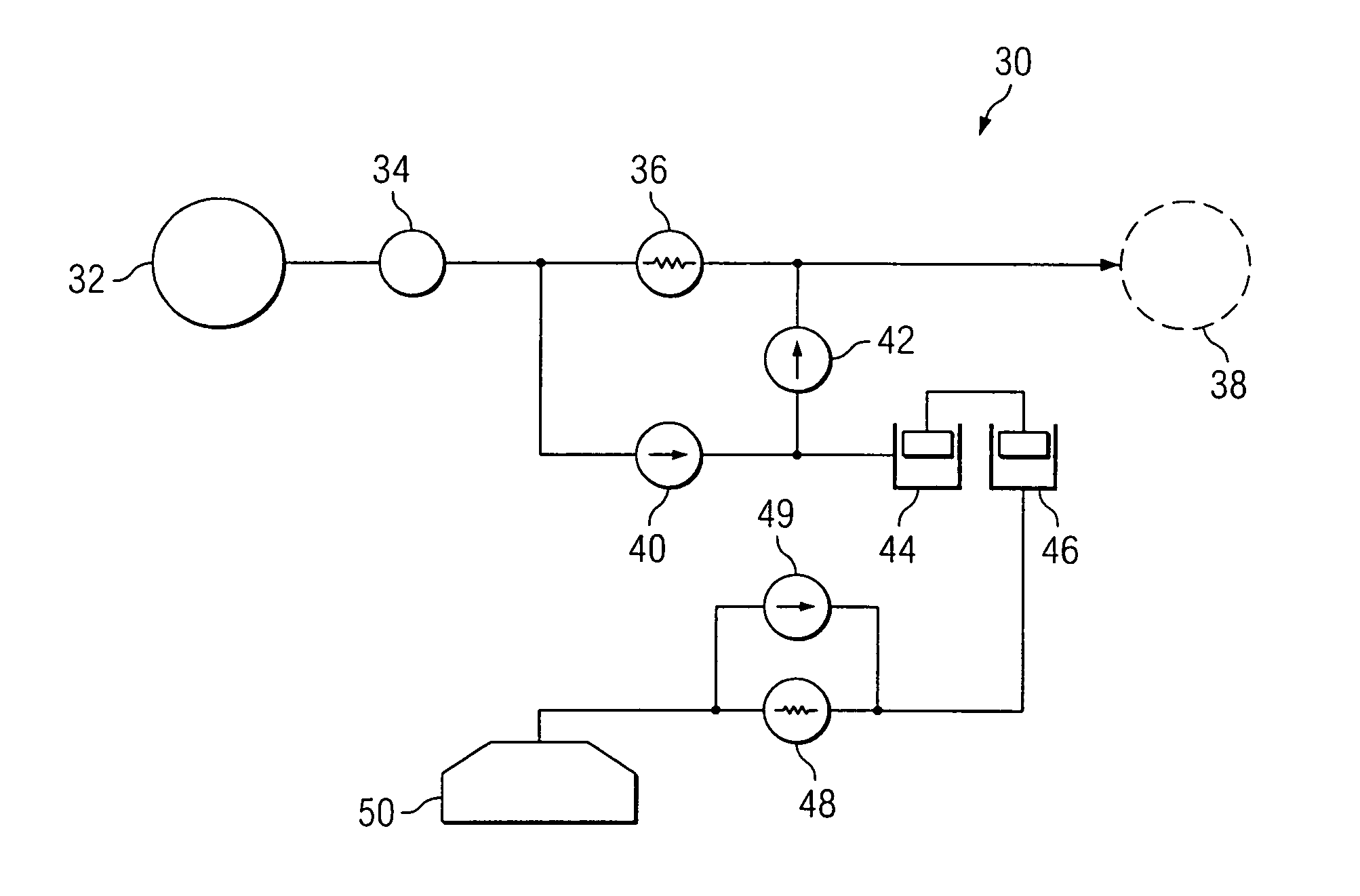
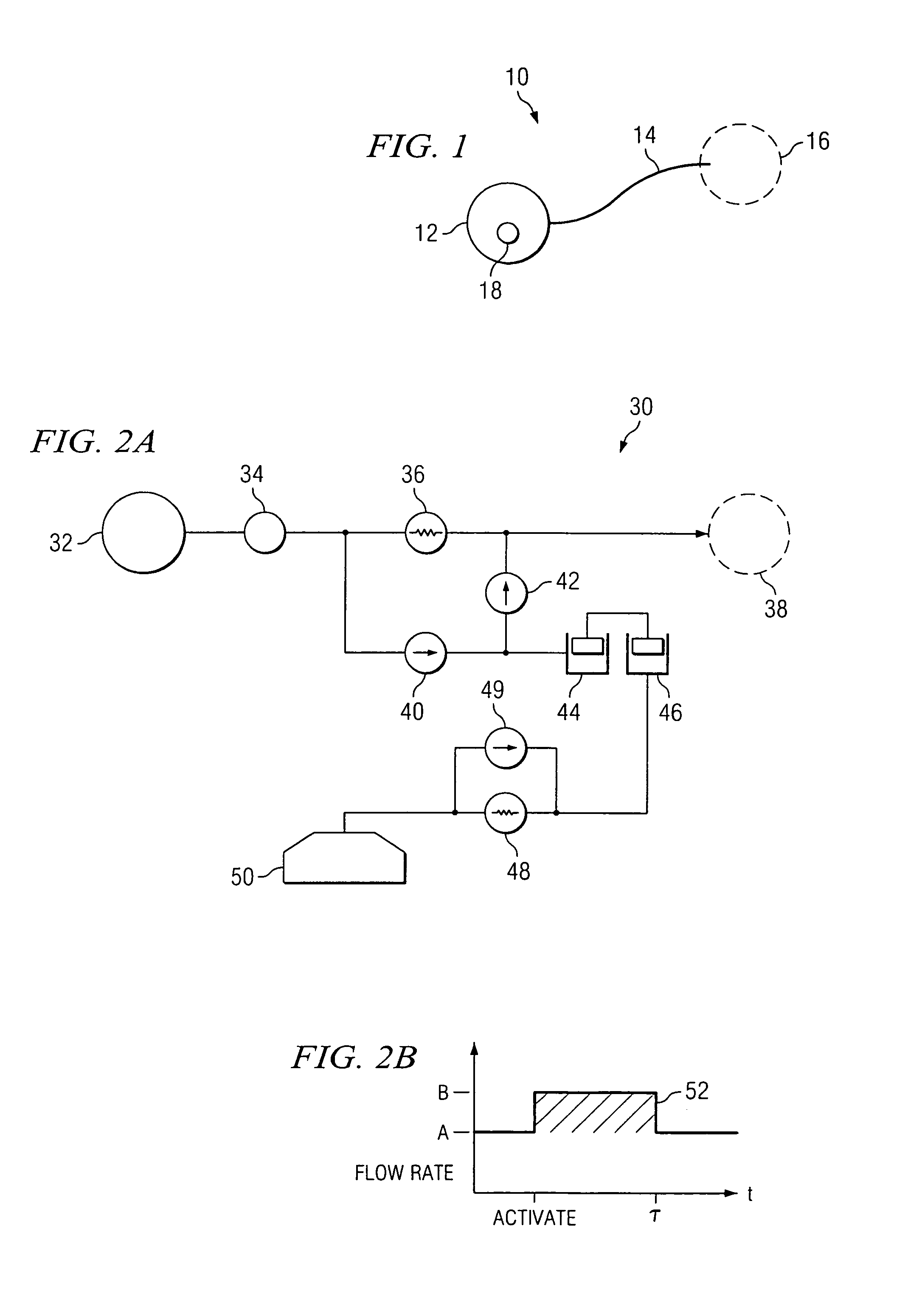
An implantable infusion pump system is disclosed. The pump system preferably includes an implantable pump and a removable module. The module may provide for varying flow rates of fluid being dispensed from the pump or may provide for a constant flow rate of such fluid. In the case of varying flow rate capabilities, the module preferably includes one or more sensors to determine information relating to the flow rate, electronics for analyzing the flow rate information, and a mechanism for physically altering the flow rate. Methods of dispensing a medicament to a patient are also disclosed, as are variations of the pump system.
Owner:AQULINX MEDICAL
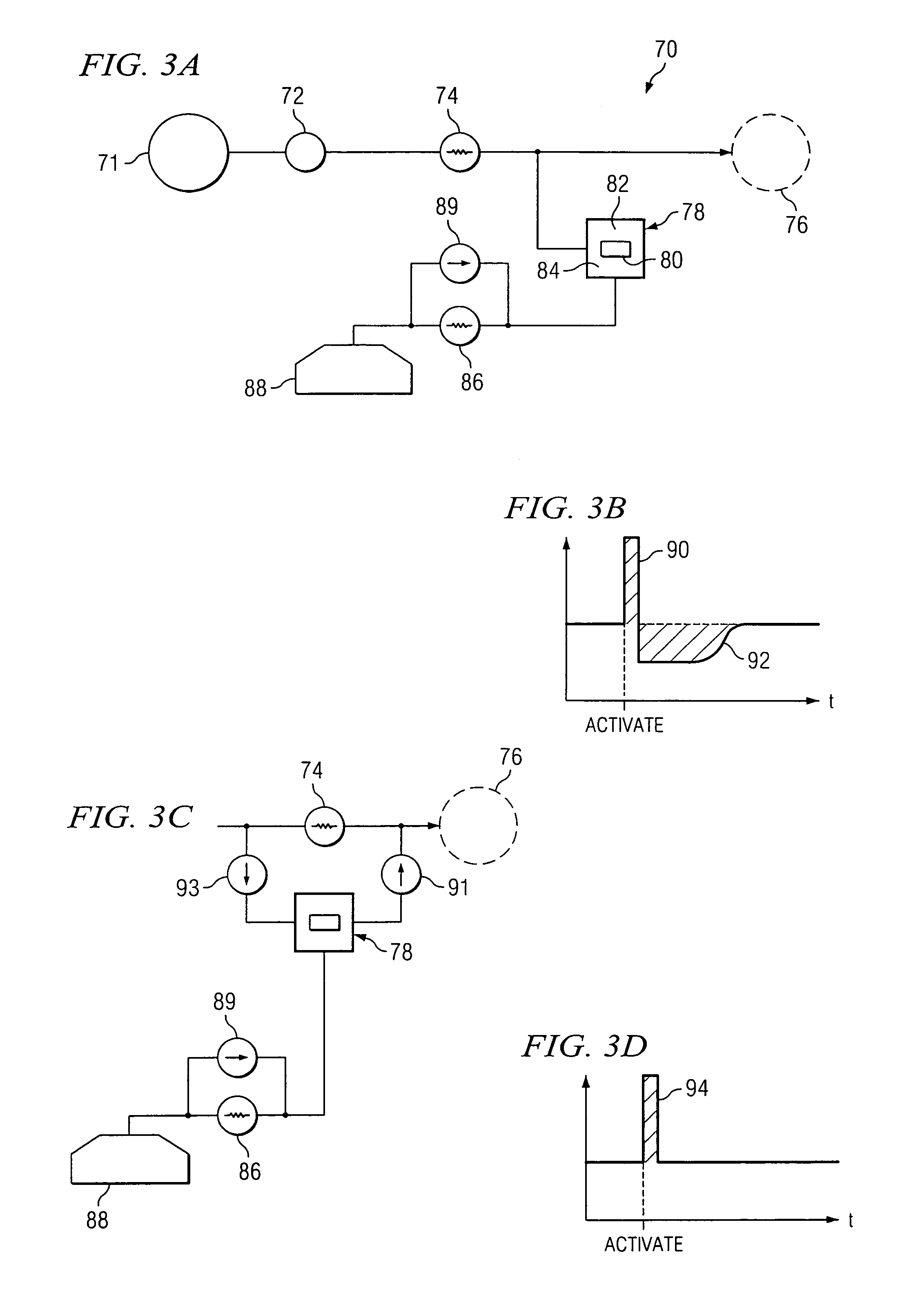
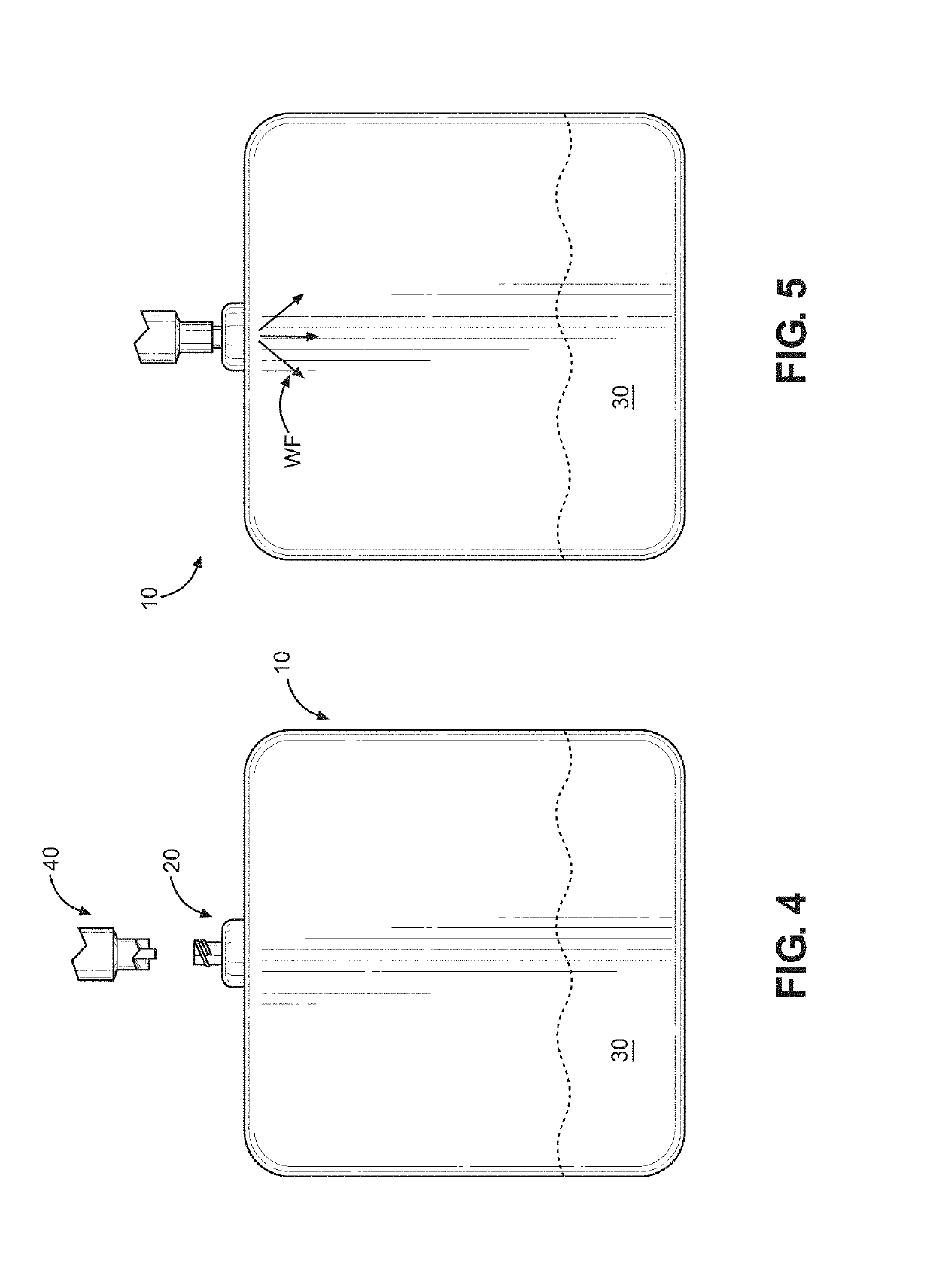
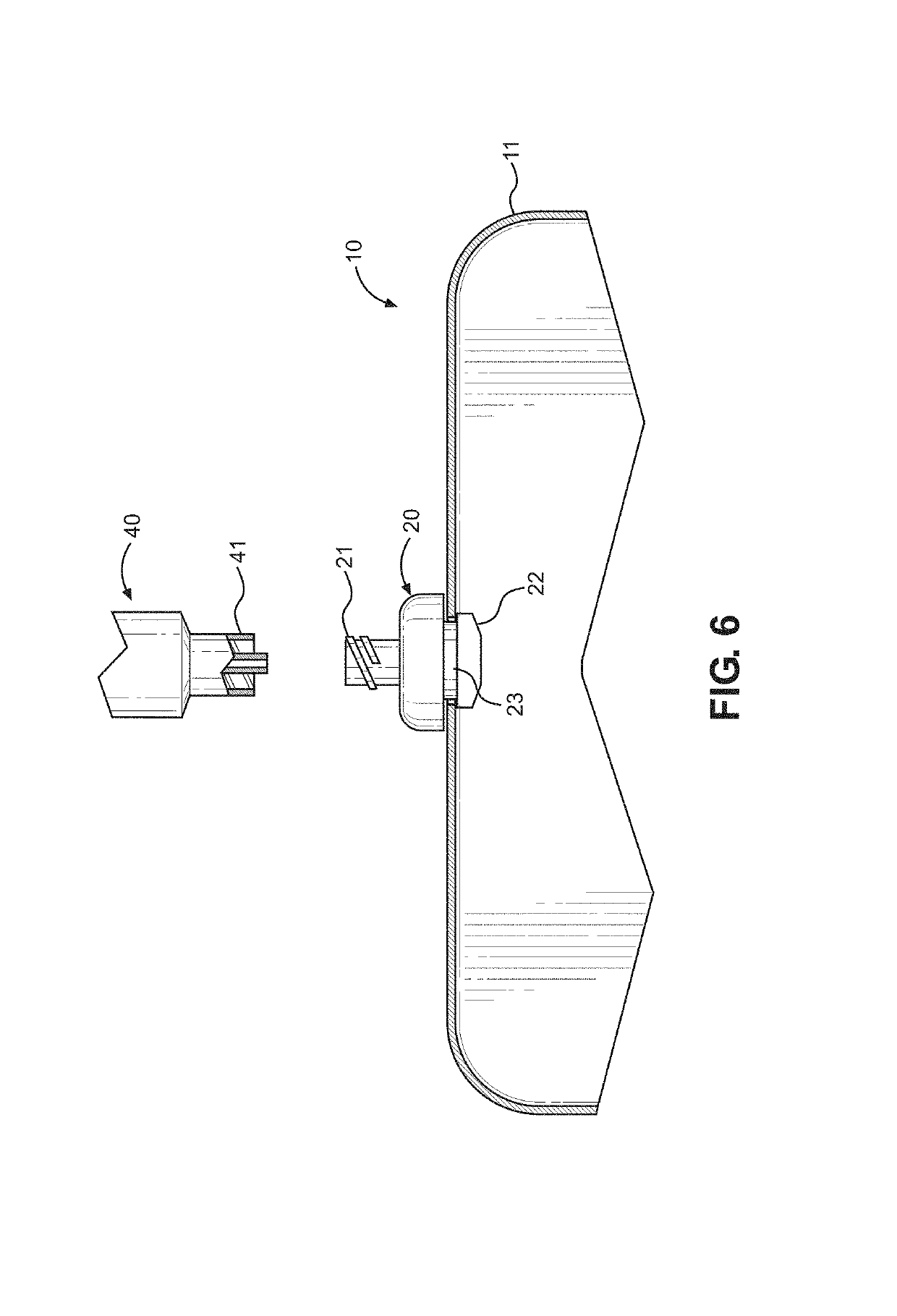
Actuation system and method for an implantable infusion pump
The invention relates to an actuation system and method for an implantable infusion pump. In an example, a working fluid is placed in an actuator. Upon actuation, the working fluid is driven into a piston cylinder. Upon deactuation, the actuator draws the fluid from the cylinder through a restrictor at a rate dictated by the motivating force, fluid viscosity, and restriction. Driving of the piston may produce a bolus dosage or fill a supplemental flow chamber for subsequent delivery. The exemplary system may be configured by selecting a fluid volume and a viscosity. These, in combination, produce a prescribed fluid delivery rate (or recharge rate) and cumulative flow volume provided to a patient over a time period or in a bolus dose. The system may also be configured to limit the total dosage of a bolus injection, or the rate of a supplemental dosage. In this manner, the system is safe, preventing overdose.
Owner:ADVANCED NEUROMODULATION SYST INC
Variable flow infusion pump system
InactiveUS7637892B2Increase and decrease flow rateFacilitate communicationInfusion devicesMedical devicesElectronicsElectronic equipment
An implantable infusion pump system is disclosed. The pump system preferably includes an implantable pump and a removable module. The module may provide for varying flow rates of fluid being dispensed from the pump or may provide for a constant flow rate of such fluid. In the case of varying flow rate capabilities, the module preferably includes one or more sensors to determine information relating to the flow rate, electronics for analyzing the flow rate information, and a mechanism for physically altering the flow rate. Methods of dispensing a medicament to a patient are also disclosed, as are variations of the pump system.
Owner:AQULINX MEDICAL
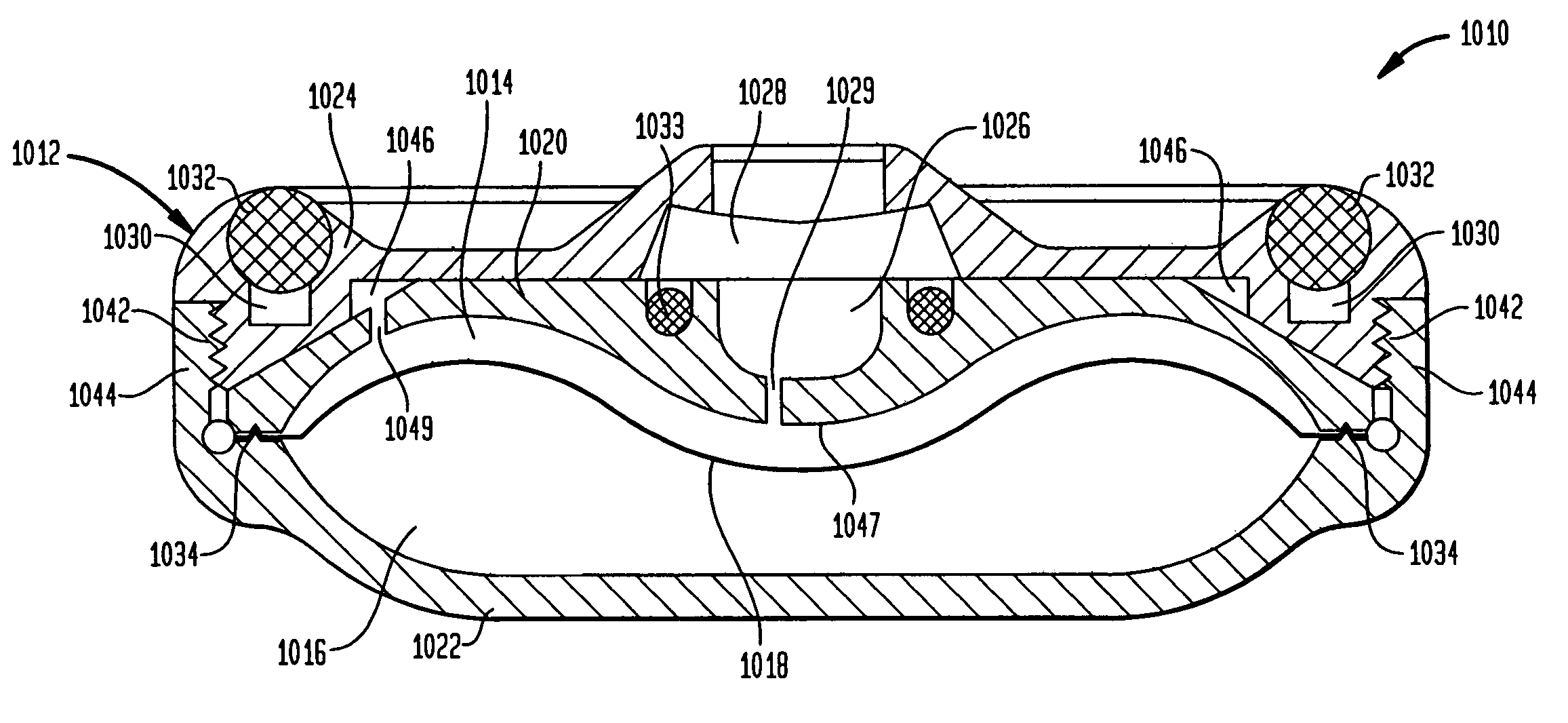
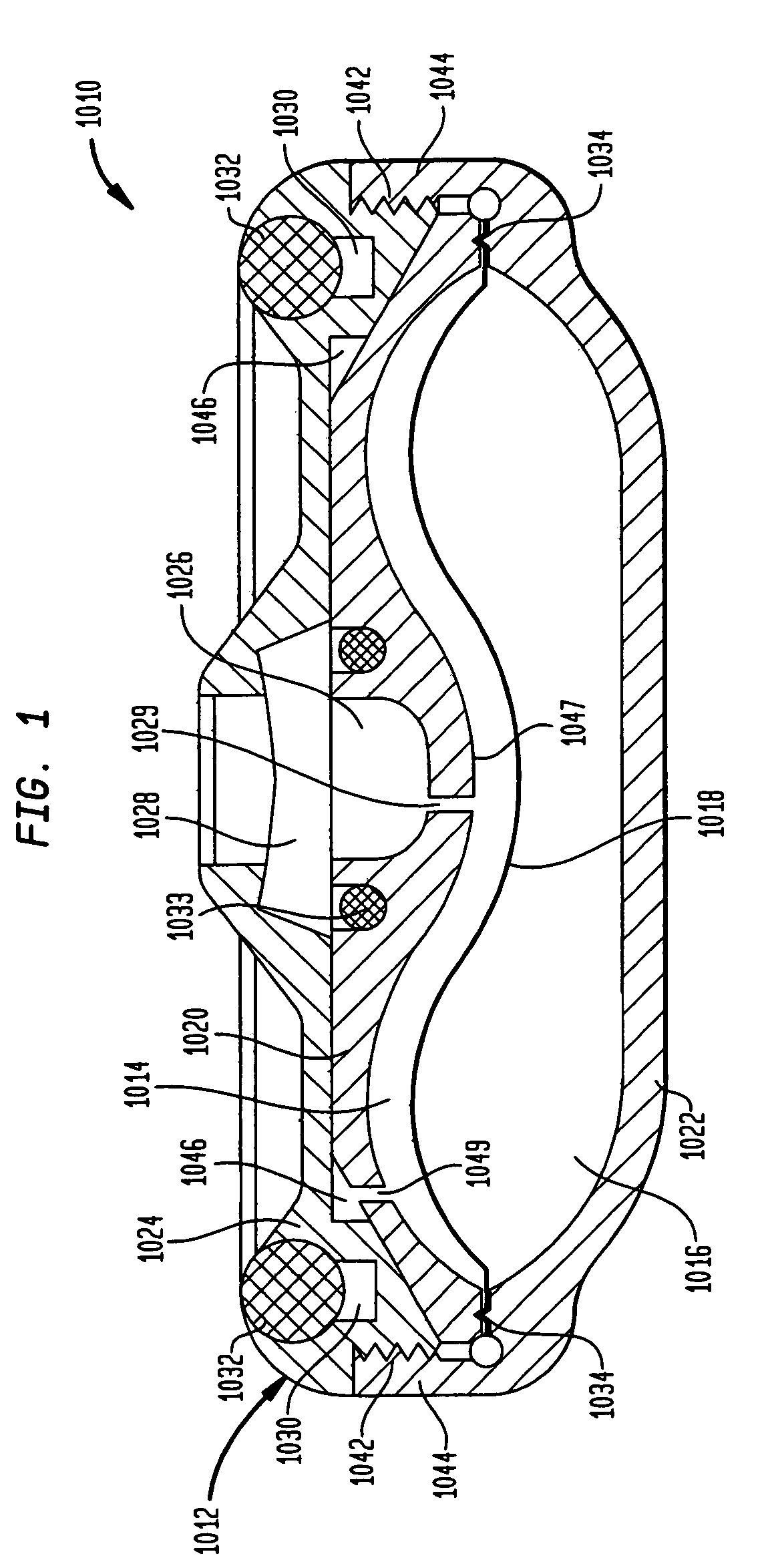
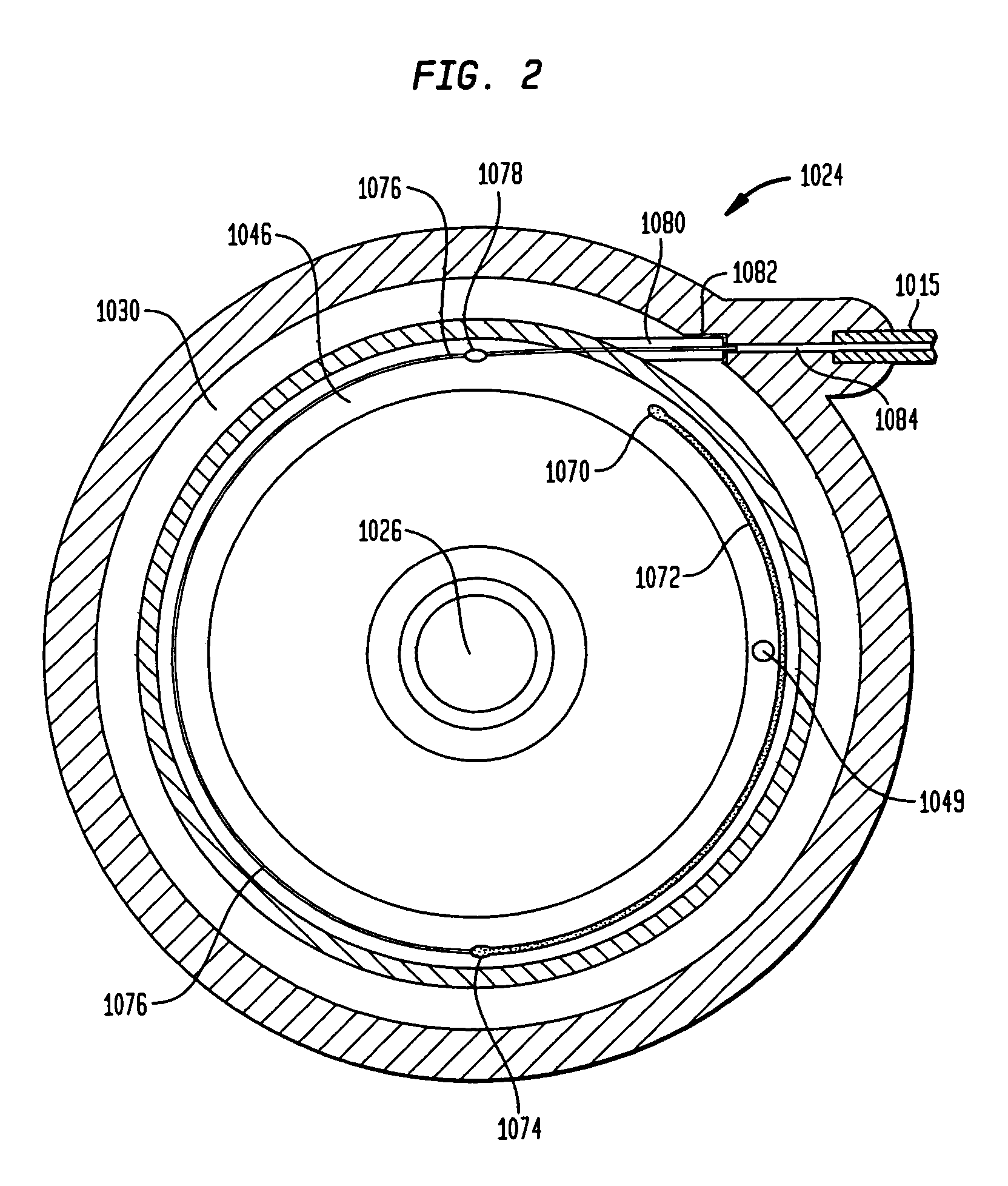
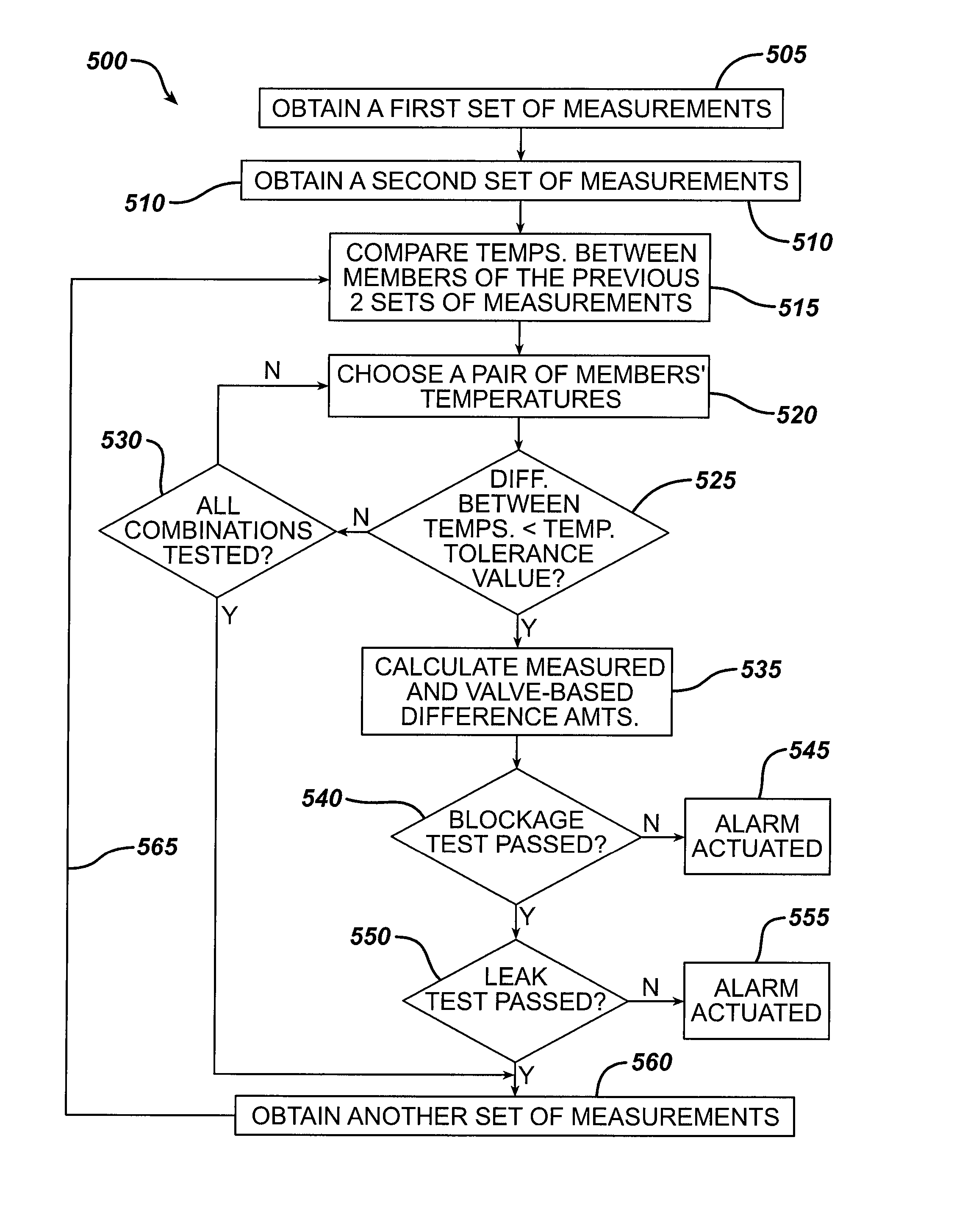
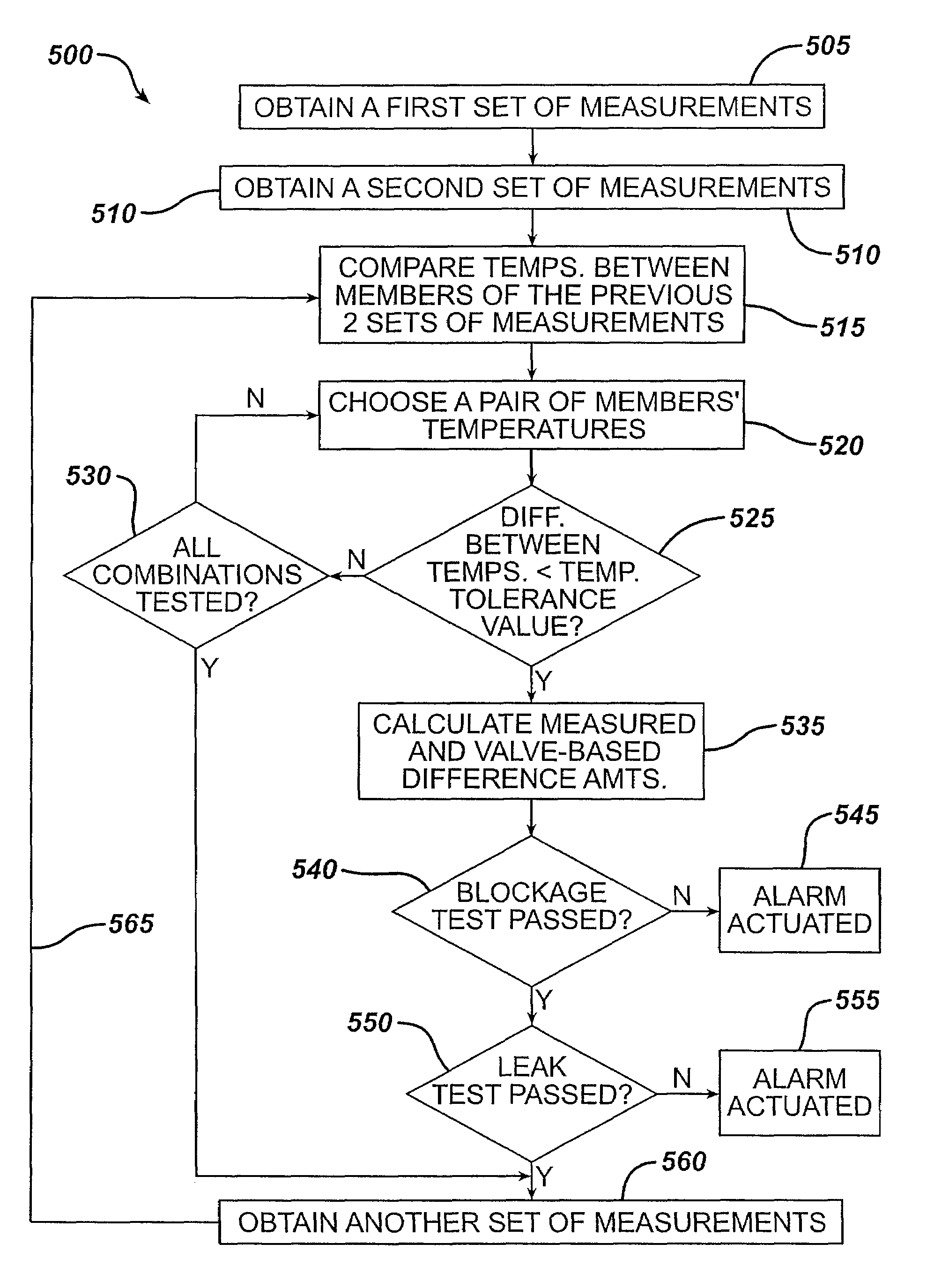
Methods and devices for monitoring fluid of an implantable infusion pump
Methods and devices are described for monitoring reservoir fluid in an implantable infusion pump. Such methods and devices can be used when the infusion pump has a temperature-dependent component. For example, a method of monitoring reservoir fluid can include obtaining measurements from the reservoir that include corresponding data related to the amount of fluid and temperature in the reservoir. A pair of measurements can be used to monitor the fluid level, or flow rate out of, the reservoir when the corresponding temperatures of the measurements are within a temperature tolerance value. In such an instance, a measured amount difference can be calculated based on the fluid amounts corresponding to the pair of measurements. Other variations of fluid monitoring methods, and implantable infusion pump systems that can perform fluid monitoring, are also described.
Owner:MEDOS INT SARL
Delivery of a sympatholytic cardiovascular agent to the central nervous system
A sympatholytic cardiovascular agent delivered by a drug delivery pump to a central nervous system site to alleviate symptoms of acute or chronic cardiac insult or impaired cardiac performance. The drug delivery pump can be external or implantable infusion pump (IIP) coupled with a drug infusion catheter extending to the site. A patient activator can command delivery of a dosage and / or an implantable heart monitor (IHM) coupled with a sensor can detect physiologic parameters associated with cardiac insult or impaired cardiac performance and trigger dosage delivery. The IIP and IHM can be combined into a single implantable medical device (IMD) or can constitute separate IMDs that communicate by any of known communication mechanisms. The sympatholytic cardiovascular agent is one of the group consisting of an alpha-adrenergic agonist and an alpha2-adrenergic agonist (e.g., clonidine, p-aminoclonidine, guanabenz, lidamidine, tizanidine, moxonidine, methyldopa, xylazine, guanfacine, detomidine, medetomidine, and dexmedetomidine).
Owner:MEDTRONIC INC
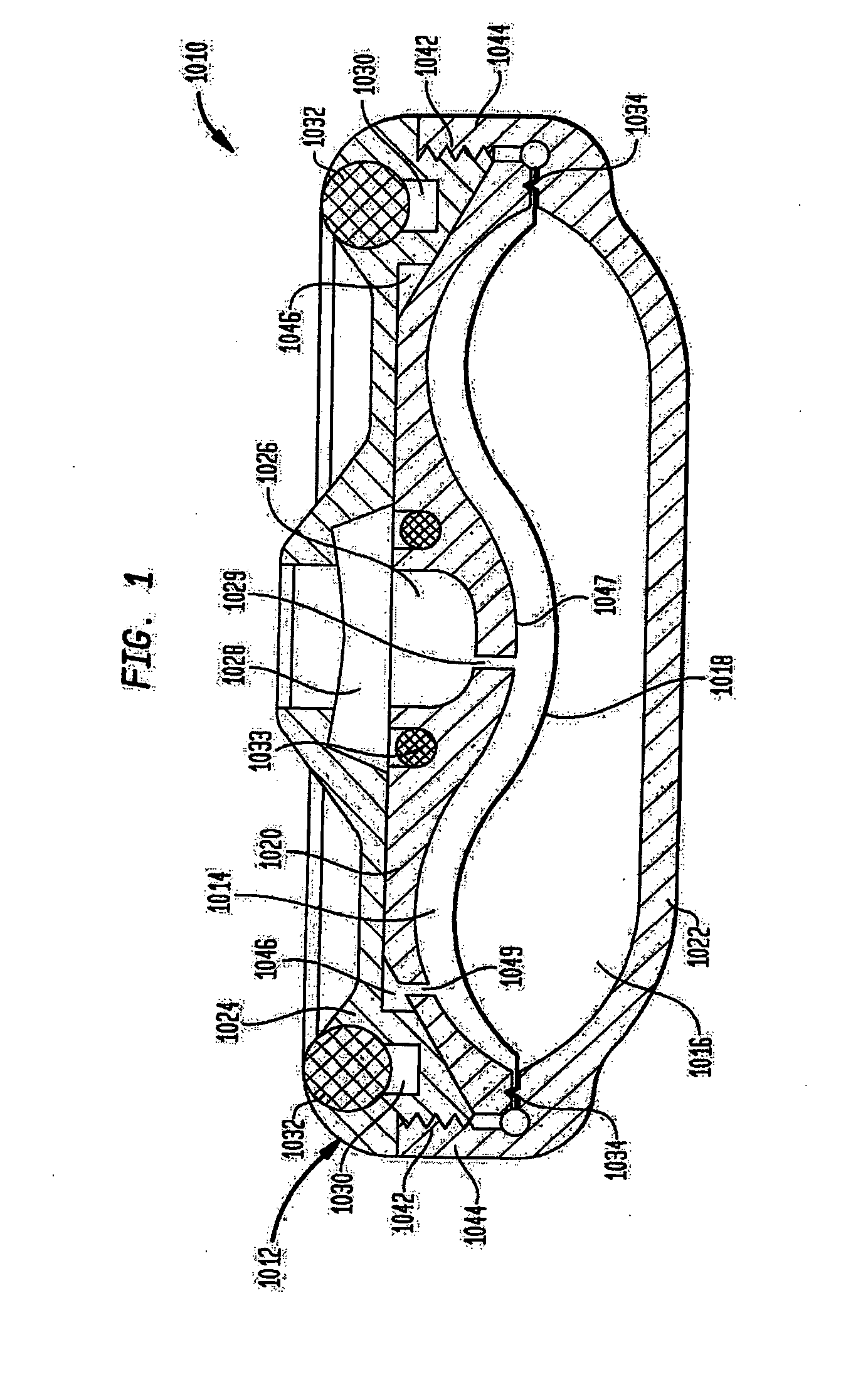
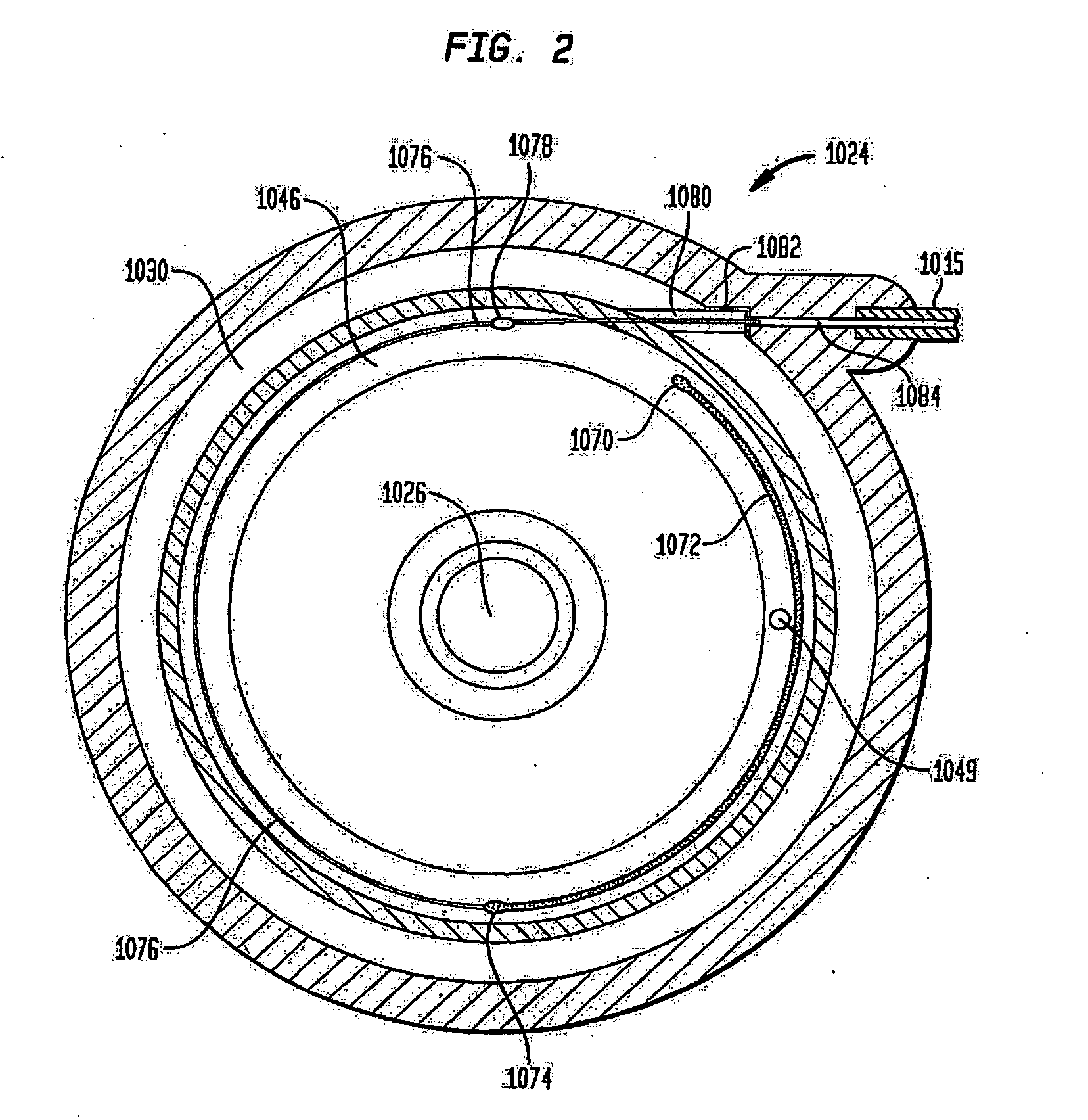
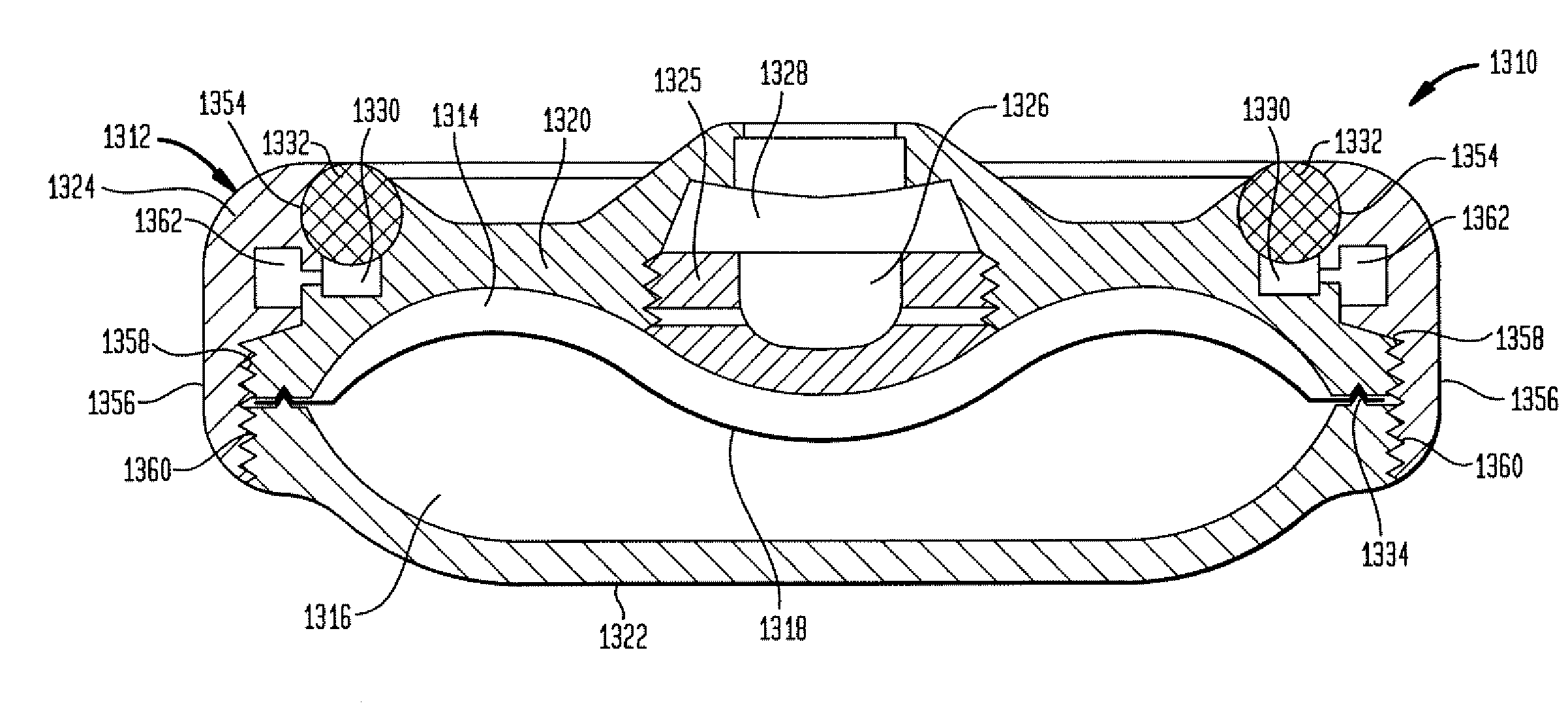
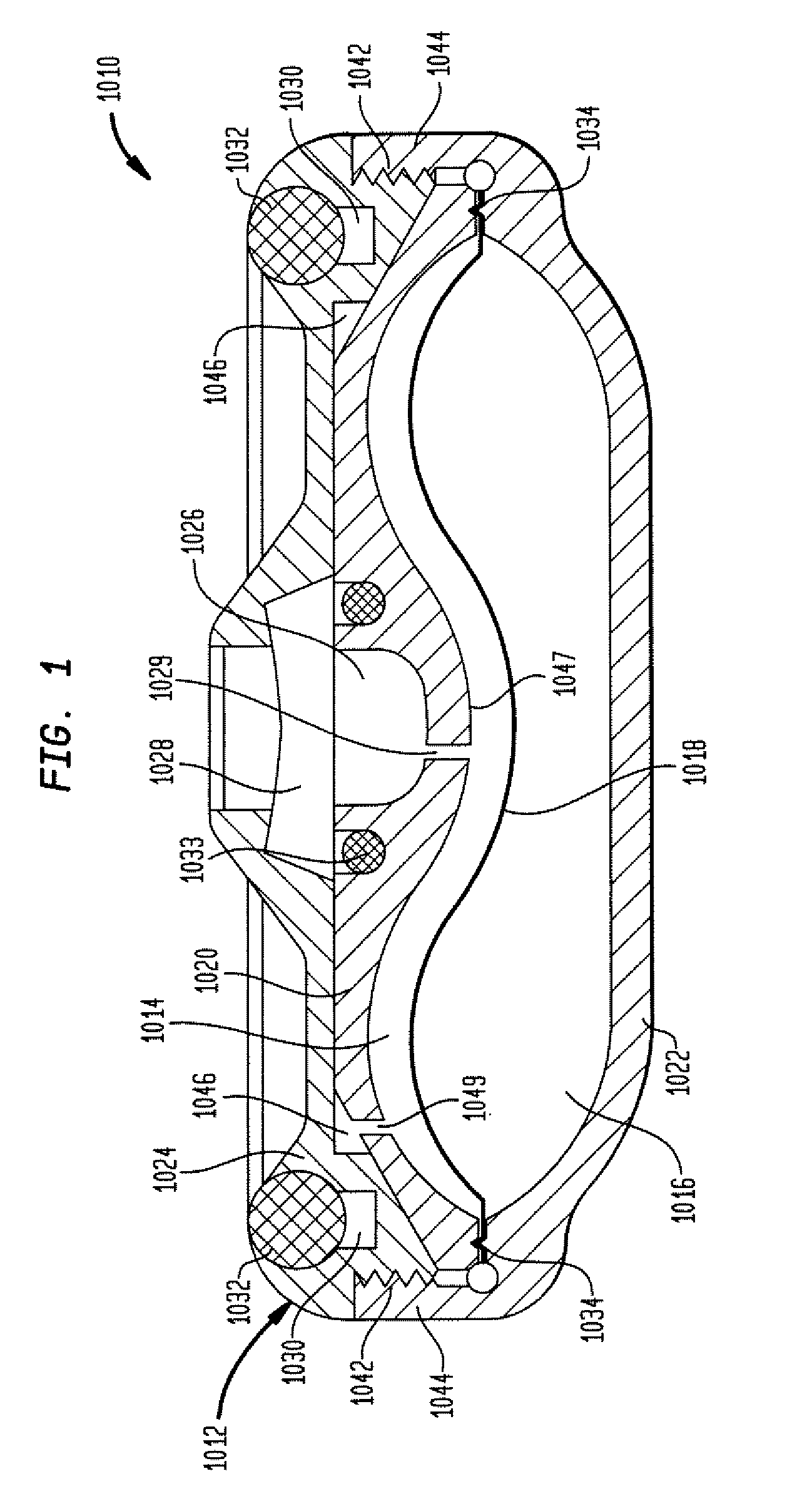
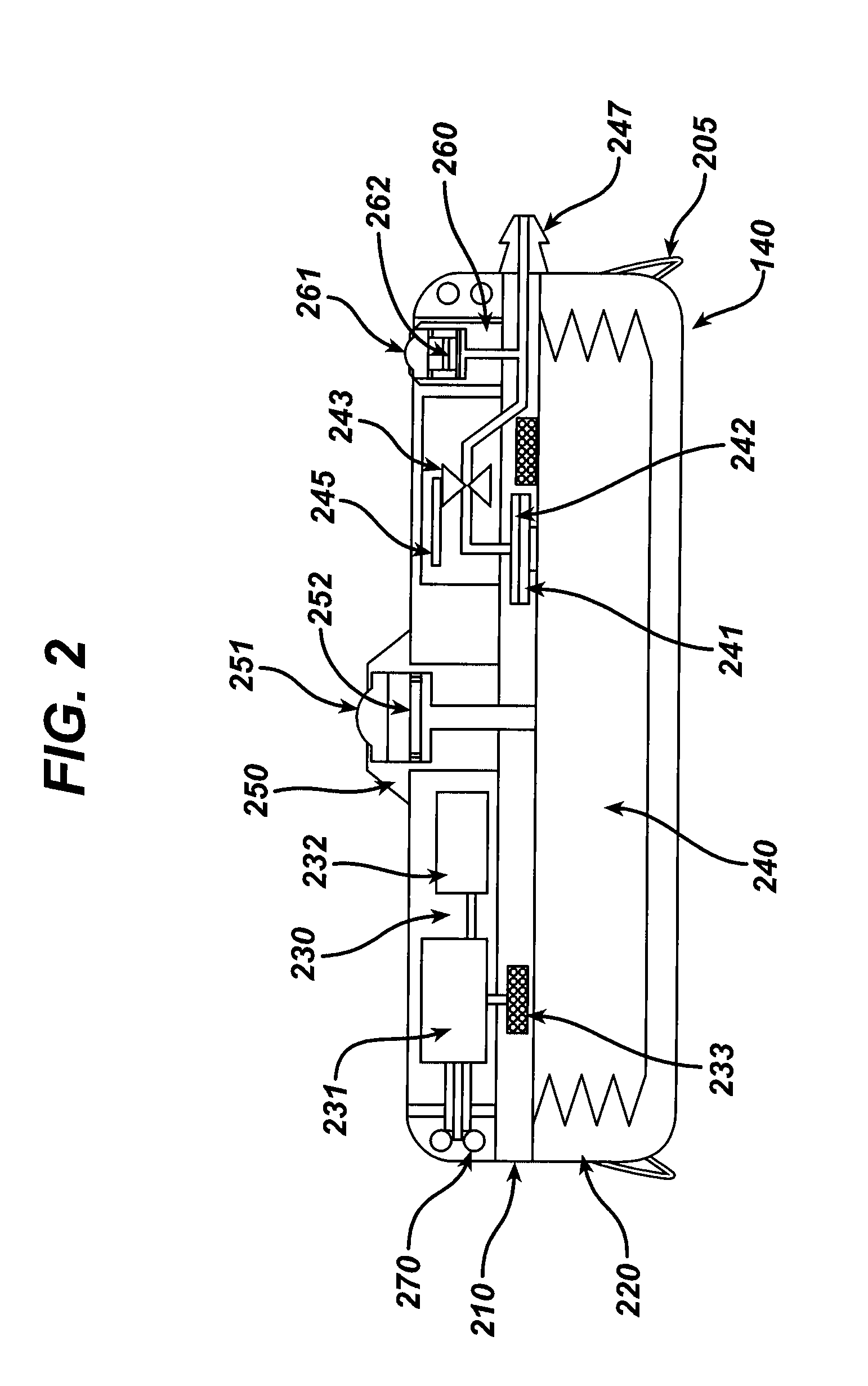
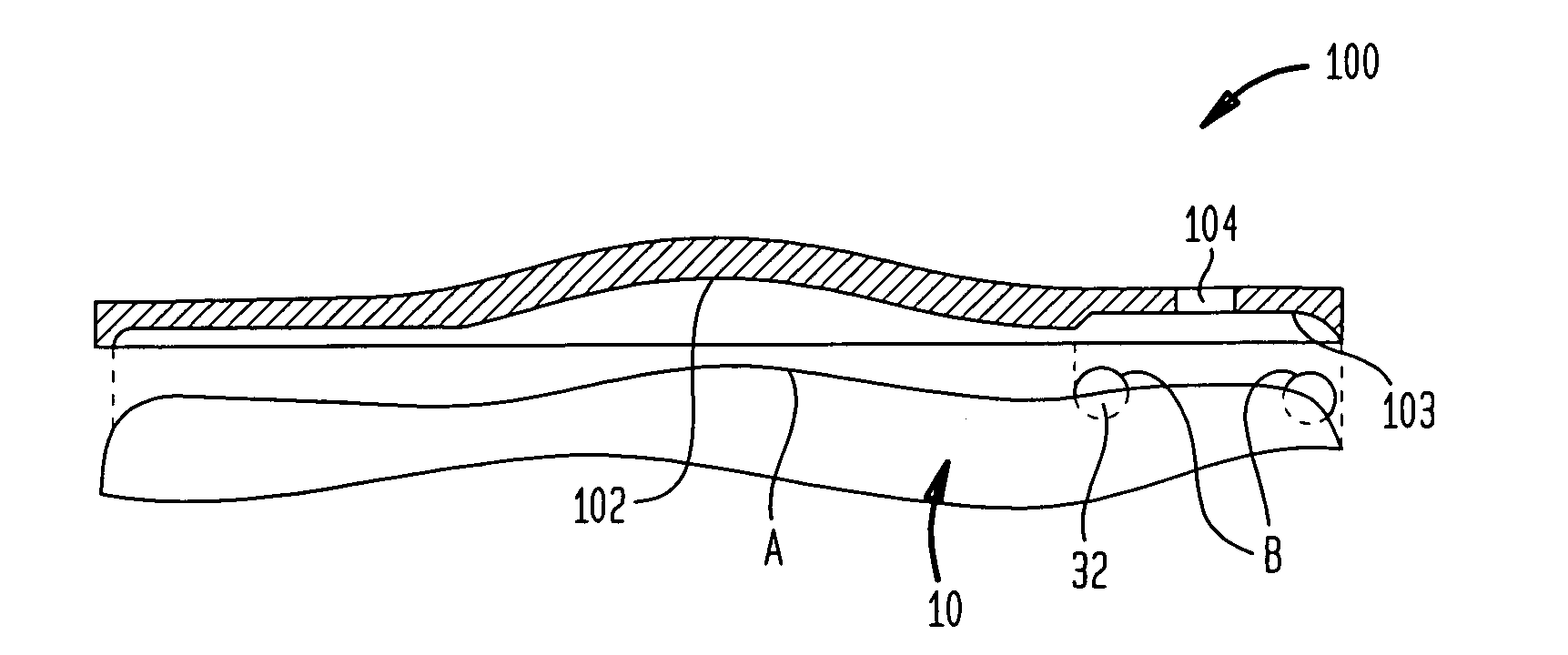
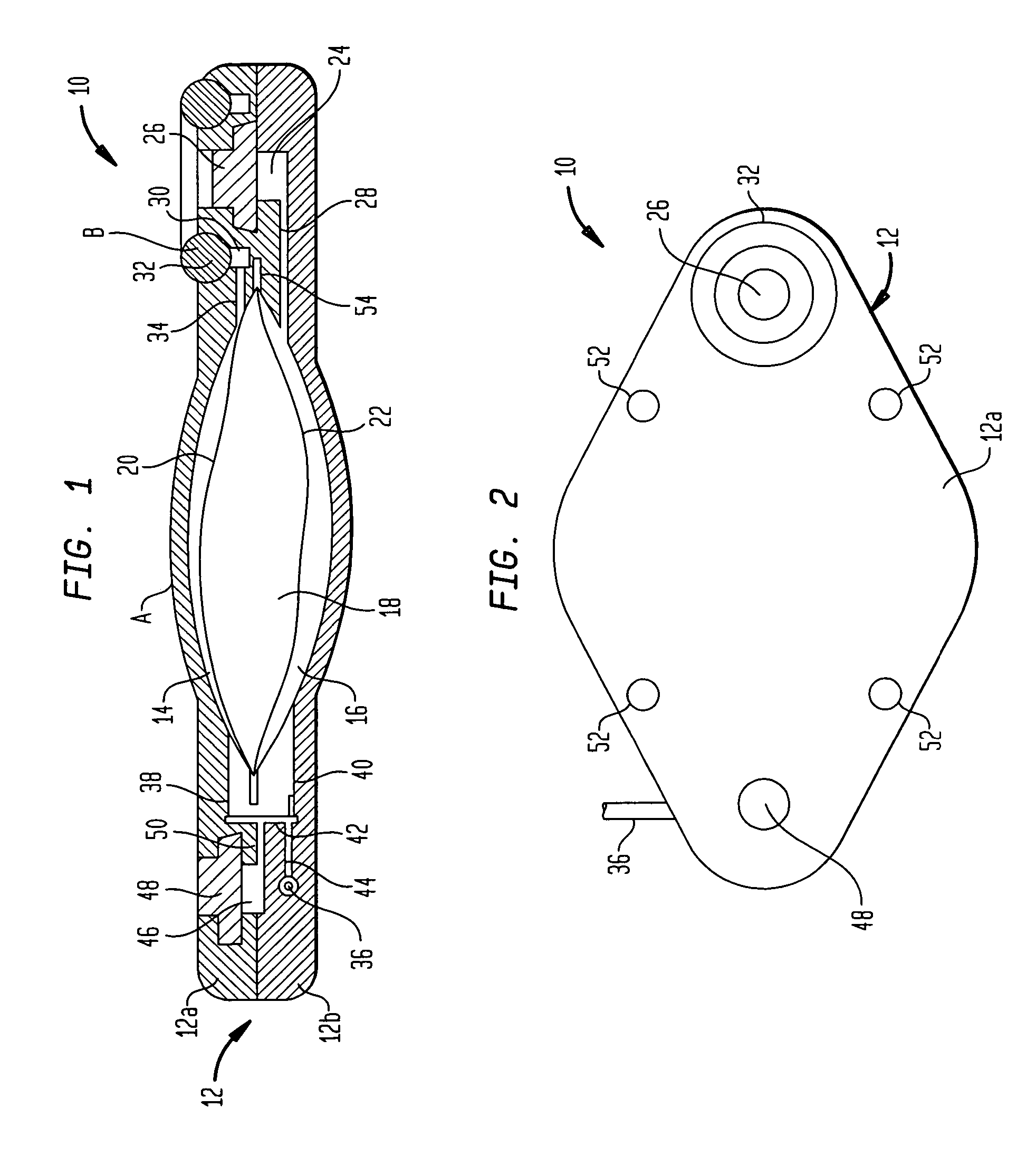
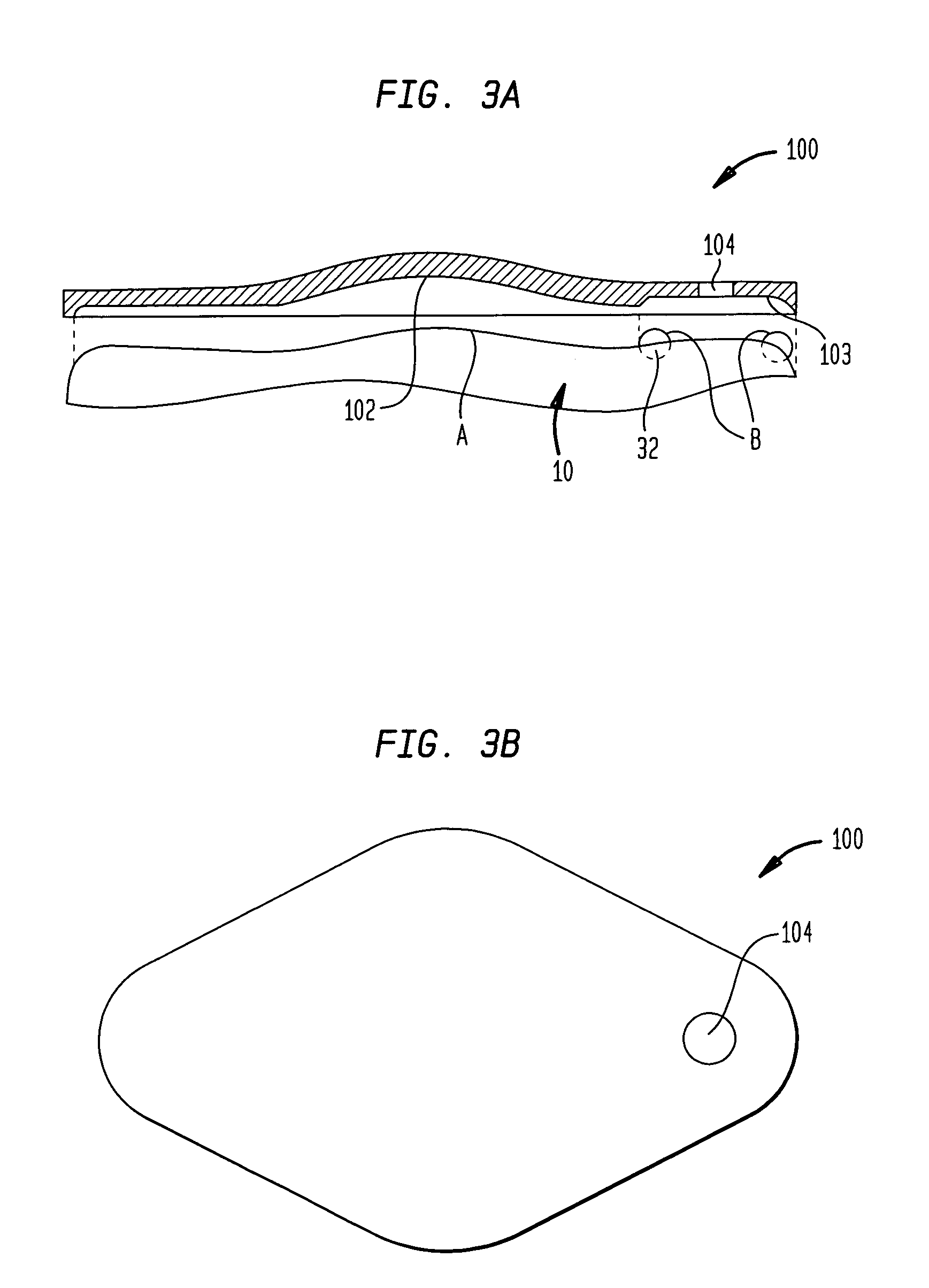
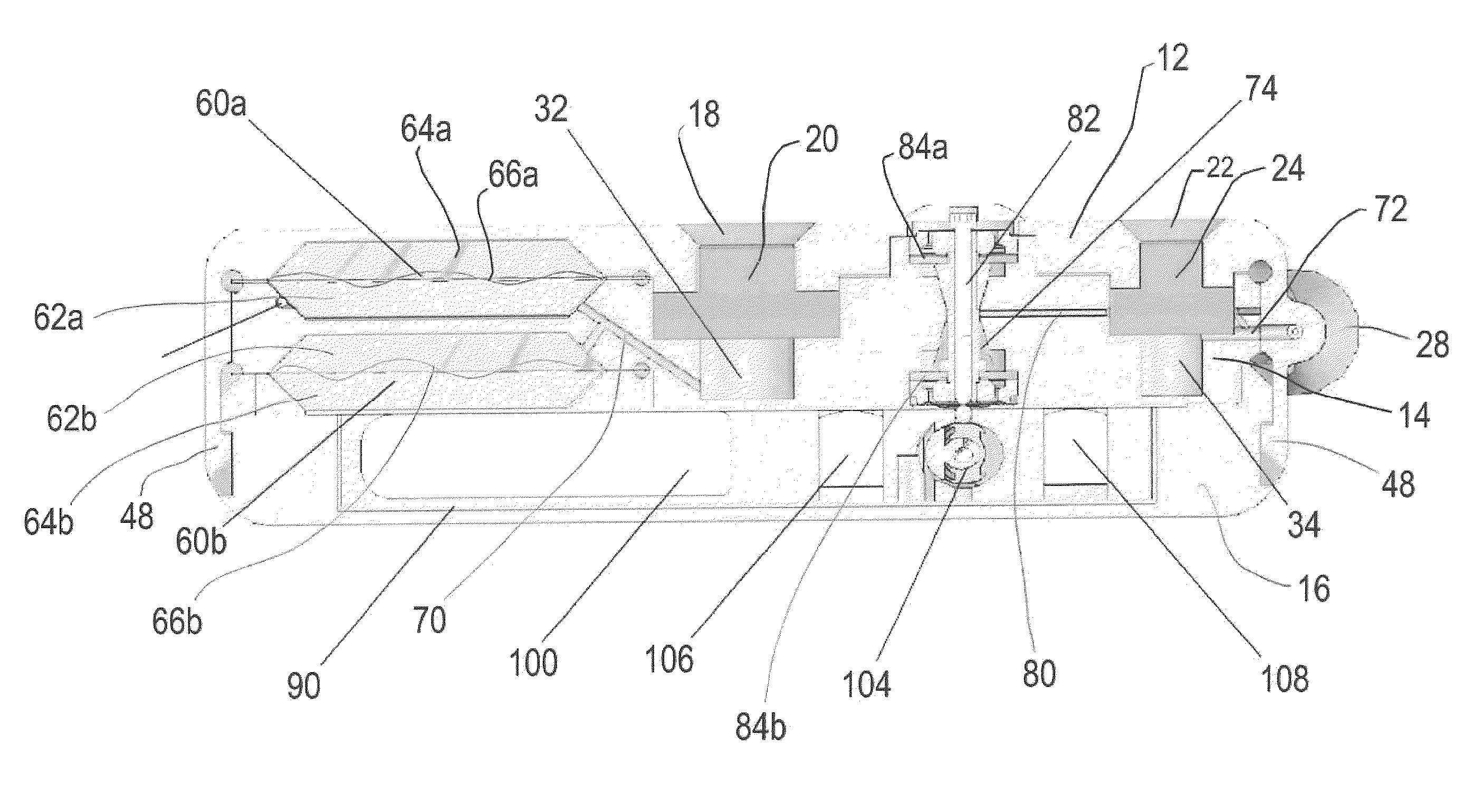
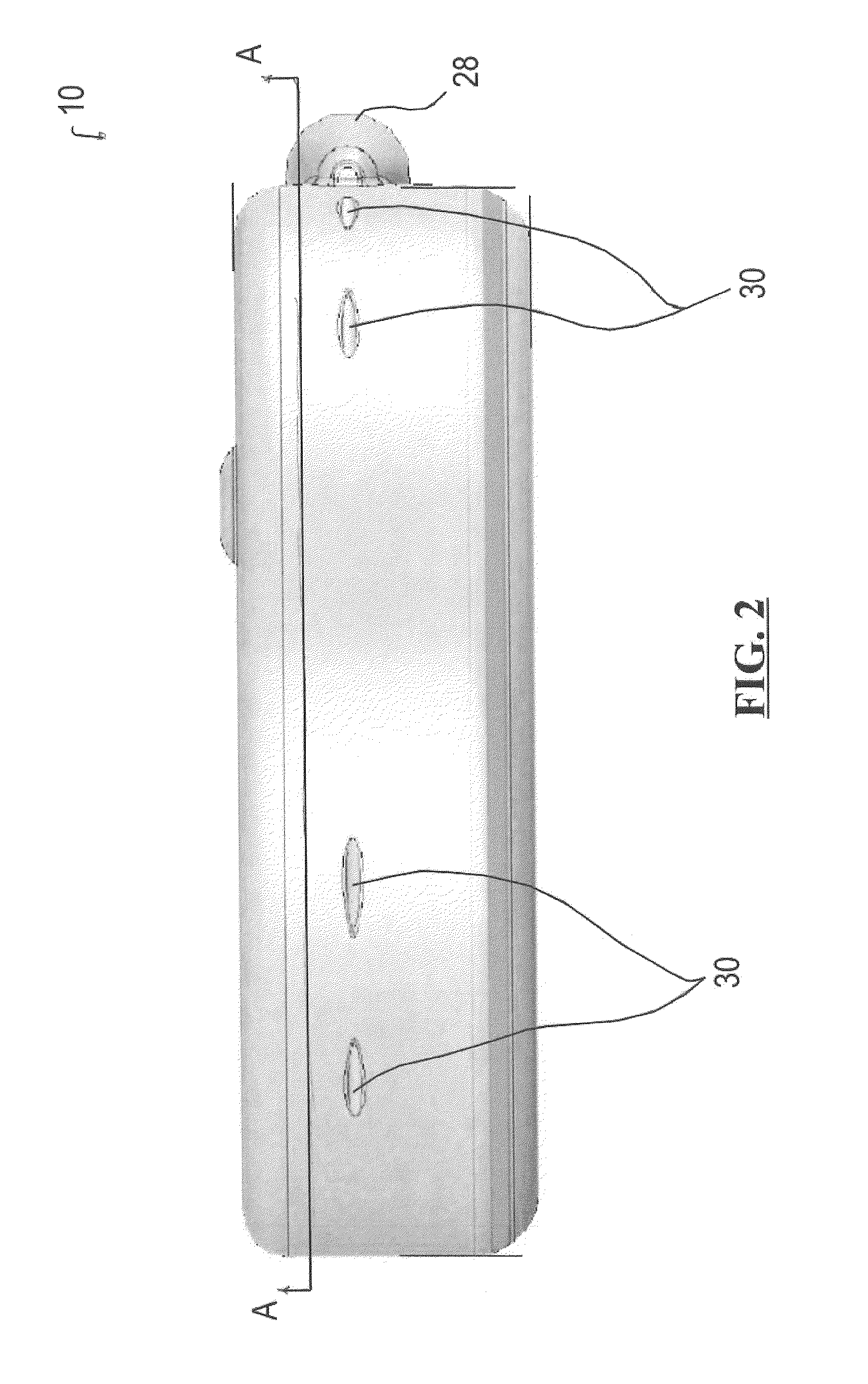
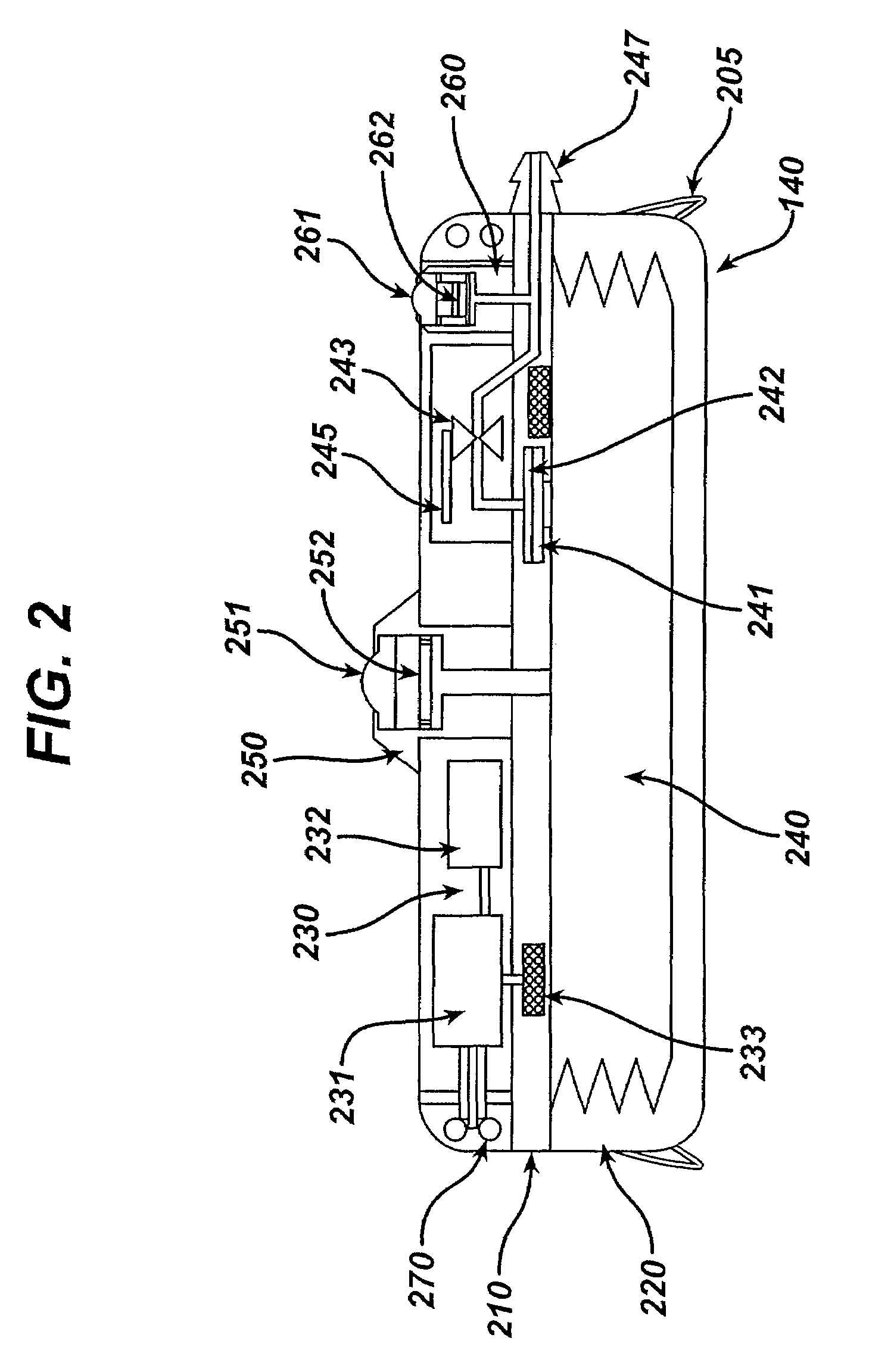
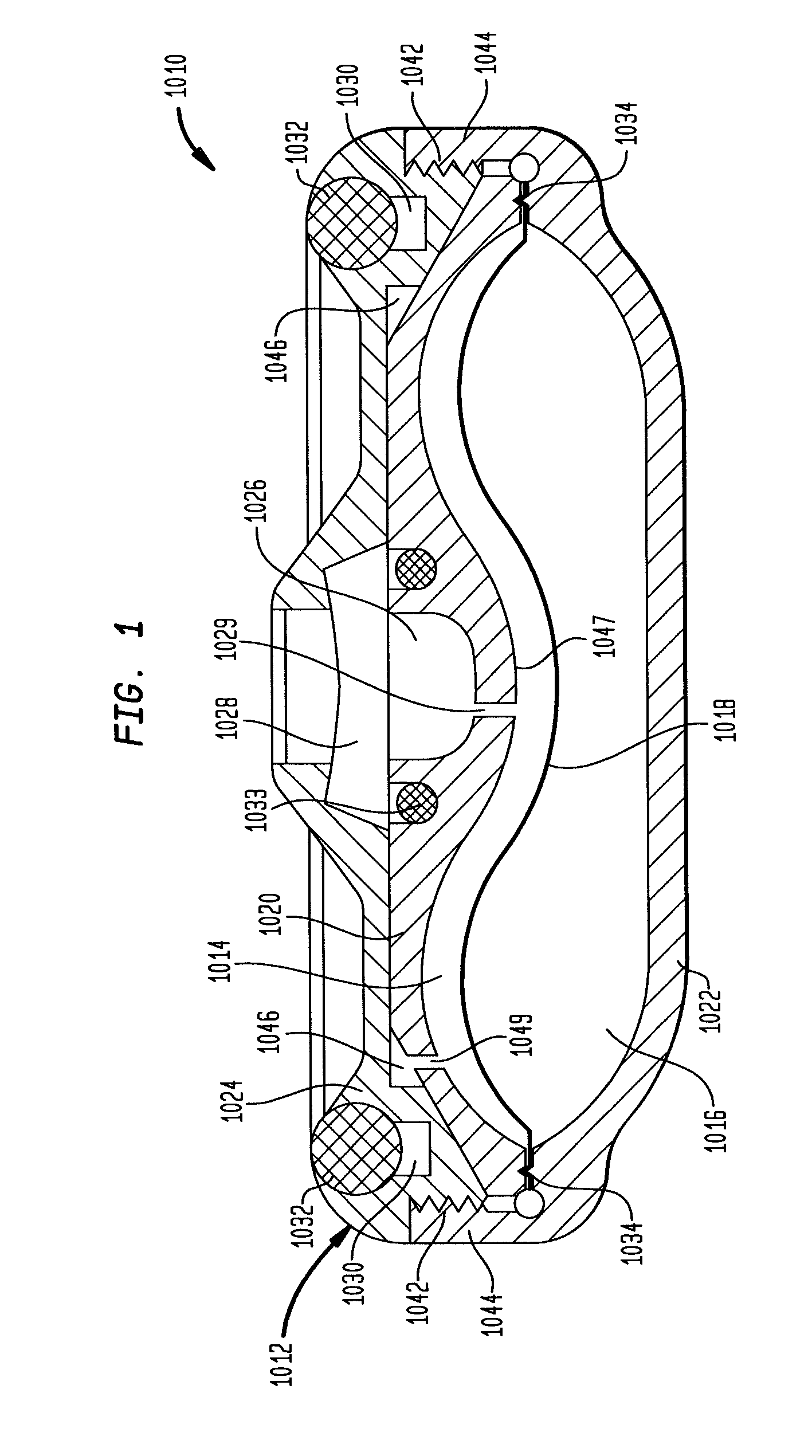
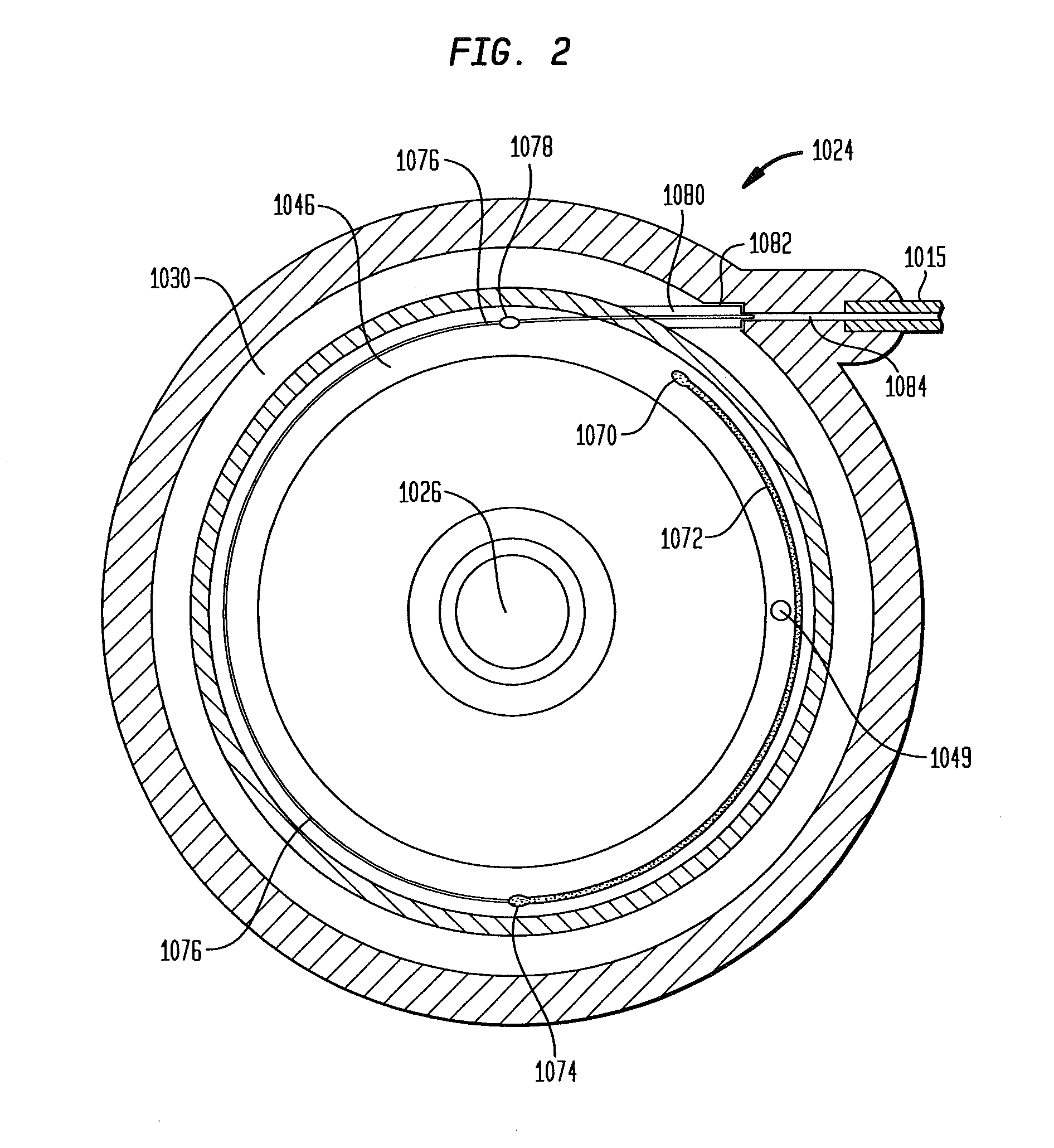
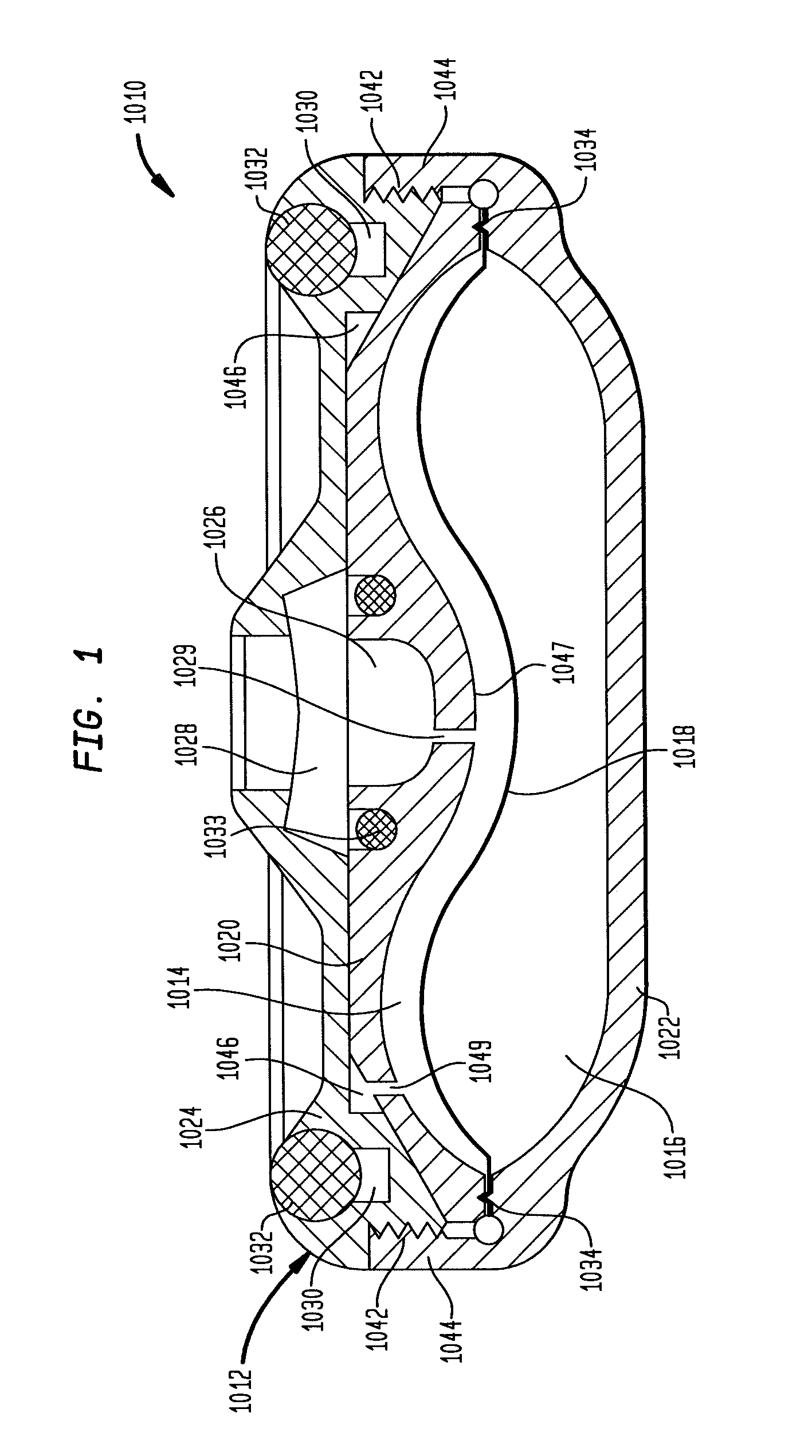
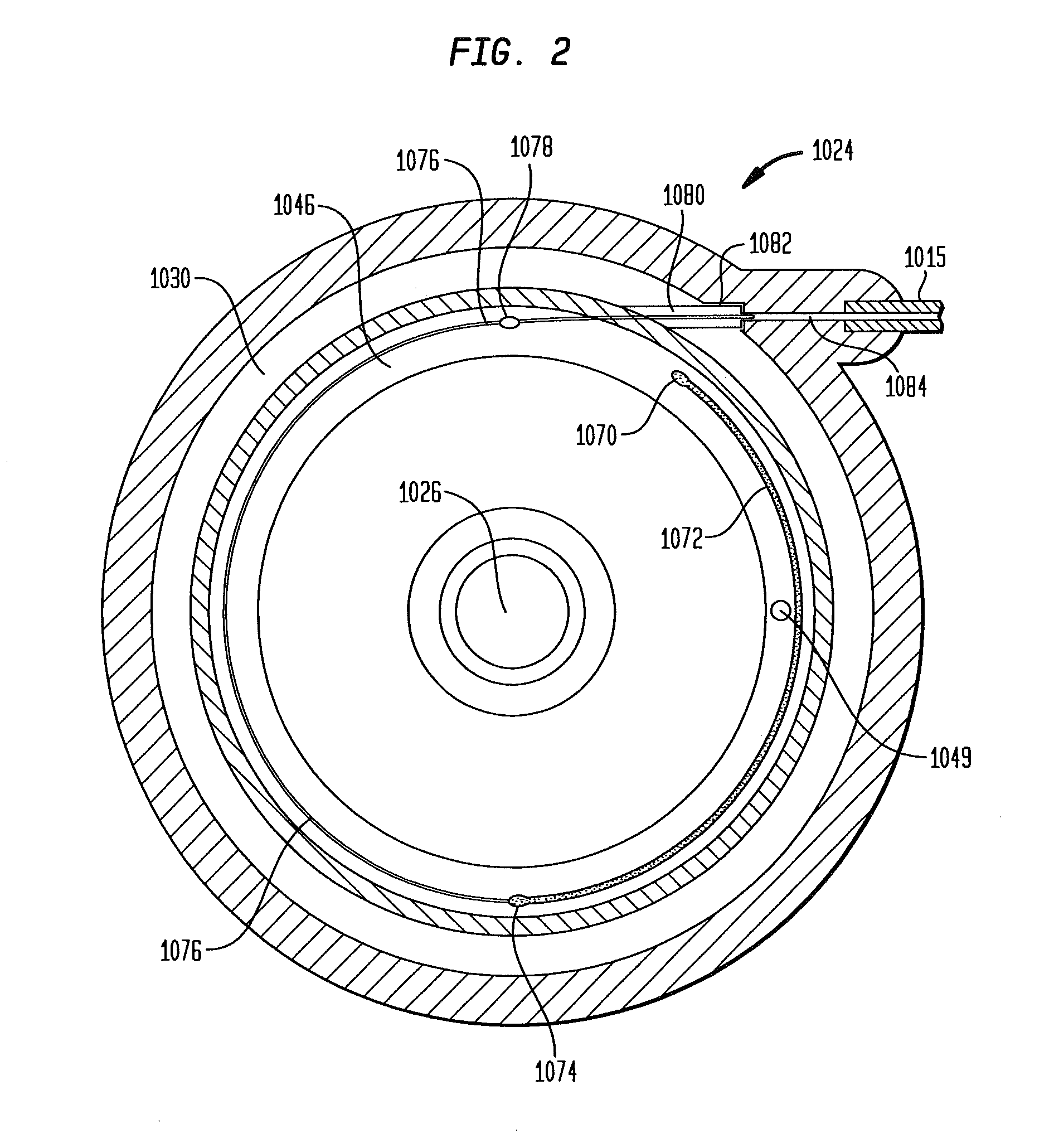
Dynamic hermetic barrier for use with implantable infusion pumps
InactiveUS20070090321A1Stress minimizationOperating means/releasing devices for valvesFlow monitorsFluid compartmentsActuator
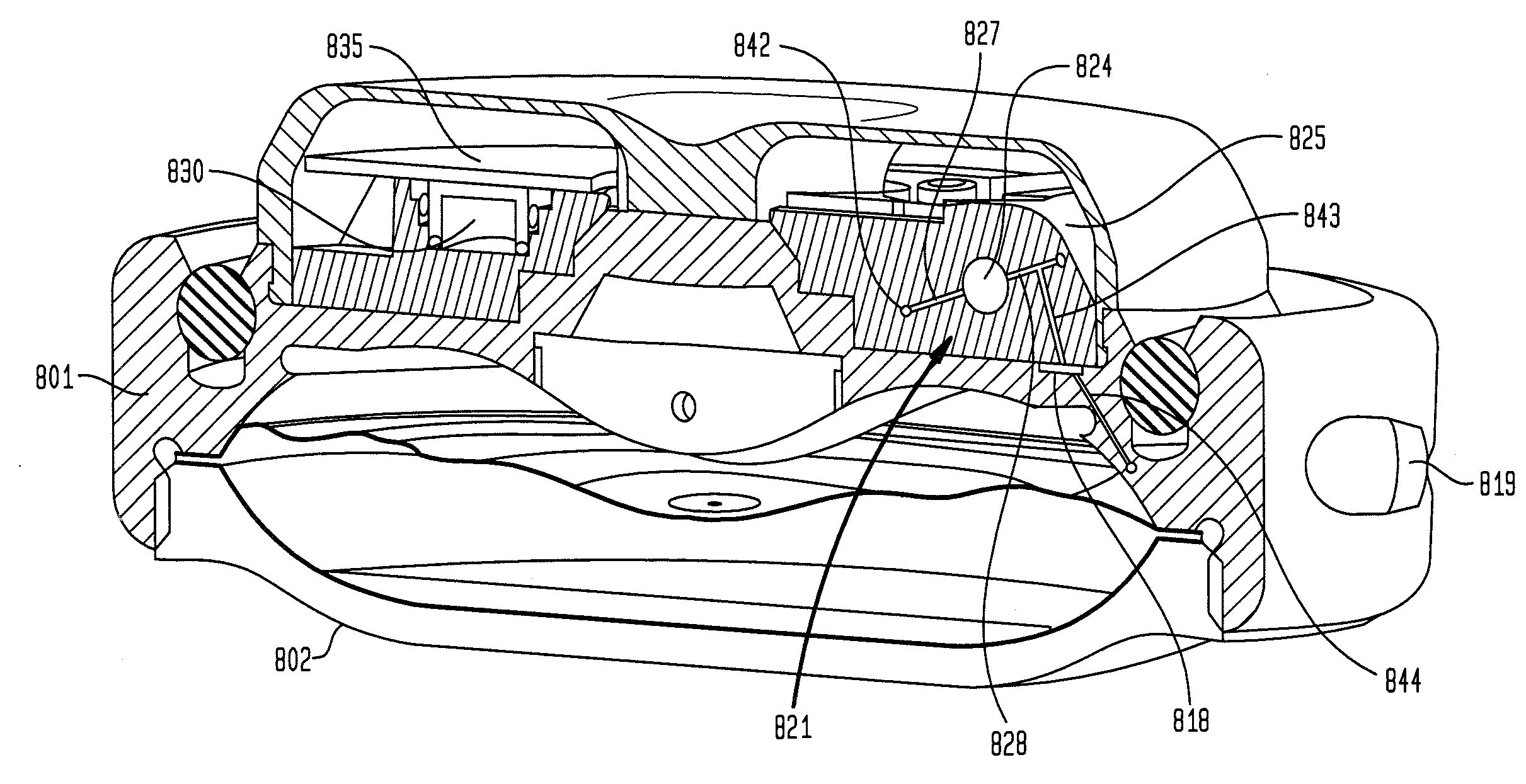
A valve mechanism for use in an implant able infusion pump includes a fluid compartment and a dry-component compartment. The compartments are sealed so that fluid cannot pass between compartments. A flexible membrane is located between the compartments and allows limited mechanical displacement between the compartments, yet prevents any fluid communication therebetween. The fluid compartment includes a valve that is positioned between the inlet chamber and the outlet chamber. The valve includes a movable trigger member that selectively causes the valve to move between an open position and a closed position. The trigger member is positioned adjacent to the first surface of the membrane. The dry-component compartment includes an actuator, which is positioned against the membrane so that generated movement of the actuator may selectively transfer to the trigger member through non-invasive deformation of the flexible membrane. In this arrangement, the valve located within the hermitically-sealed fluid compartment is effectively controlled from the dry component compartment. The flexible membrane includes at least one deformed region that extends beyond the membrane plane, which can be ripple-shaped or bellows-shaped.
Owner:CODMAN & SHURTLEFF INC
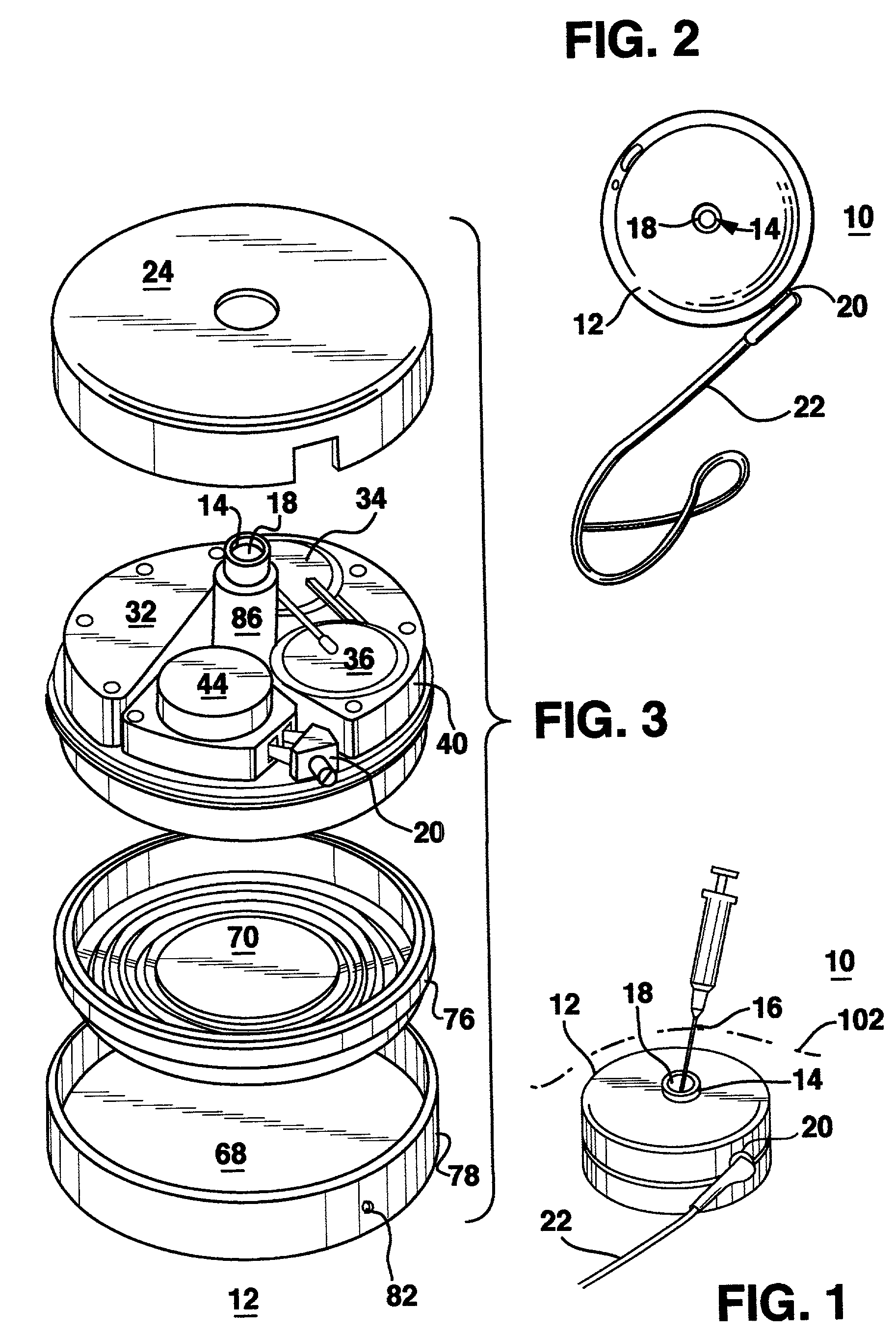
Template system for multi-reservoir implantable pump
A template system for use in conjunction with a multiple reservoir or chambered implantable infusion pump is disclosed. The template system preferably includes at least one template having opening(s) for guiding a needle or syringe to various ports of the multiple reservoir pump. Preferably, each template includes at least two surfaces for cooperating with a like portions of the implantable pump, for properly seating the template on the pump. A kit is also disclosed including three templates for guiding injections into different ports of the pump.
Owner:AQULINX MEDICAL
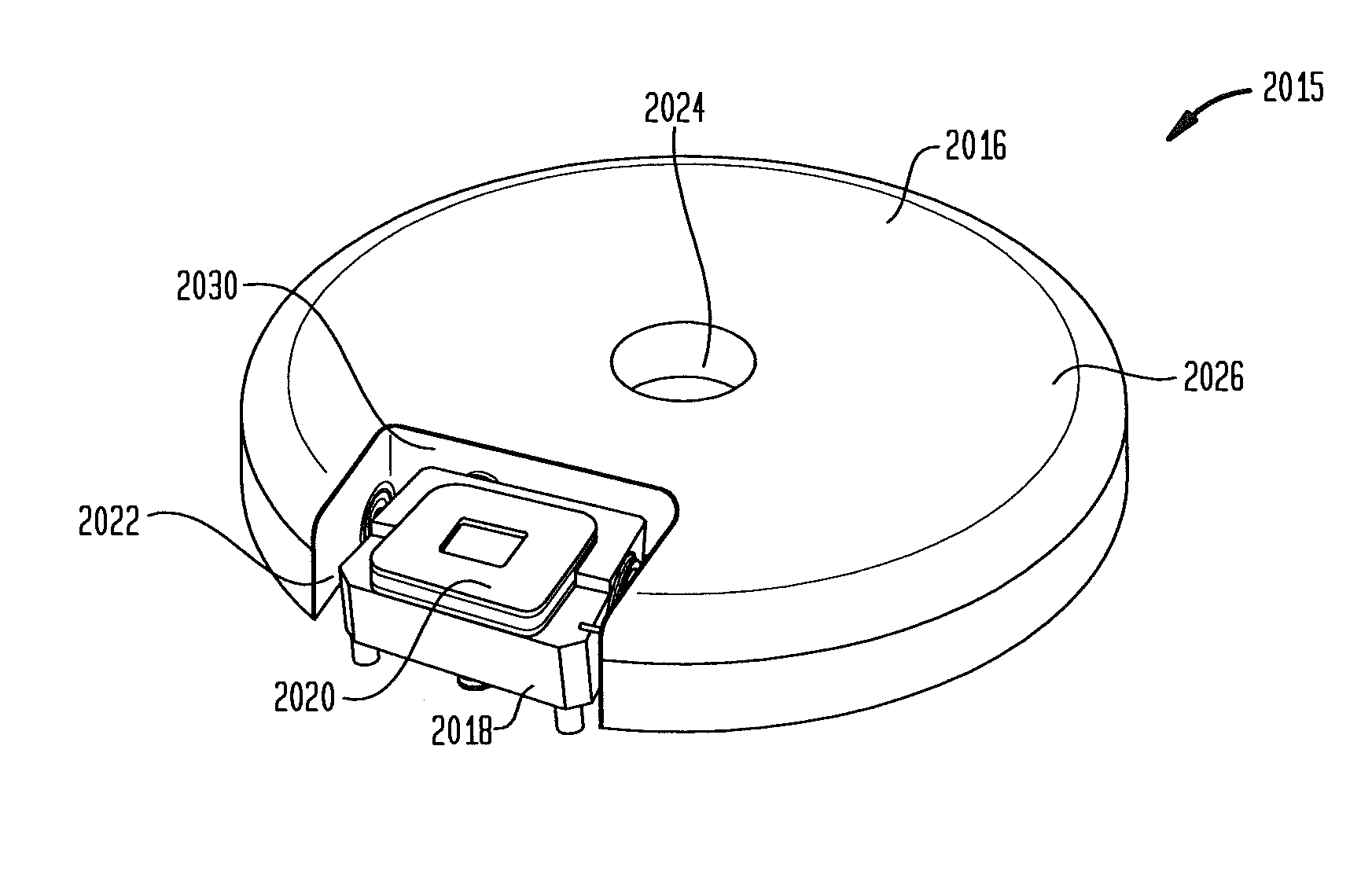
Reduced sized programmable pump
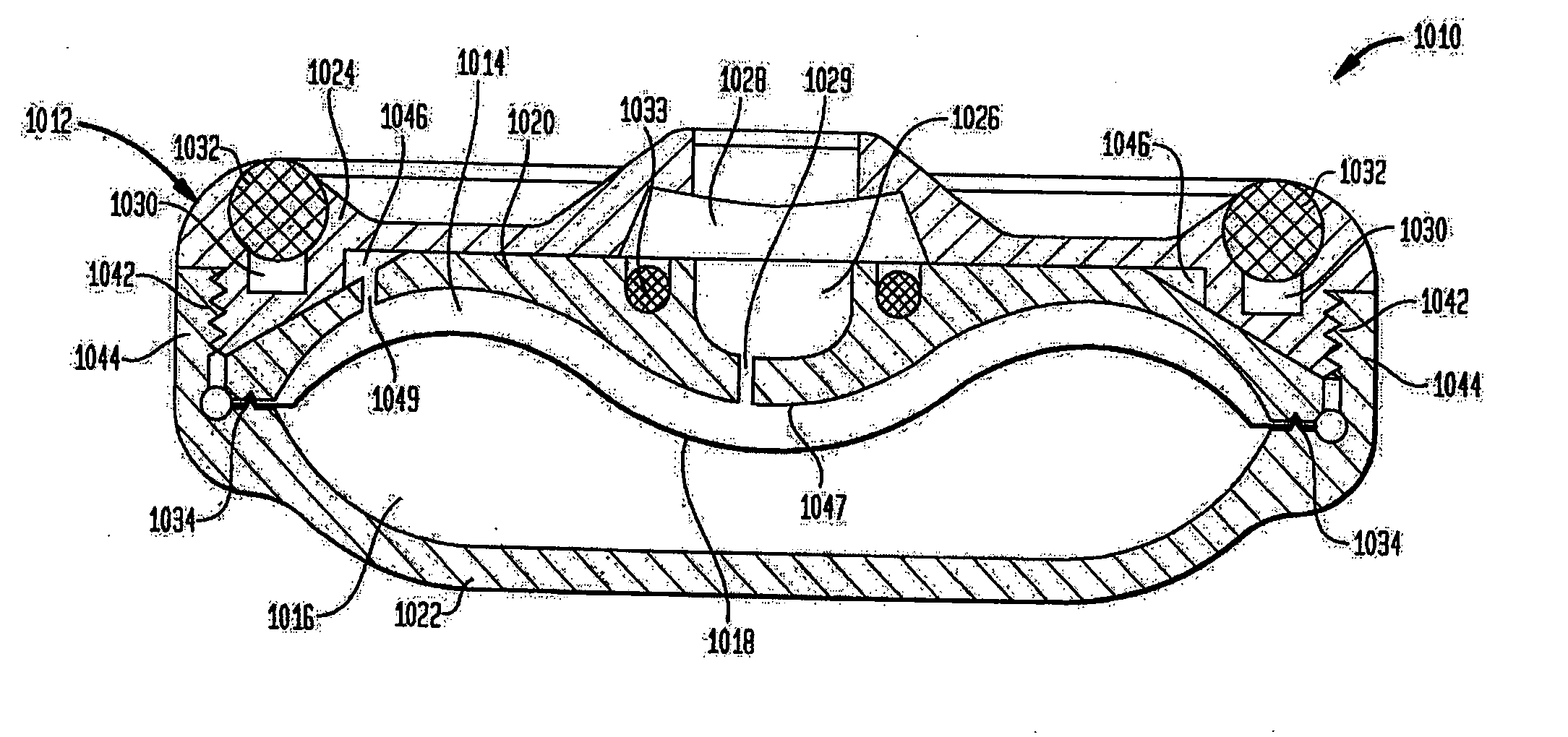
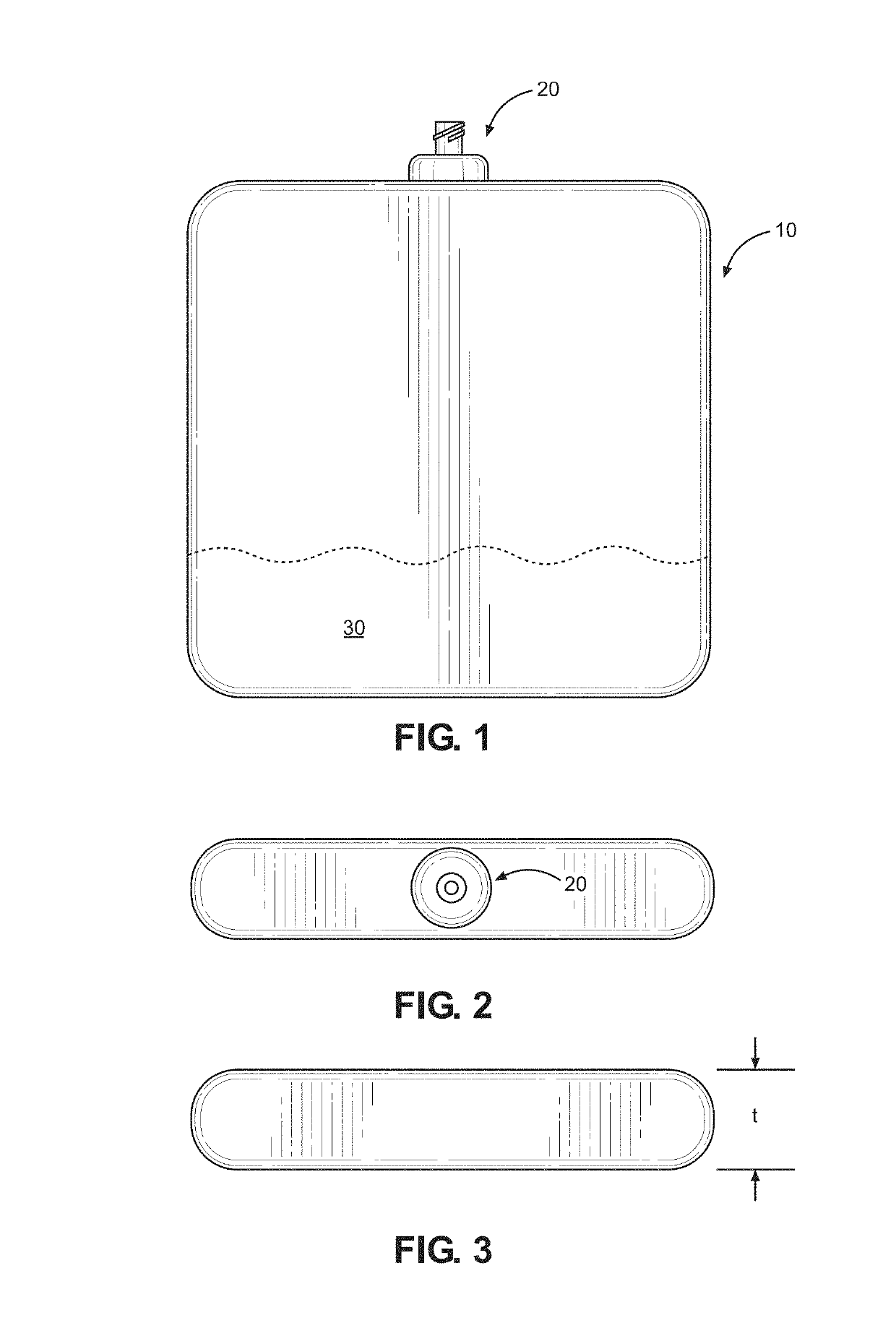
A reduced size implantable infusion pump is disclosed. The pump preferably includes a lower profile pump housing facilitated by specific propellant envelope configurations. For instance, the pump may include one or more c-shaped propellant envelopes that each define active substance and propellant chambers.
Owner:AQULINX MEDICAL
Methods and devices for monitoring fluid of an implantable infusion pump
Methods and devices are described for monitoring reservoir fluid in an implantable infusion pump. Such methods and devices can be used when the infusion pump has a temperature-dependent component. For example, a method of monitoring reservoir fluid can include obtaining measurements from the reservoir that include corresponding data related to the amount of fluid and temperature in the reservoir. A pair of measurements can be used to monitor the fluid level, or flow rate out of, the reservoir when the corresponding temperatures of the measurements are within a temperature tolerance value. In such an instance, a measured amount difference can be calculated based on the fluid amounts corresponding to the pair of measurements. Other variations of fluid monitoring methods, and implantable infusion pump systems that can perform fluid monitoring, are also described.
Owner:MEDOS INT SARL
Non-constant pressure infusion pump
InactiveUS20080125702A1Easy constructionMedical devicesPharmaceutical delivery mechanismPeriod effectsReservoir pressure
Owner:ADVANCED NEUROMODULATION SYST INC
Template system for multi-reservoir implantable pump
InactiveUS20070185470A1Opening closed containersBottle/container closureImplantable infusion pumpsImplantable Pump
A template system for use in conjunction with a multiple reservoir or chambered implantable infusion pump is disclosed. The template system preferably includes at least one template having opening(s) for guiding a needle or syringe to various ports of the multiple reservoir pump. Preferably, each template includes at least two surfaces for cooperating with a like portions of the implantable pump, for properly seating the template on the pump. A kit is also disclosed including three templates for guiding injections into different ports of the pump.
Owner:AQULINX MEDICAL
Variable flow infusion pump system
InactiveUS20120253270A1Increase and decrease flow rateFacilitate communicationInfusion devicesMedical devicesMedicineMechanical engineering
An implantable infusion pump system is disclosed. The pump system preferably includes an implantable pump and a programmable module. The module may provide for varying flow rates of fluid being dispensed from the pump or may provide for a constant flow rate of such fluid. In the case of varying flow rate capabilities, the module preferably includes one or more sensors to determine information relating to the flow rate, electronics for analyzing the flow rate information, and a mechanism for physically altering the flow rate. In certain embodiments, the module includes a hermetically sealed housing. Methods of dispensing a medicament to a patient utilizing such a system are also disclosed, as are variations of the pump system.
Owner:AQULINX MEDICAL
Test method and apparatus for verification of medical device functionality
This invention relates to methods, apparatuses and systems for testing the functionality of the pumping mechanism of a medical device, e.g., an implantable infusion pump, while the medical device is contained within a shipping package. The test apparatus enables such functional verification, without opening the shipping package, when the medical device is still contained within the package that has been appropriately sealed to maintain sterility of the medical device.
Owner:MEDTRONIC MIMIMED INC
Variable flow infusion pump system
InactiveUS8915893B2Increase and decrease flow rateFacilitate communicationMedical devicesPharmaceutical delivery mechanismHermetic sealElectronics
An implantable infusion pump system is disclosed. The pump system preferably includes an implantable pump and a programmable module. The module may provide for varying flow rates of fluid being dispensed from the pump or may provide for a constant flow rate of such fluid. In the case of varying flow rate capabilities, the module preferably includes one or more sensors to determine information relating to the flow rate, electronics for analyzing the flow rate information, and a mechanism for physically altering the flow rate. In certain embodiments, the module includes a hermetically sealed housing. Methods of dispensing a medicament to a patient utilizing such a system are also disclosed, as are variations of the pump system.
Owner:AQULINX MEDICAL
Test Method and Apparatus for Verification of Medical Device Functionality
This invention relates to methods, apparatuses and systems for testing the functionality of the pumping mechanism of a medical device, e.g., an implantable infusion pump, while the medical device is contained within a shipping package. The test apparatus enables such functional verification, without opening the shipping package, when the medical device is still contained within the package that has been appropriately sealed to maintain sterility of the medical device.
Owner:MEDTRONIC MIMIMED INC
Kit and method of reducing human error during implanted infusion pump refilling
ActiveUS10434248B1Improve securityMedical devicesPressure infusionProtein detectionVisual perception
Owner:AMNEAL PHARMA
Test method and apparatus for verification of medical device functionality
Owner:MEDTRONIC MIMIMED INC
Template system for multi-reservoir implantable pump
Owner:AQULINX MEDICAL
Methods and systems for providing therapies into the pericardial space
Owner:MEDTRONIC INC
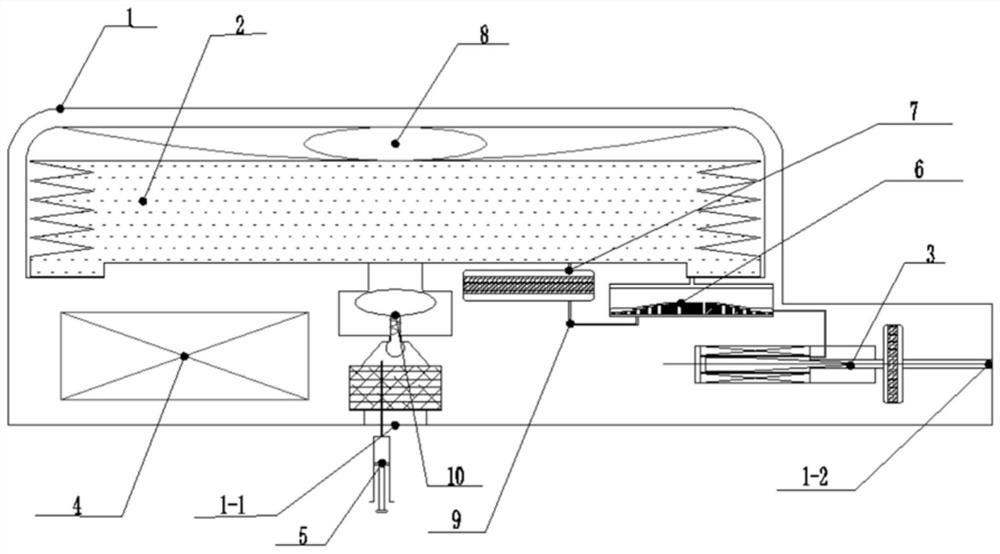
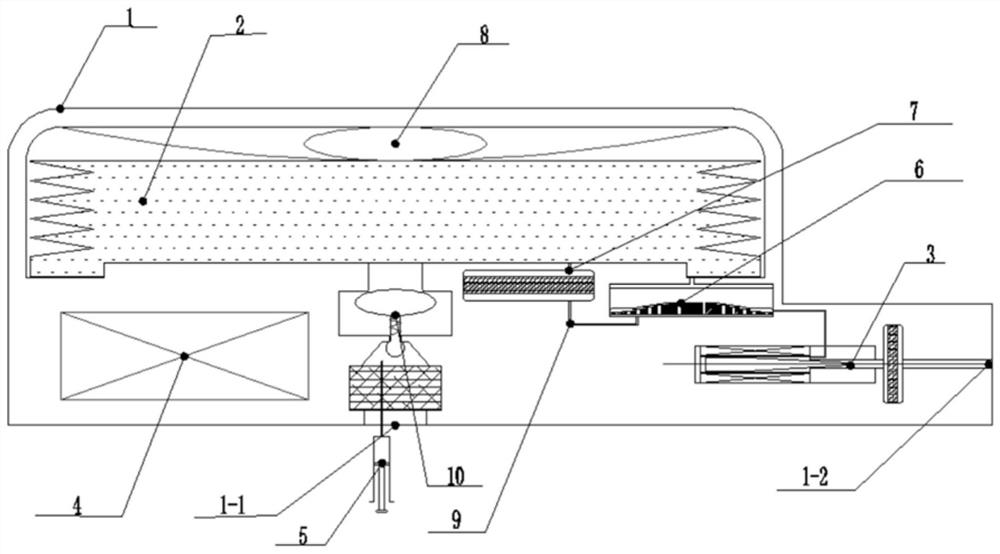
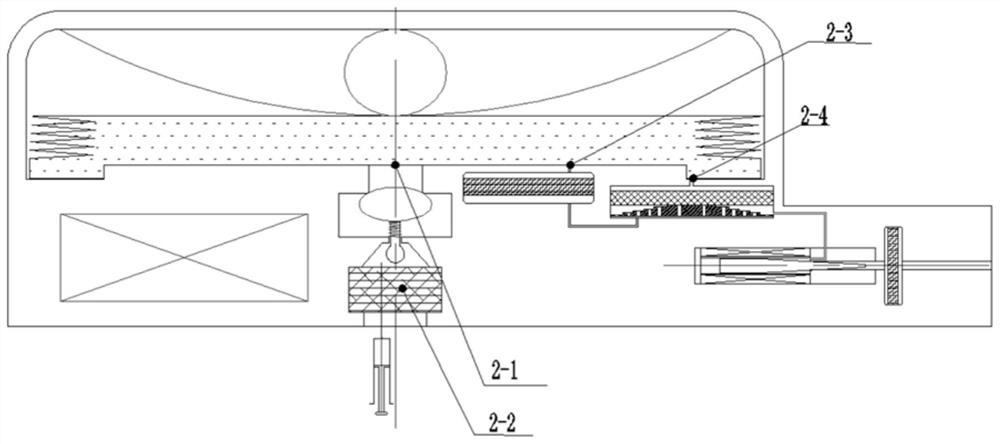
Implantable infusion pump
PendingCN114652917AExtended service lifeReduce volumeMedical devicesPressure infusionMedical equipmentExternal energy
The invention discloses an implantable infusion pump and belongs to the technical field of microfluid control medical equipment.The implantable infusion pump comprises a shell, an elastic medicine bag capable of storing energy repeatedly, an active flow valve and a pump control module are arranged in the shell, a liquid medicine inlet and a liquid medicine outlet are formed in the shell, the liquid medicine inlet is connected with an inlet of the elastic medicine bag, and the active flow valve is connected with the pump control module. An inlet of the elastic medicine bag is provided with sealing silica gel, an outlet of the elastic medicine bag is connected with the liquid medicine outlet of the shell through the active flow valve, and the pump control module is in control connection with the active flow valve. Power comes from positive pressure generated when liquid medicine is stored in the elastic medicine bag, micro-flow adjustment is conducted through the active flow valve, medicine is stably output, external energy is not needed, continuous power supply of a battery is not needed in the medicine output period, and therefore consumption of the battery is greatly reduced, and the service life of the pump is greatly prolonged; the safety valve is used for protecting the pressure of the elastic medicine bag from exceeding the set pressure, so that the use safety is improved.
Owner:北京安诺流控科技有限公司
Emergency management implantable drug delivery systems
ActiveUS20210330884A1Reduce riskImprove delivery capabilitiesOrganic active ingredientsPeptide/protein ingredientsDrug overdosePharmaceutical drug
An implantable emergency management drug delivery system configured to deliver a medicament to reverse the effects of a drug overdose. The implantable emergency management drug delivery system including an implantable infusion pump configured to infuse a first medicament, and an emergency handling device having at least one physiological sensor configured to monitor a condition of a patient for a possibility of an overdose from the first medicament, a communication module configured to communicate the possibility of an overdose to the implantable infusion pump, and an implantable medicament delivery mechanism configured to deliver a second medicament to reduce one or more adverse physiological effects of the first medicament.
Owner:MEDTRONIC INC
Kit and method of reducing human error during implanted infusion pump refilling
Protein detection kit usable with implanted infusion pumps. The kit includes at least one container containing a protein-detecting composition and having an interface for allowing fluid to be introduced and a visual indicating mechanism for providing a visual indicator indicative of protein detection.
Owner:AMNEAL PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com