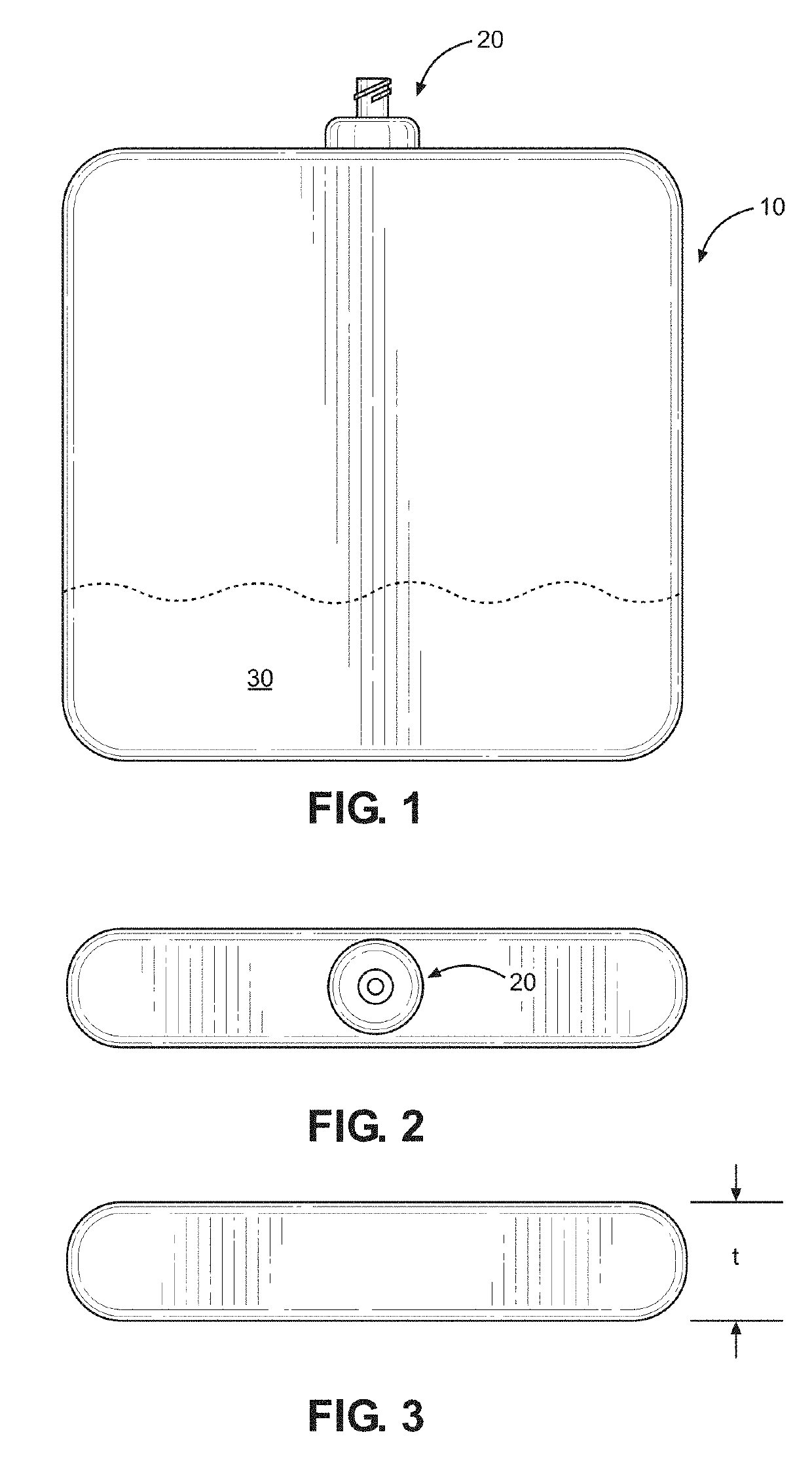
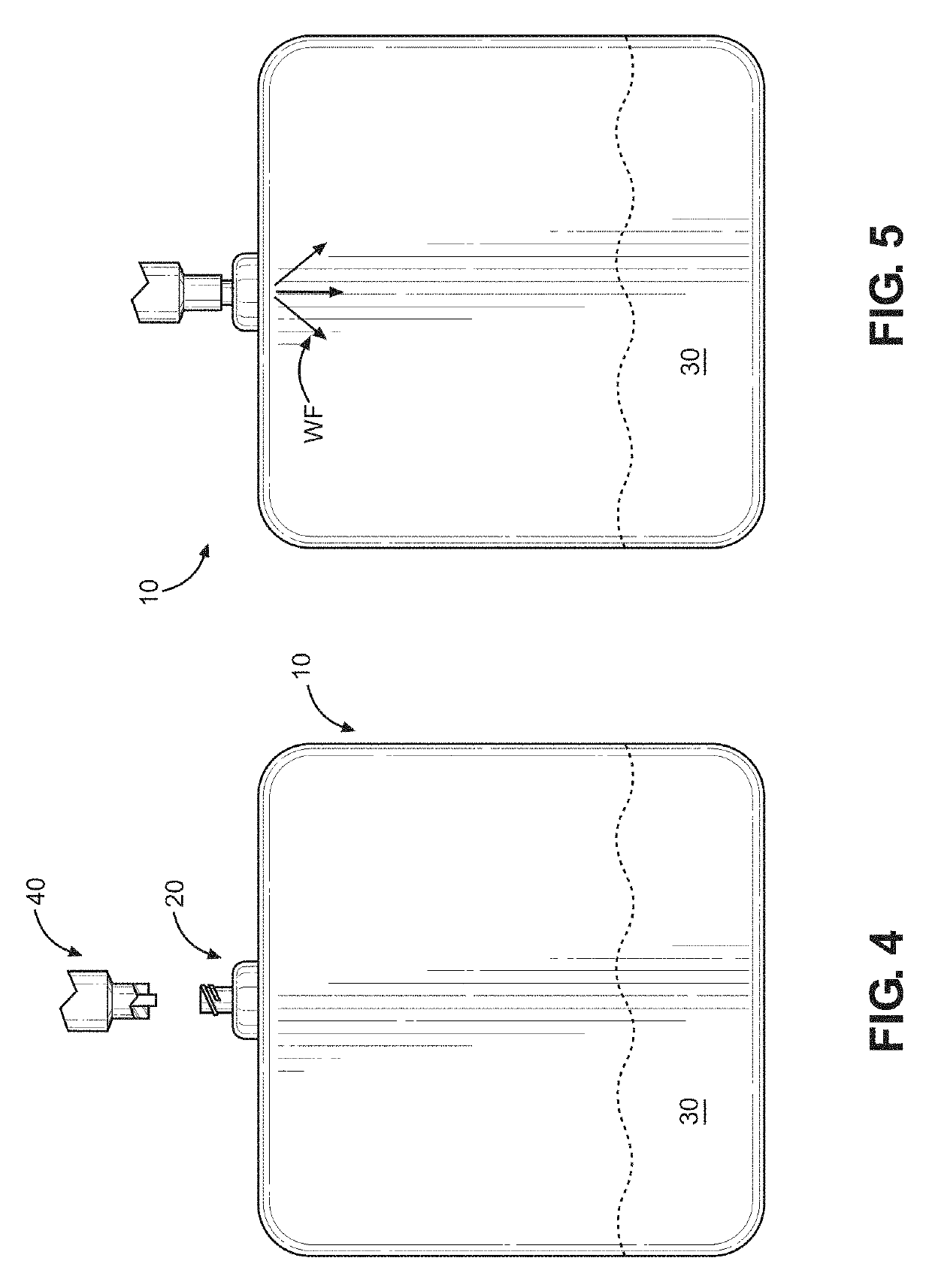
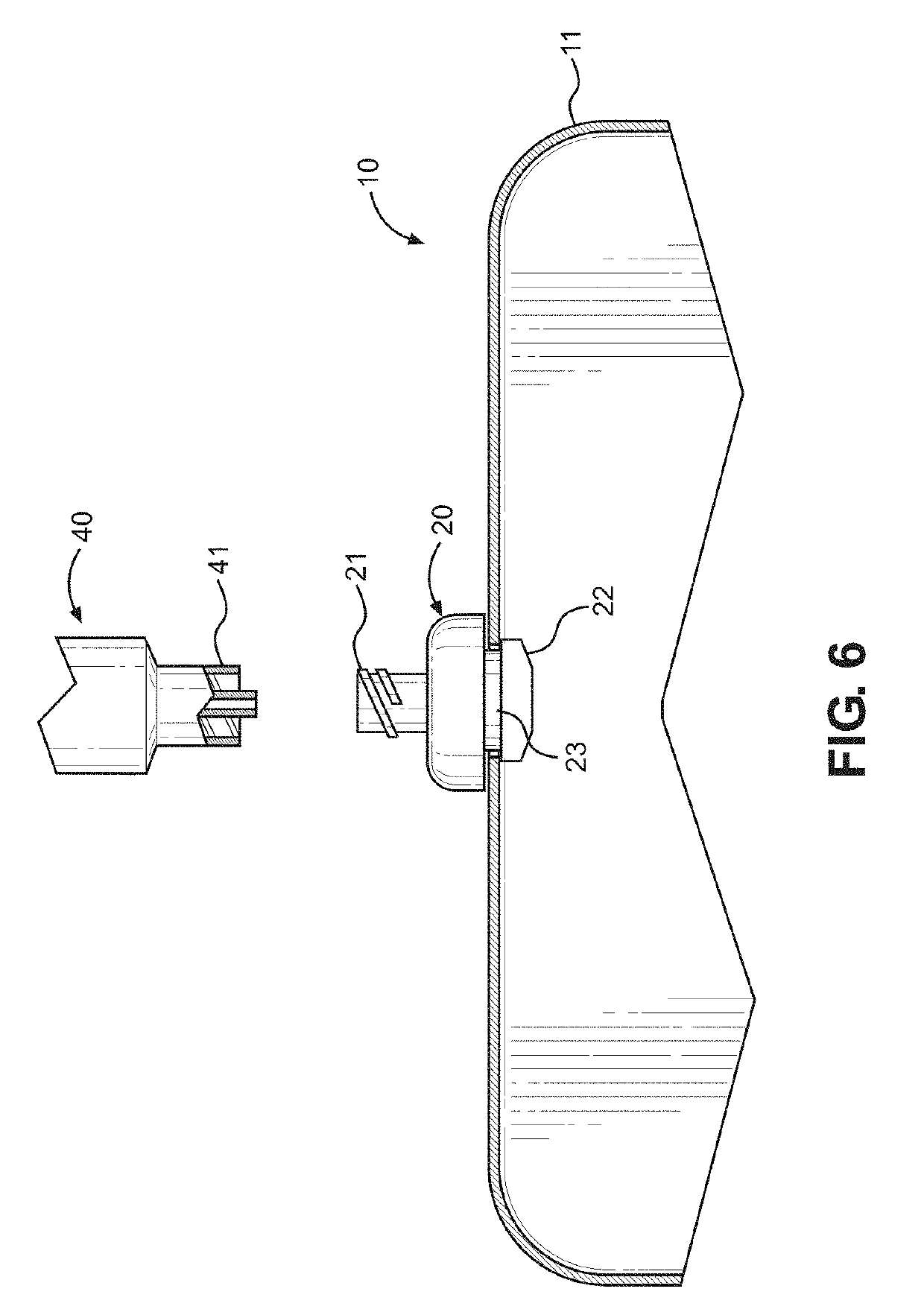
Kit and method of reducing human error during implanted infusion pump refilling
a technology for implanted infusion pumps and kits, which is applied in the field of kits or systems for more safely filling implanted infusion pumps, can solve the problems of health care professional failure, withdrawal reactions, complete withdrawal of medication from patients, etc., and achieve the effect of improving the safety of treatmen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0139]Development of a qualitative colour test for proteins in abdominal body fluid.
[0140]Sensitivity to be reached 0.03 g / mL=30 mg / mL.
[0141]The value of 30 mg / ml was determined as the target value based on reported levels of total protein in peritoneal fluid (normally exudate material is>3.0 g / dl with transudate<3.0 g / dl).
Objectives
[0142](1) Develop formulation for peritoneal fluid
[0143](2) Define appropriate Biuret solution to protein fluid ratio
[0144](3) Define protein operating range and limit of detection for the Biuret solution
[0145](5) Verify stability of the solution
[0146](6) Use of control samples with the Biuret solutions
Technical Summary
[0147](1) Biuret reagent development
[0148]Three different Biuret solutions were tested—with all solutions sensitive enough to visualize 30 mg / mL, of protein. Gornall and Weichselbaum refer to the sources of biuret found in a literature search.
[0149](1.1) Biuret Formulations
[0150]Gornall biuret solution. See FIG. 29.
[0151]Weichselbaum biure...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com