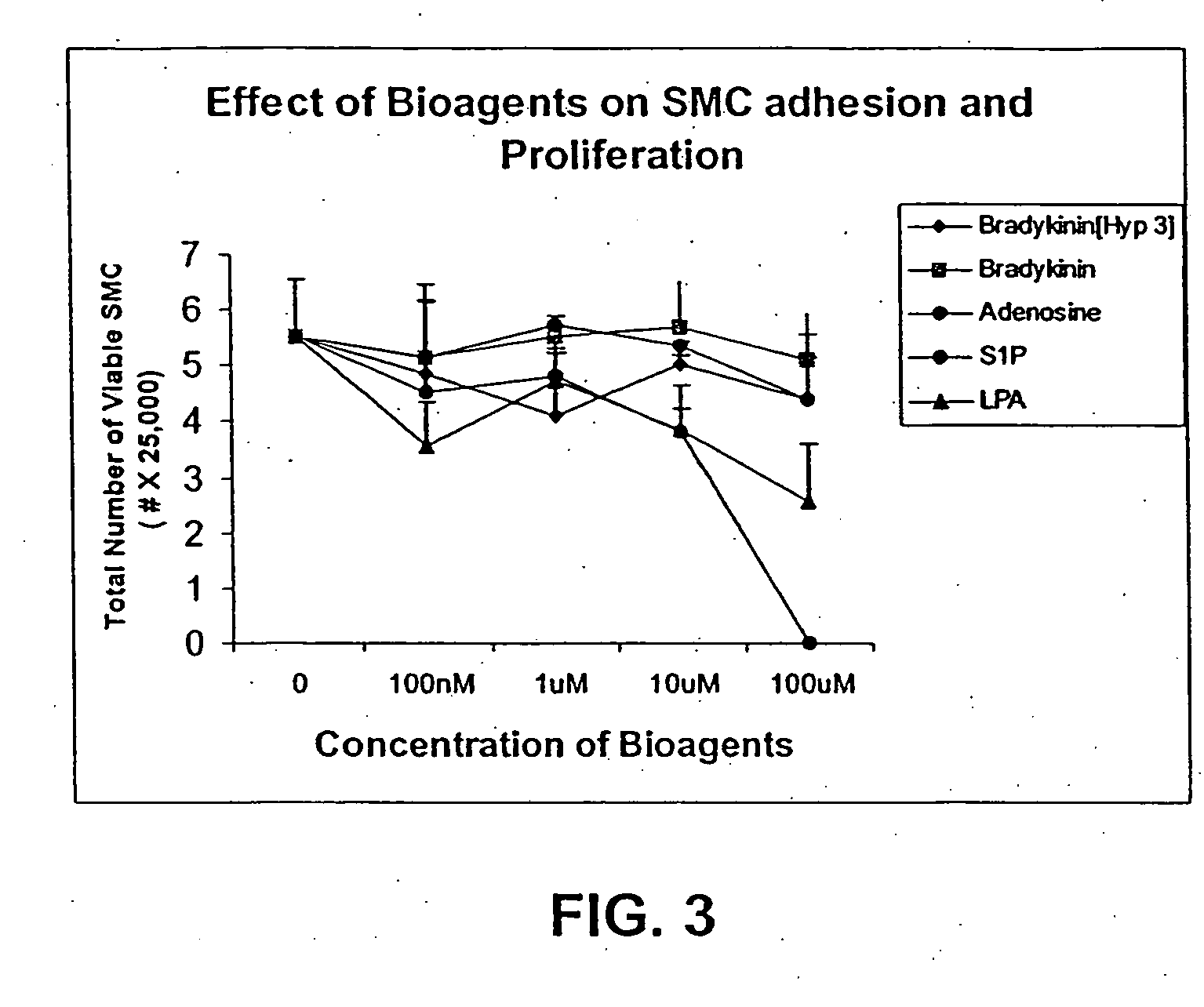
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
328 results about "Implant surgery" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
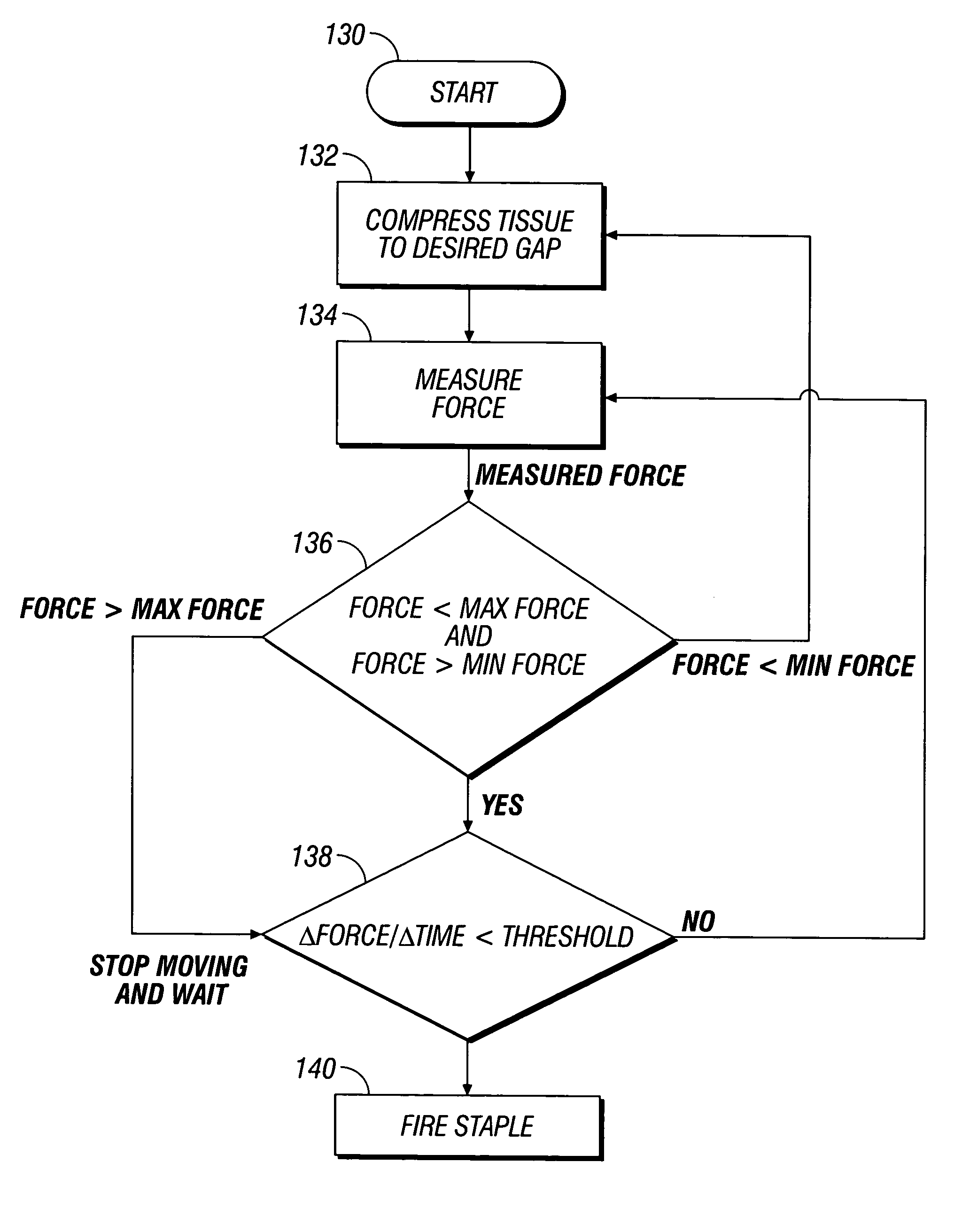
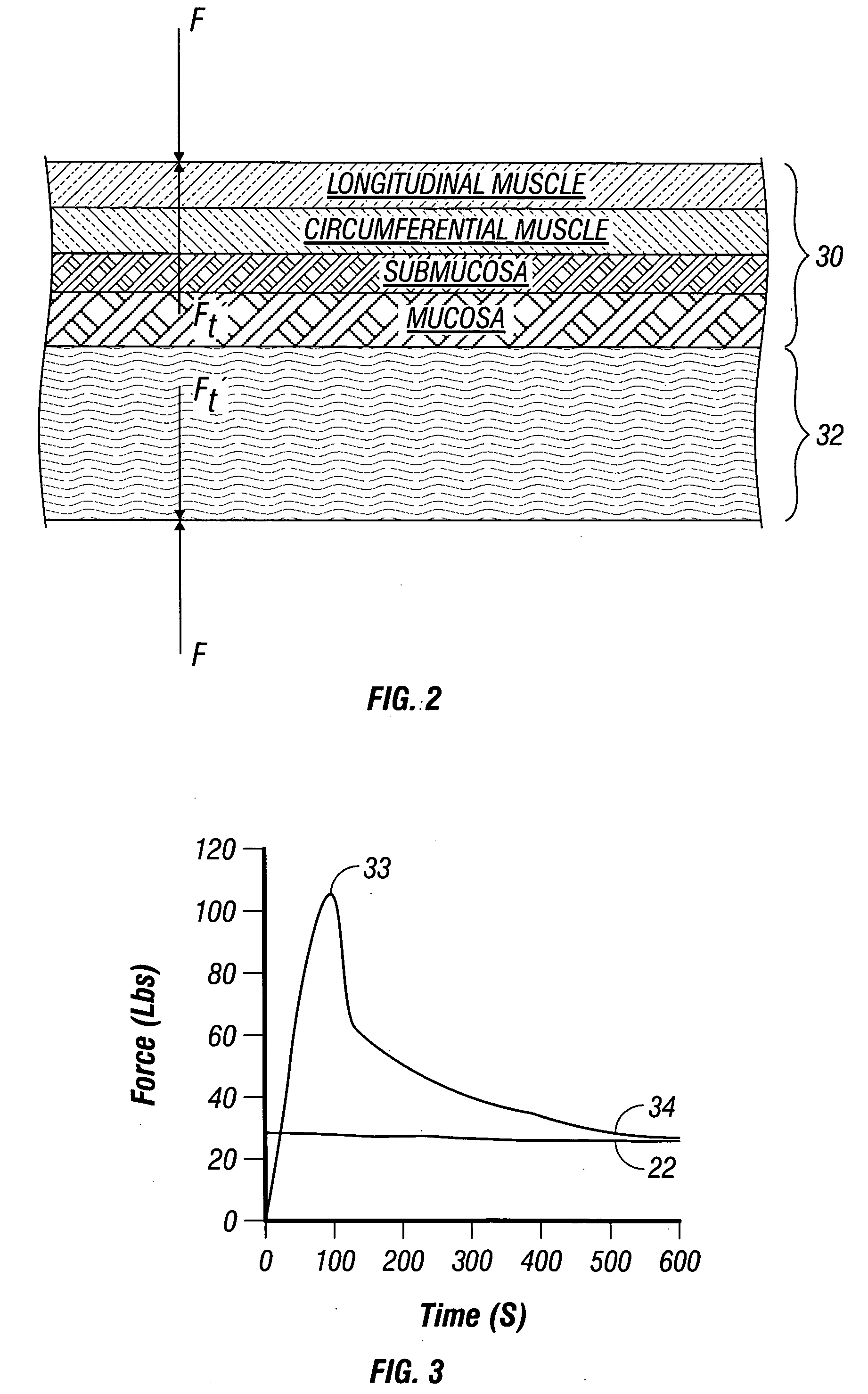
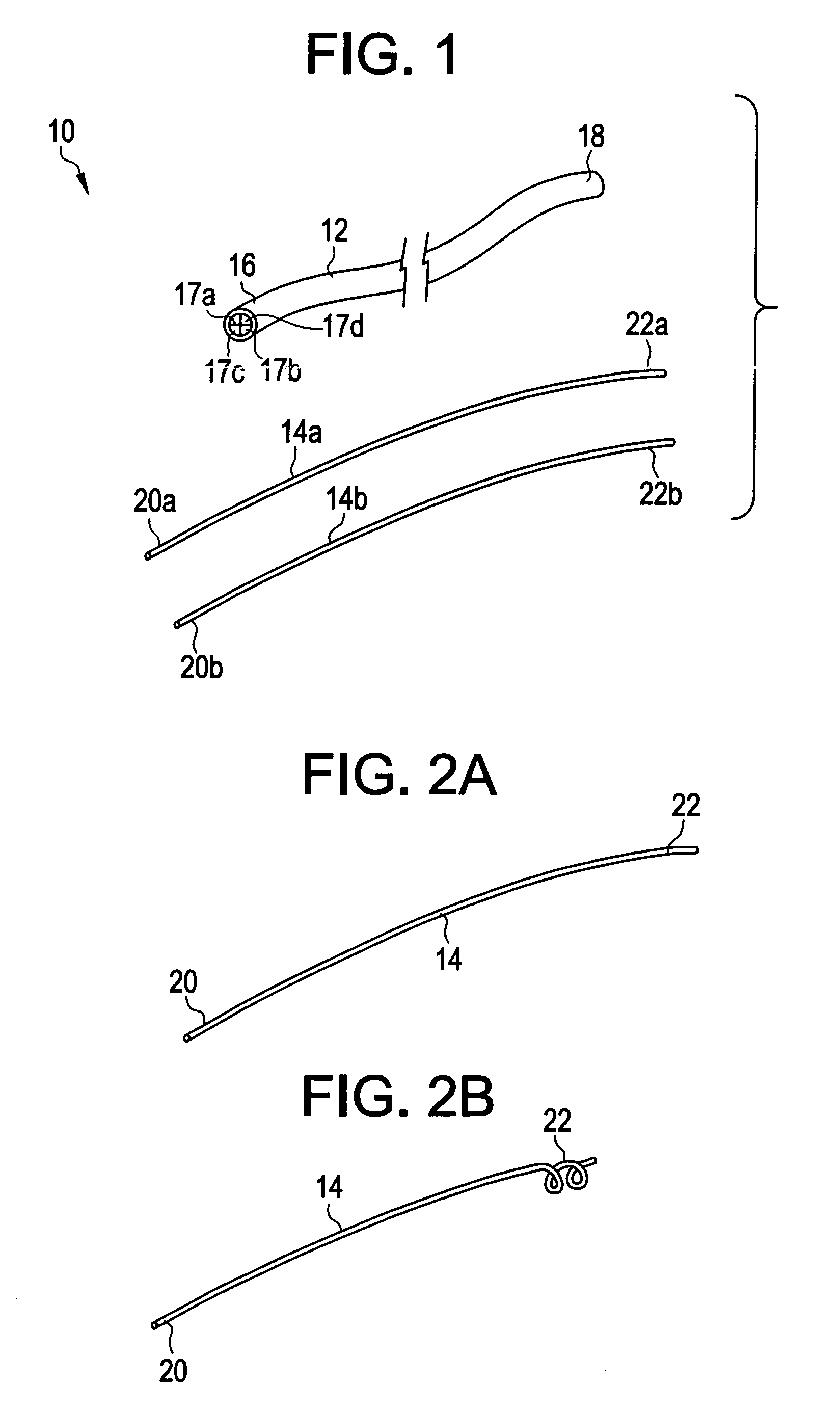
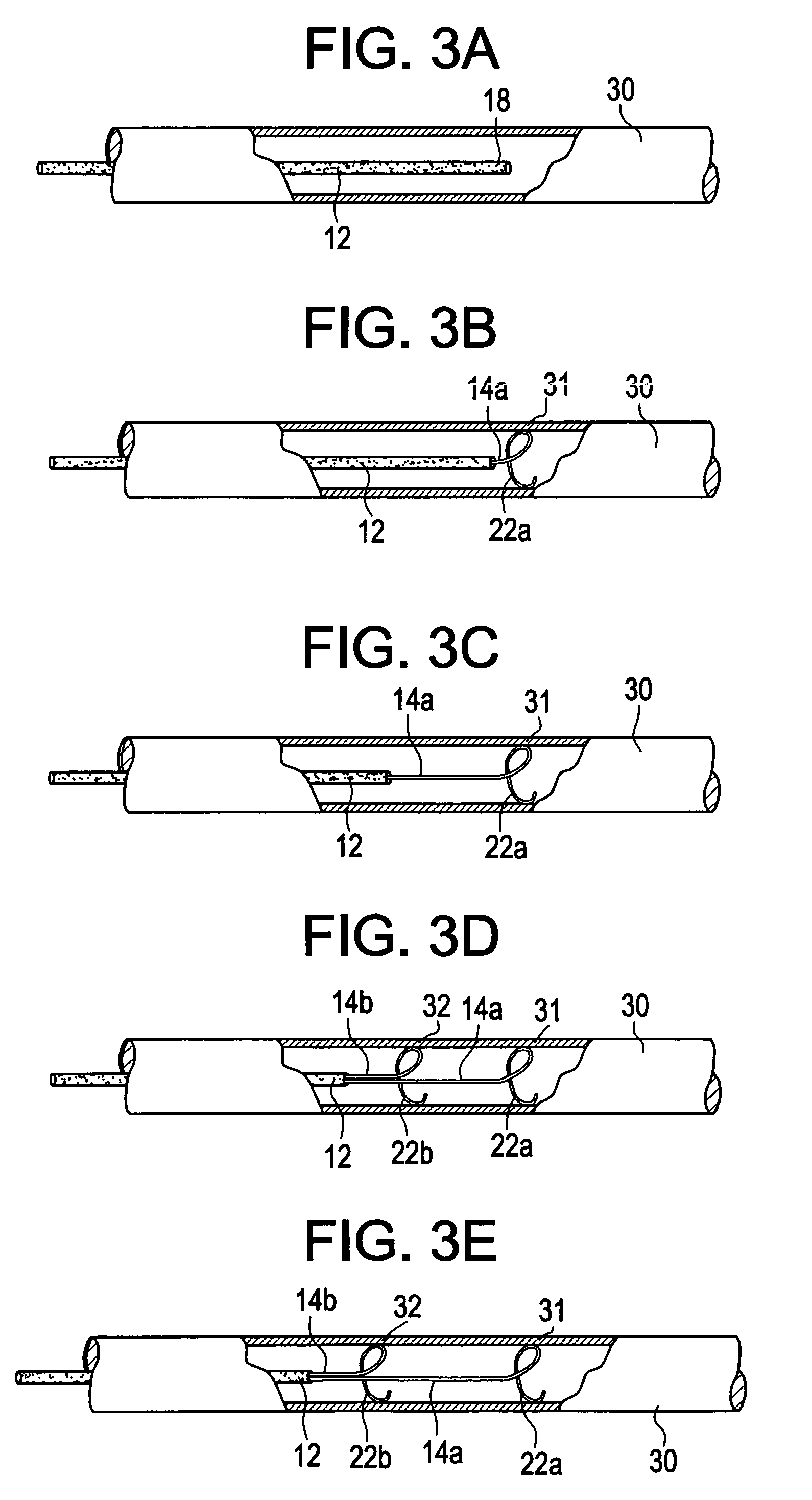
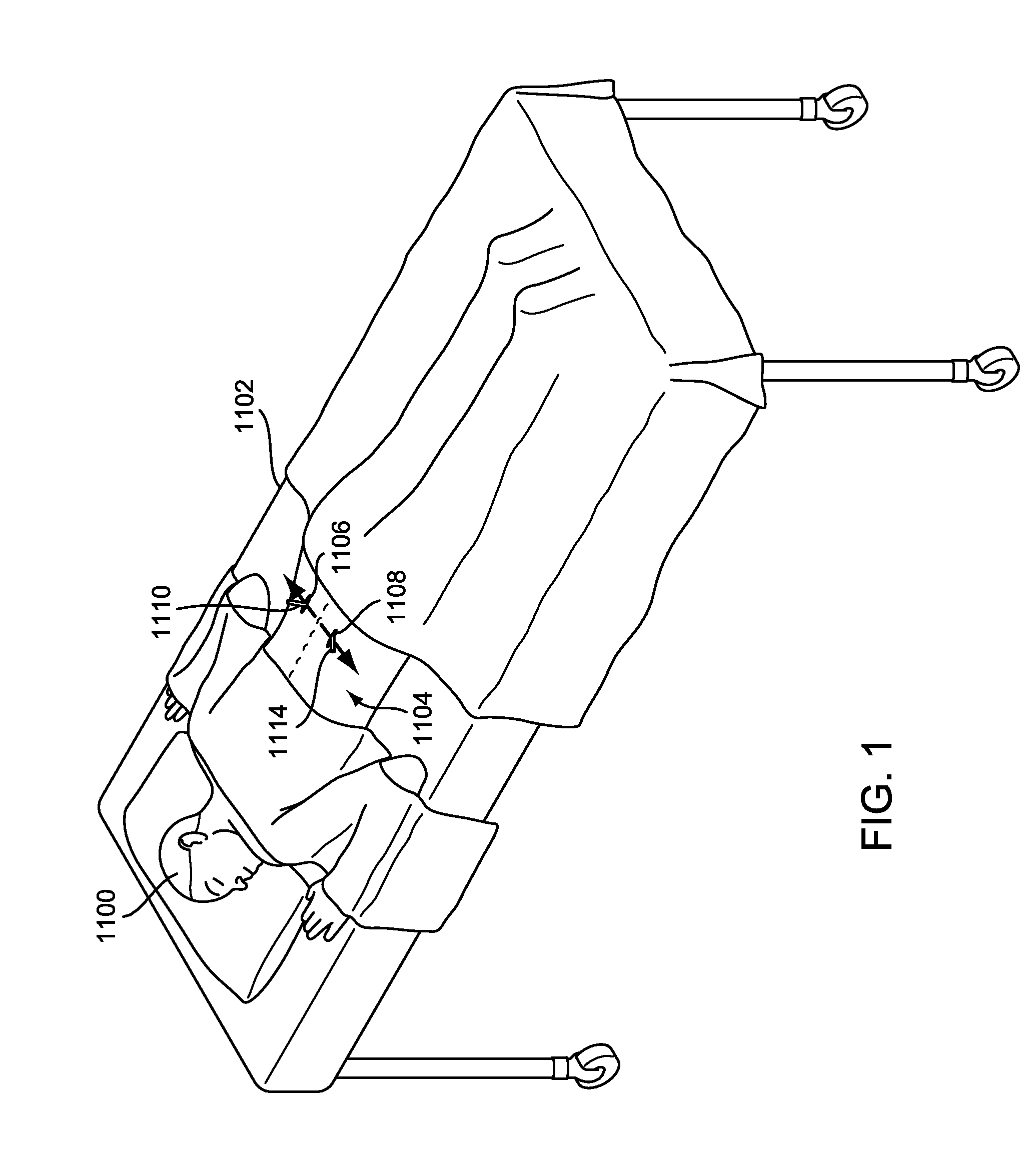
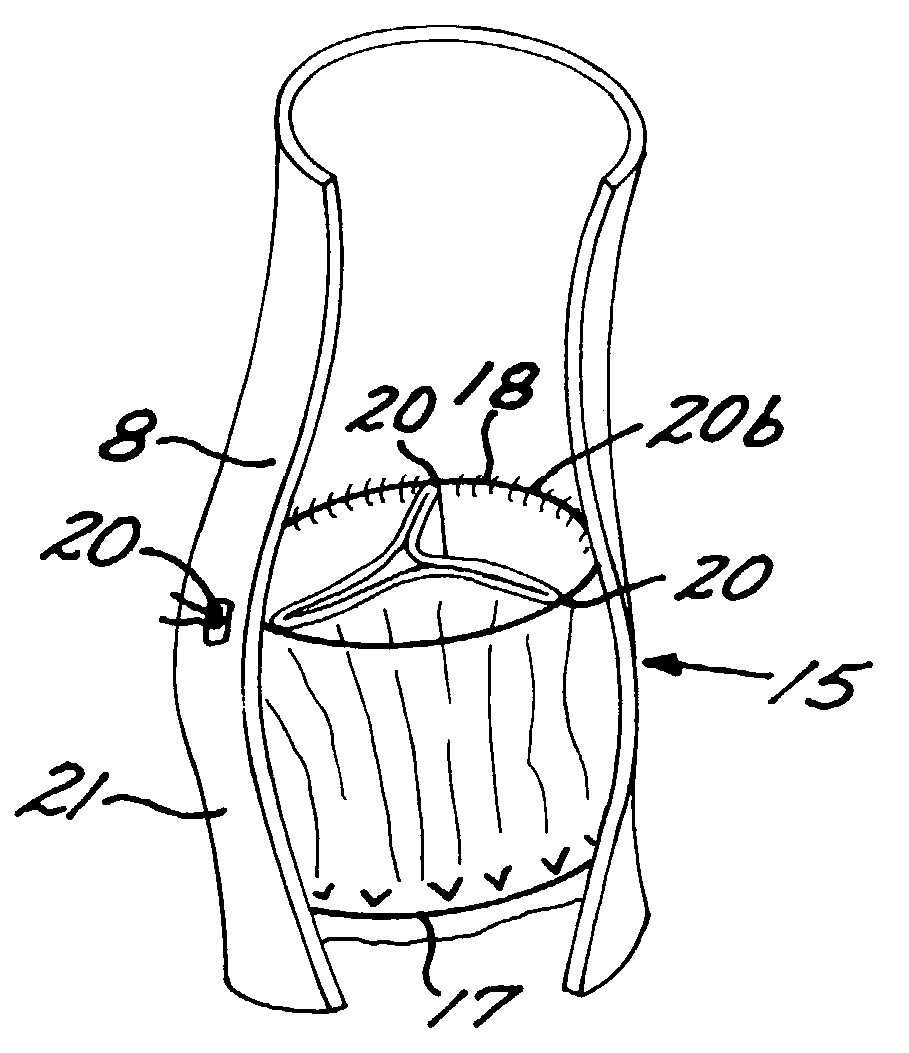
Method and system to determine an optimal tissue compression time to implant a surgical element
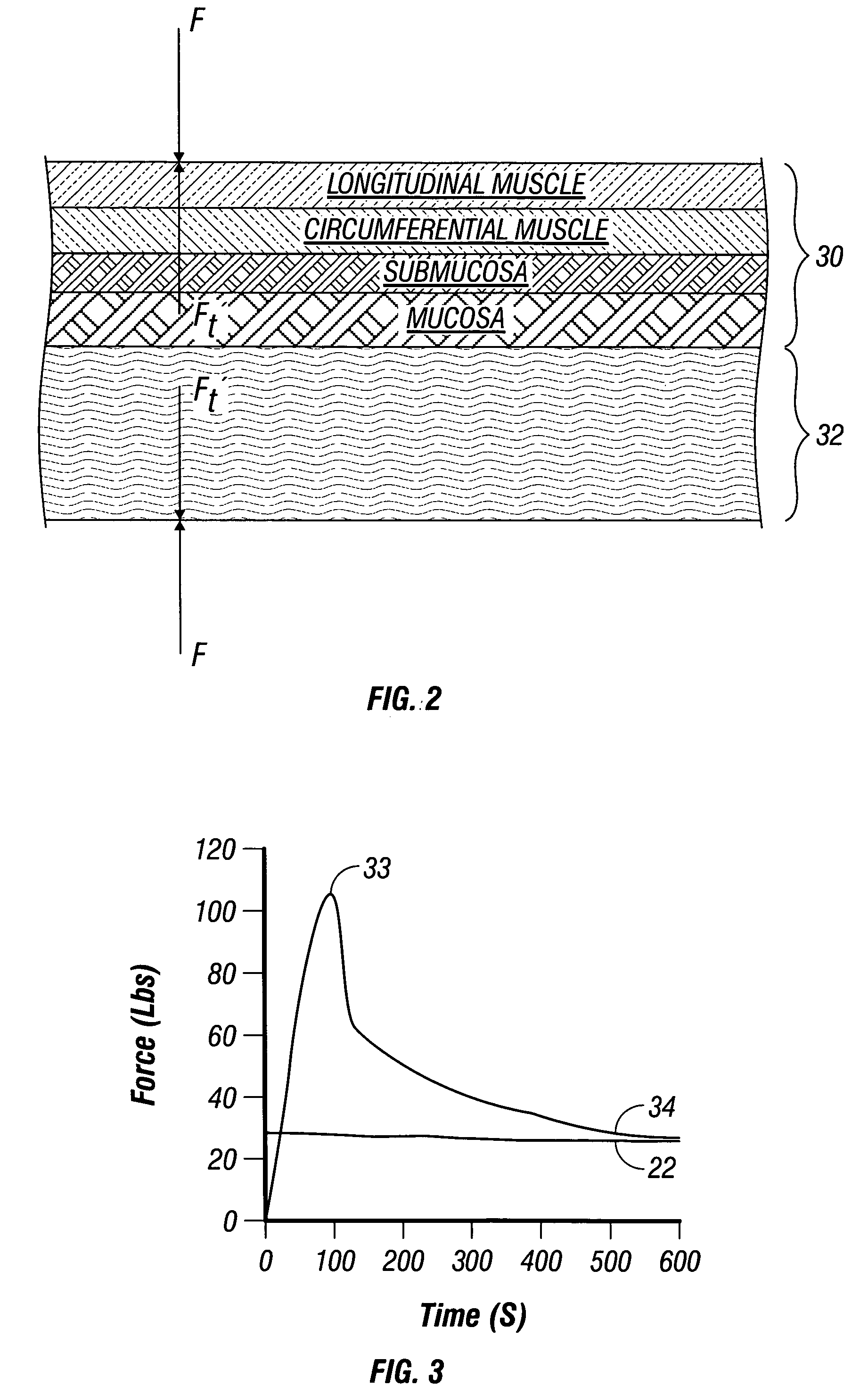
A method for determining an optimal compression of tissue to apply a surgical element has the steps of applying a load to the tissue. The method has the step of determining a reactive load applied by the tissue in response to the load. The method also has the step of determining the reactive load per unit time for a predetermined time period and determining a slope of the reactive load per unit time. The method further has the steps of evaluating the slope relative to a predetermined threshold, and signaling when the slope exceeds the predetermined threshold.
Owner:COVIDIEN LP
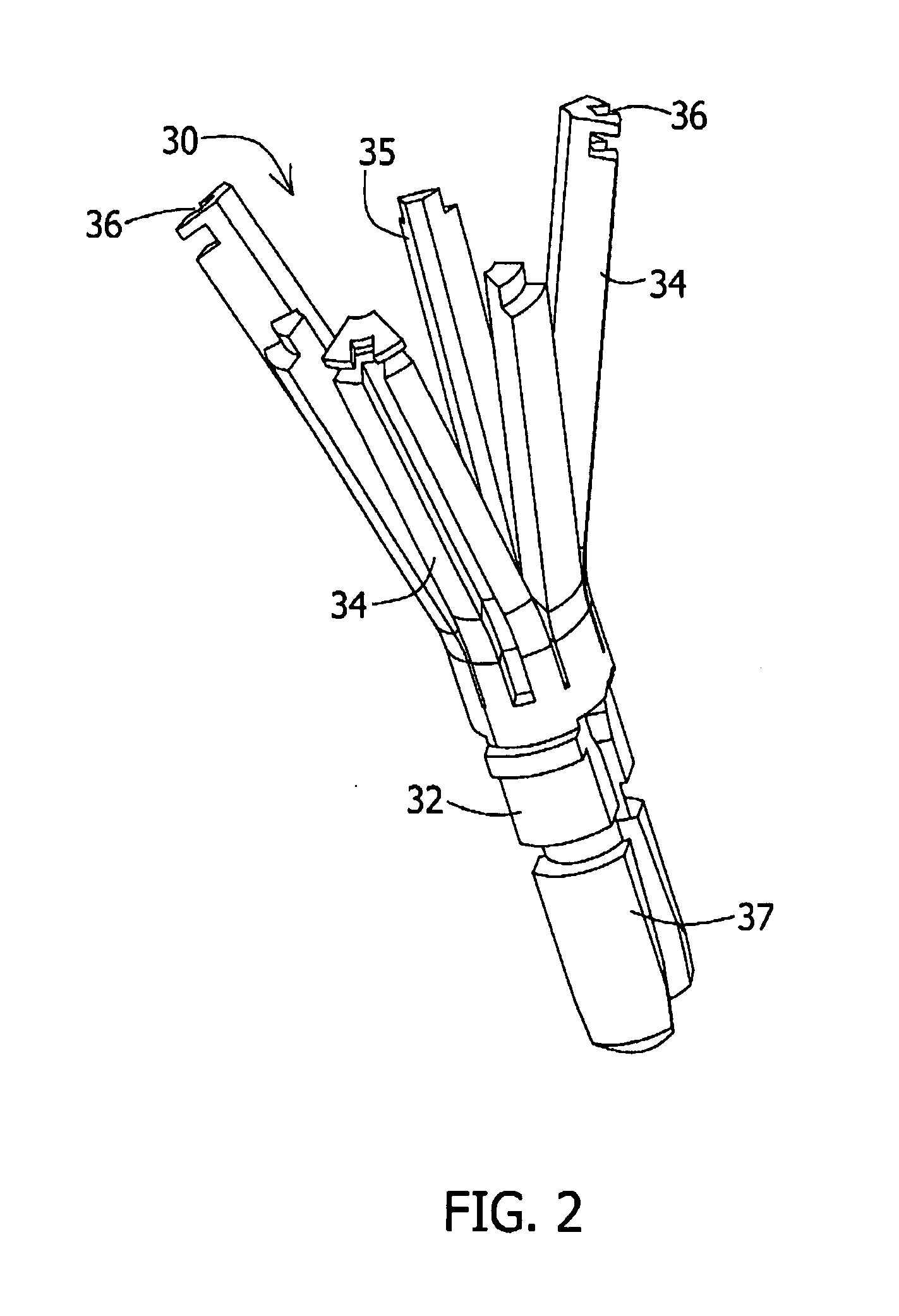
Stent loading tool and method for use thereof
ActiveUS20090054976A1The method is simple and reliableEasy to pushStentsHeart valvesVALVE PORTCatheter device
A loading tool for withdrawing, crimping, and loading a stent-mounted valve into a delivery catheter, and for pushing the stent-mounted valve from the delivery catheter into a native heart valve orifice. The loading tool comprises at least one connector adapted for being removably connected to the stent of the stent-mounted valve. A crimping tool having a generally converging shape is adapted for use with the loading tool. Following connection of the loading tool to the stent-mounted valve, the loading tool operates to allow the stent-mounted valve to be drawn through the crimping tool, and loaded, in a crimped state, into a delivery catheter. Also disclosed is a kit of the of the various components for effecting the delivery of the stent-mounted valve and a method for withdrawing, crimping, and loading a stent-mounted valve from a storage container into a delivery catheter for the performance of a transcatheter valve implantation procedure.
Owner:MEDTRONIC VENTOR TECH
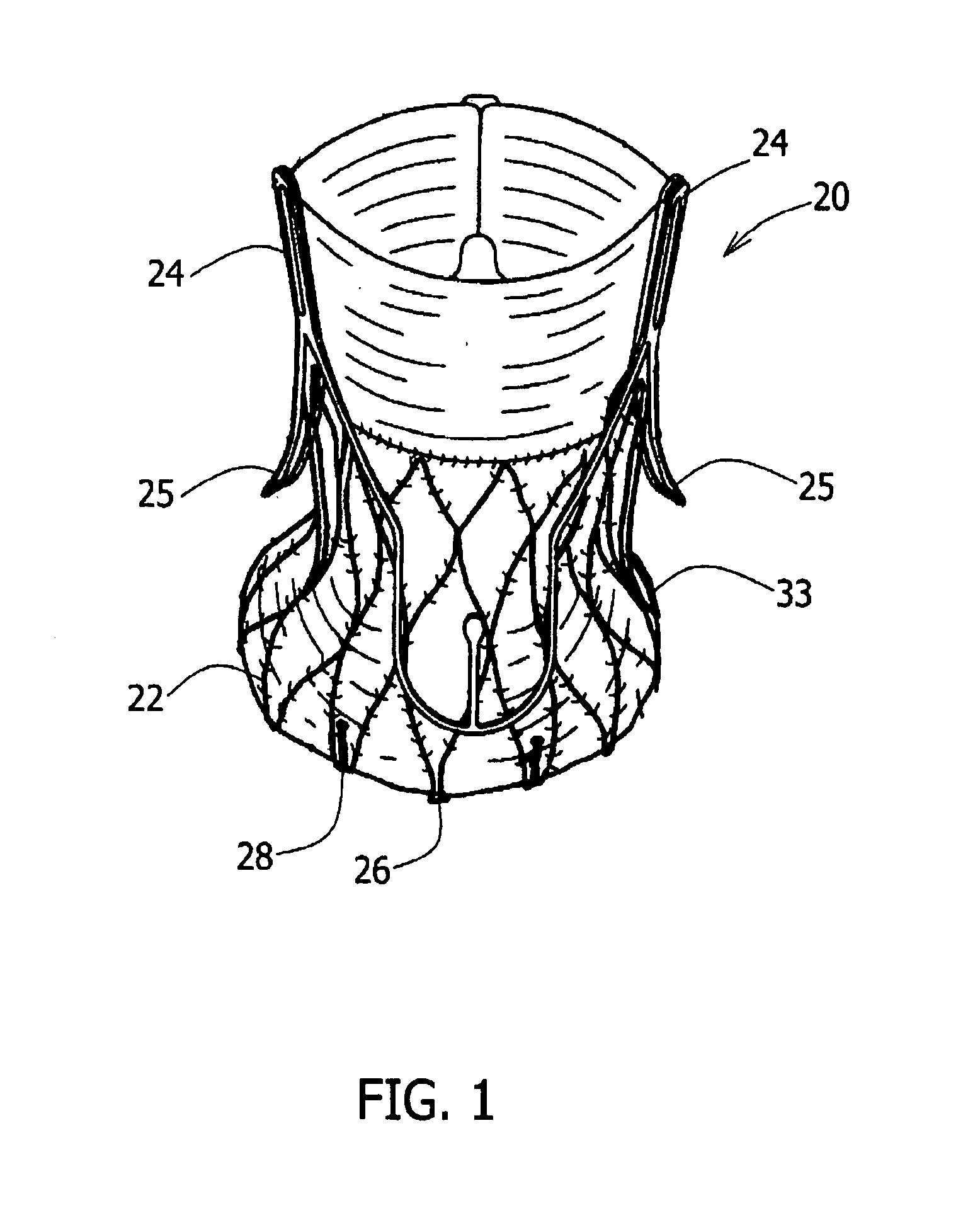
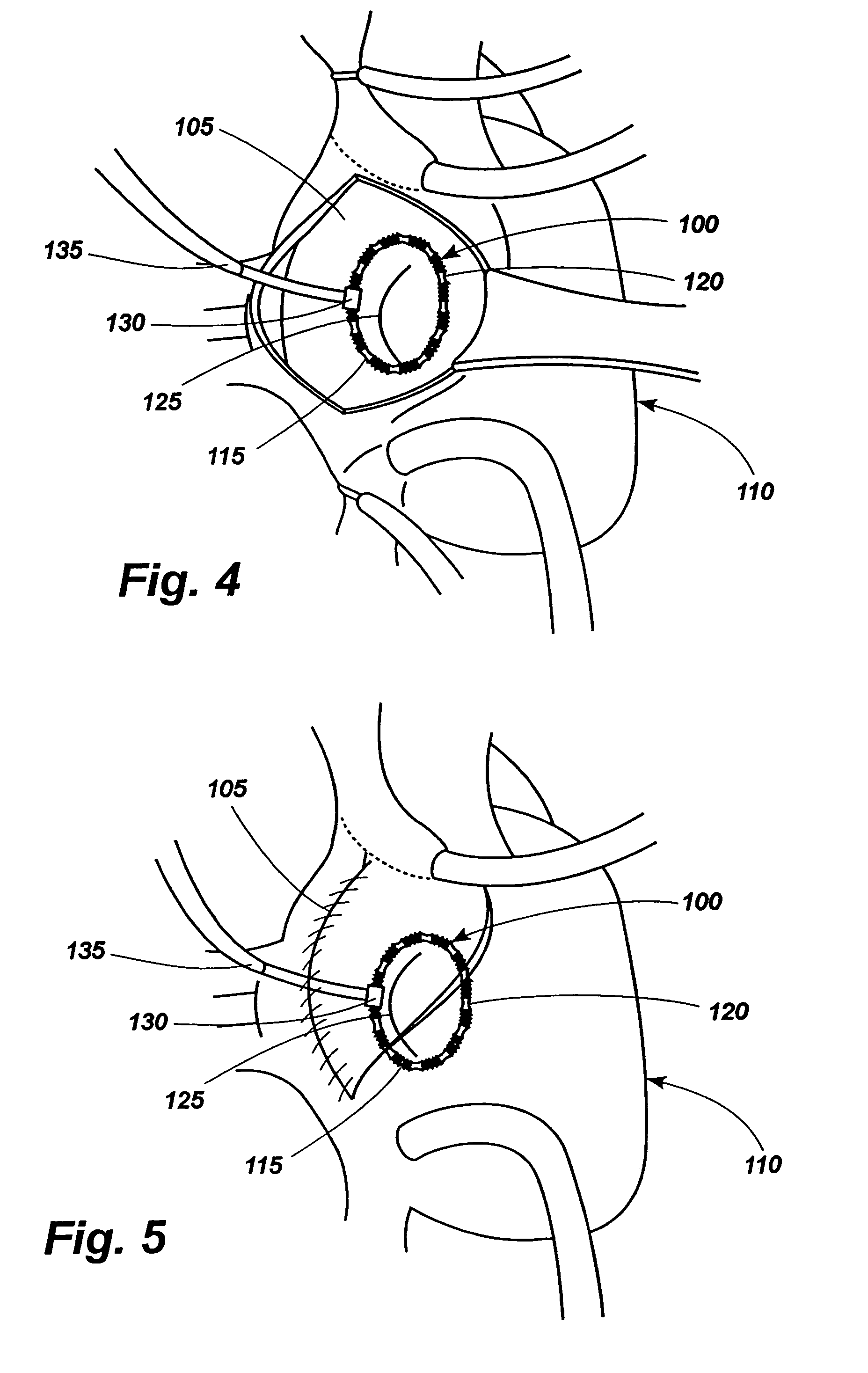
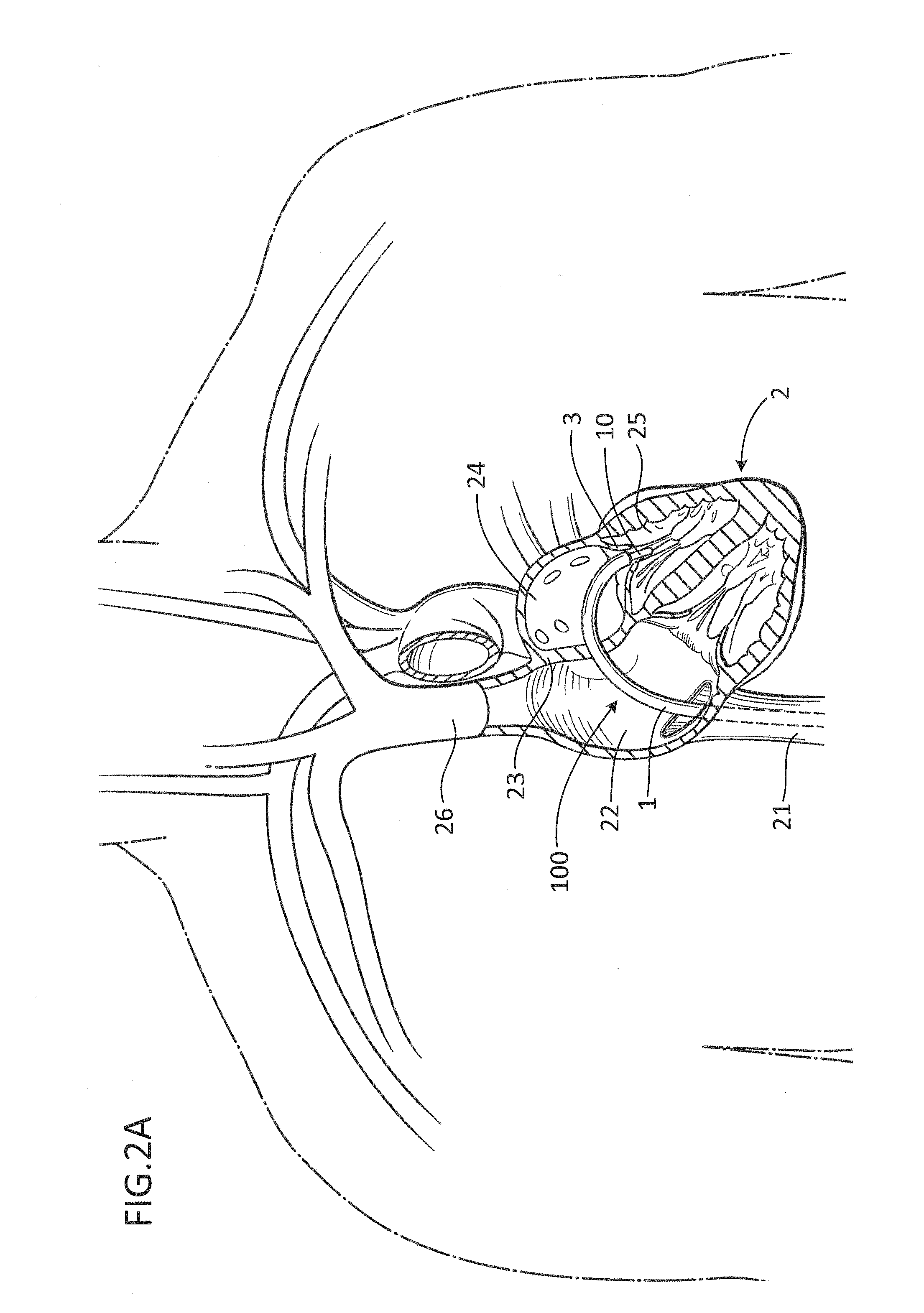
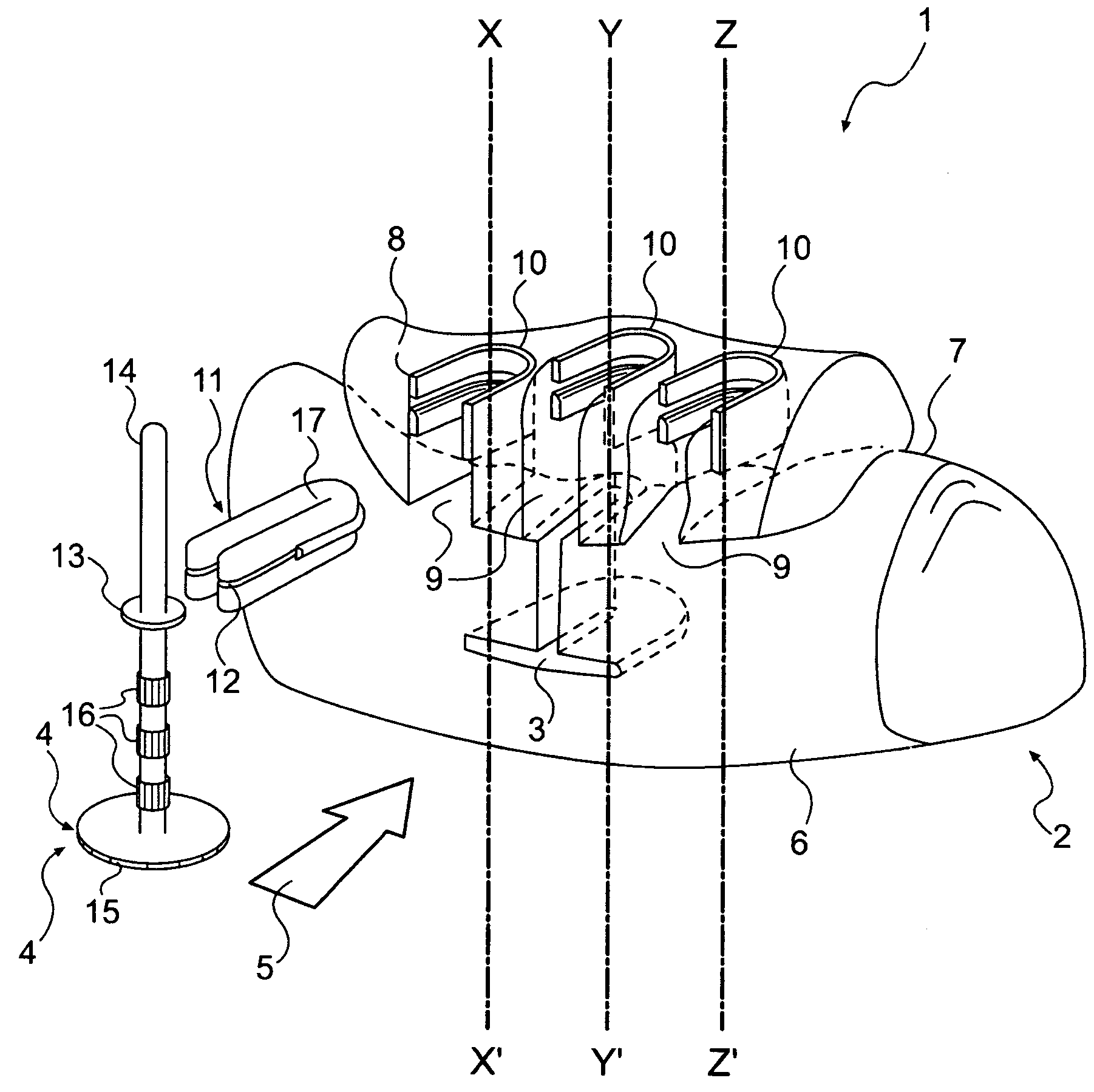
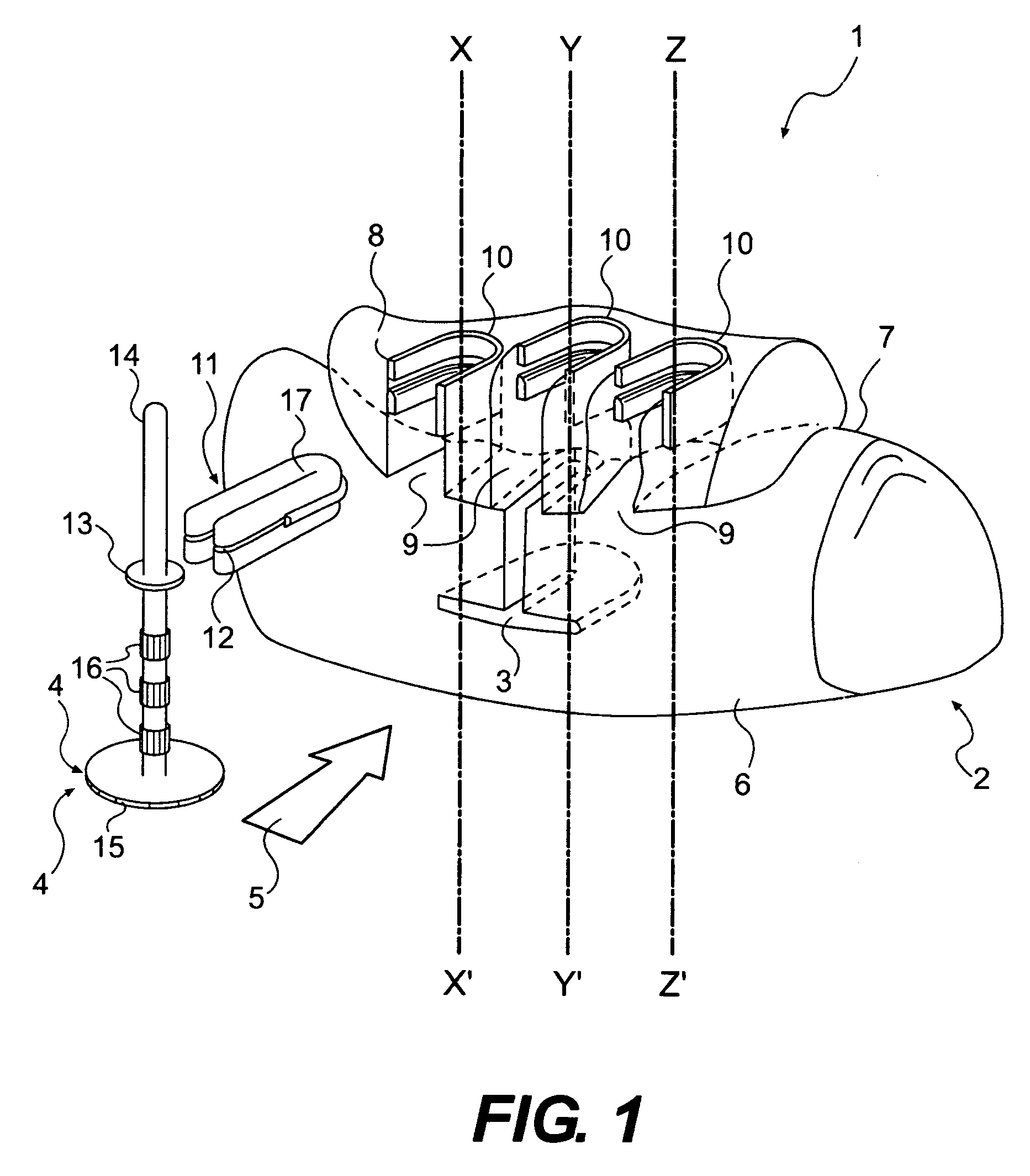
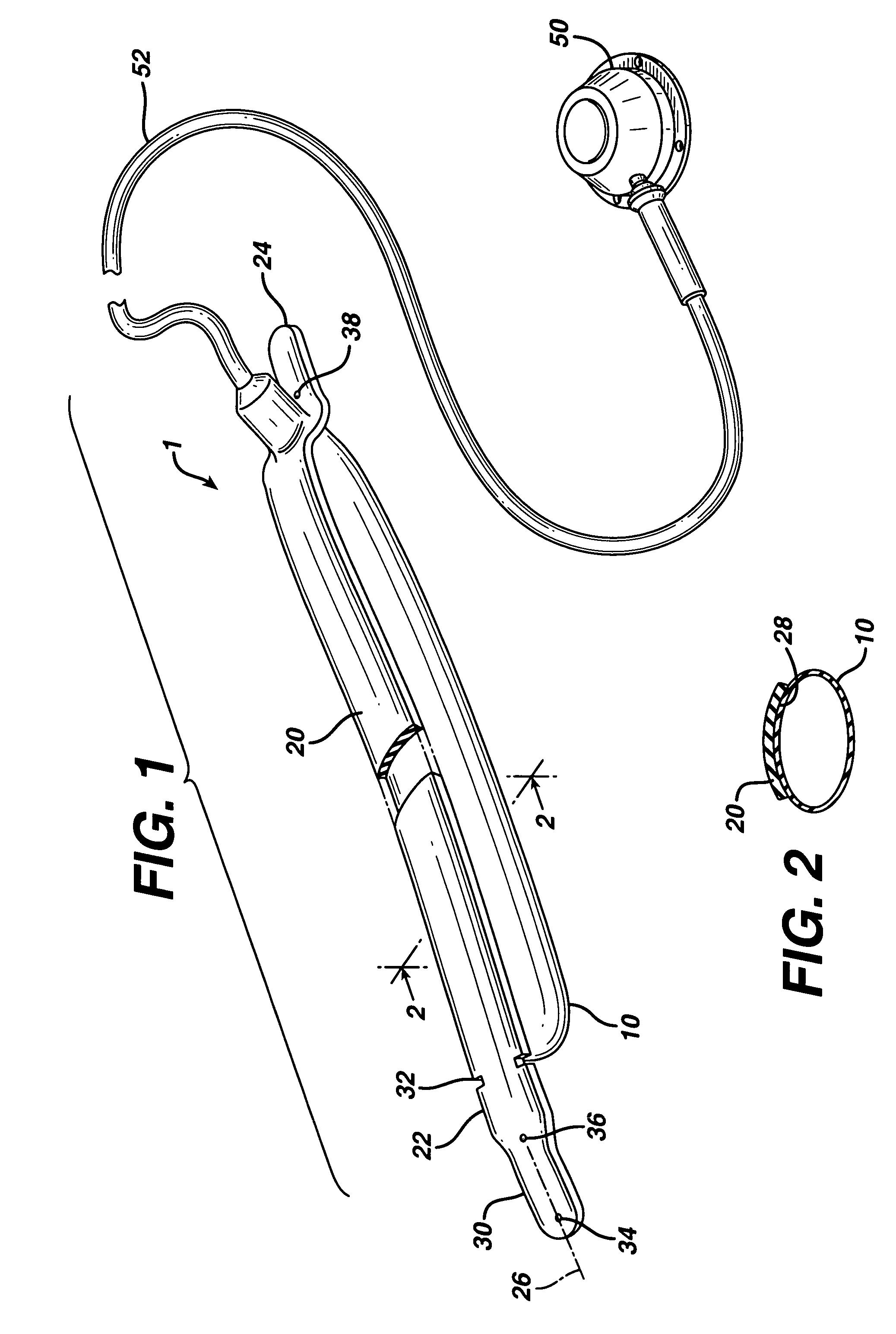
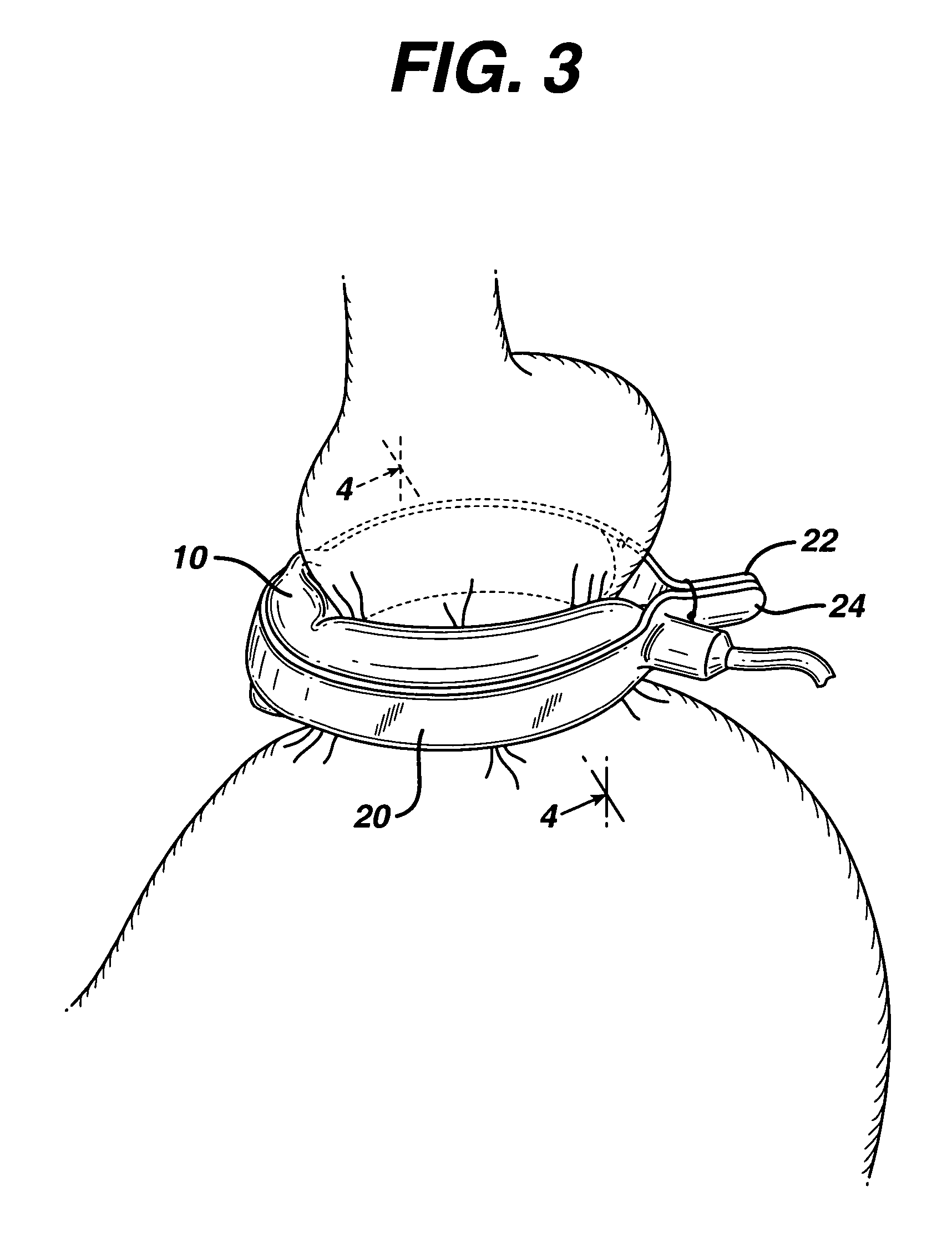
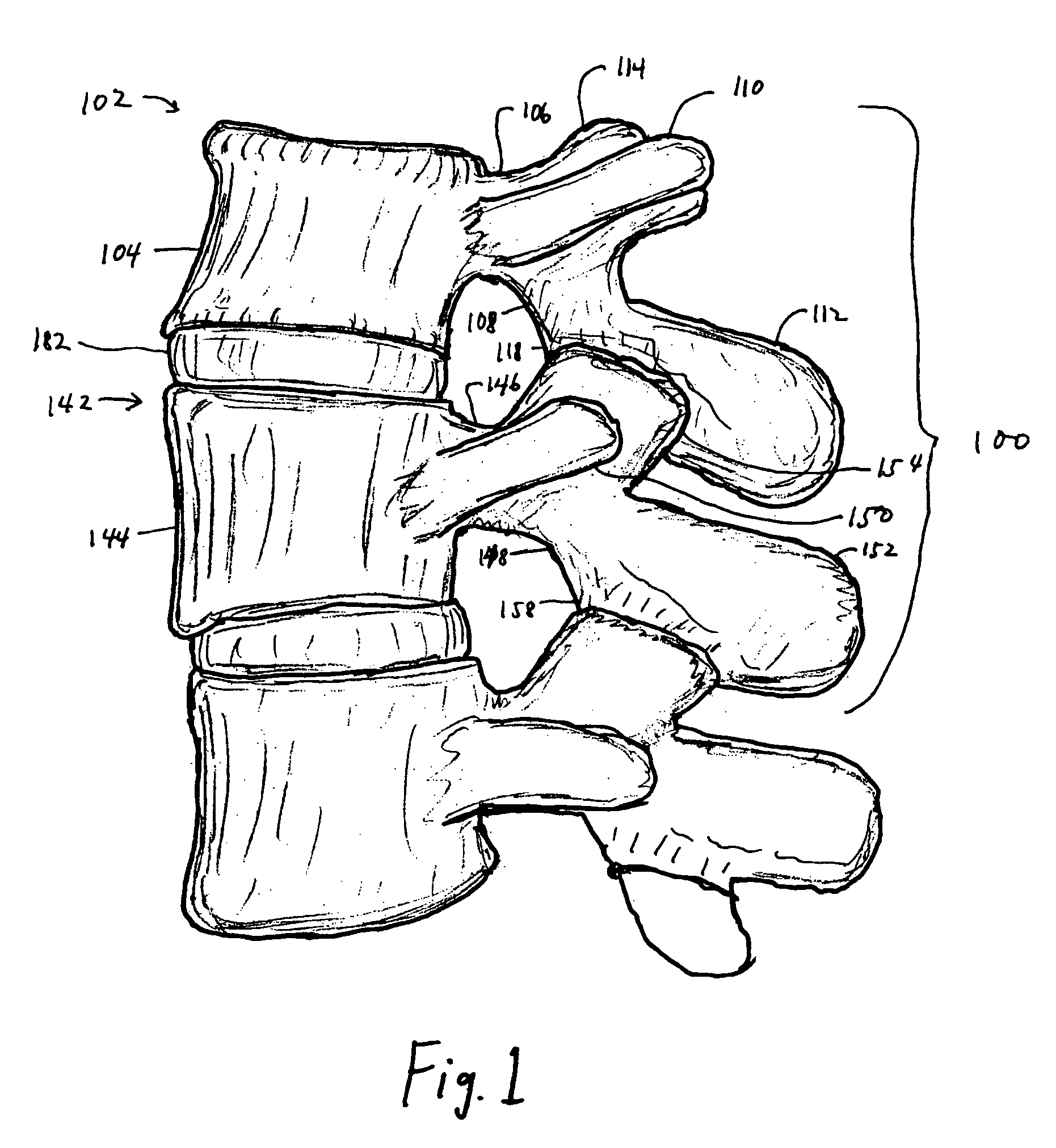
Apparatus for implanting surgical devices for controlling the internal circumference of an anatomic orifice or lumen
A system for implanting a surgical device to control the circumference of internal anatomic passages corrects physiologic dysfunctions resulting from a structural lumen which is either too large or too small. Implants are disclosed which employ various means for adjusting and maintaining the size of an orifice to which they are attached. Systems permit the implants to be implanted using minimally invasive procedures and permit final adjustments to the circumference of the implants after the resumption of normal flow of anatomic fluids in situ. Methods are disclosed for using the implants to treat heart valve abnormalities, gastroesophageal abnormalities, anal incontinence, and the like.
Owner:ST JUDE MEDICAL CARDILOGY DIV INC
Devices, systems and methods for delivering a prosthetic mitral valve and anchoring device
Prosthetic mitral heart valves and anchors for use with such valves are provided that allow for an improved implantation procedure. In various embodiments, a helical anchoring device is formed as a coiled or twisted anchor that includes one or more turns that twist or curve around a central axis. Curved arms attached to the frame of the valve guide the helical anchoring device into position beneath the valve leaflets and around the mitral valve annulus as it exits the delivery catheter, and the expandable prosthetic mitral valve is held within the coil of the anchoring device. The anchoring device and the valve can be delivered together, simplifying the valve replacement procedure.
Owner:MITRAL VALVE TECHNOLOGIES SARL
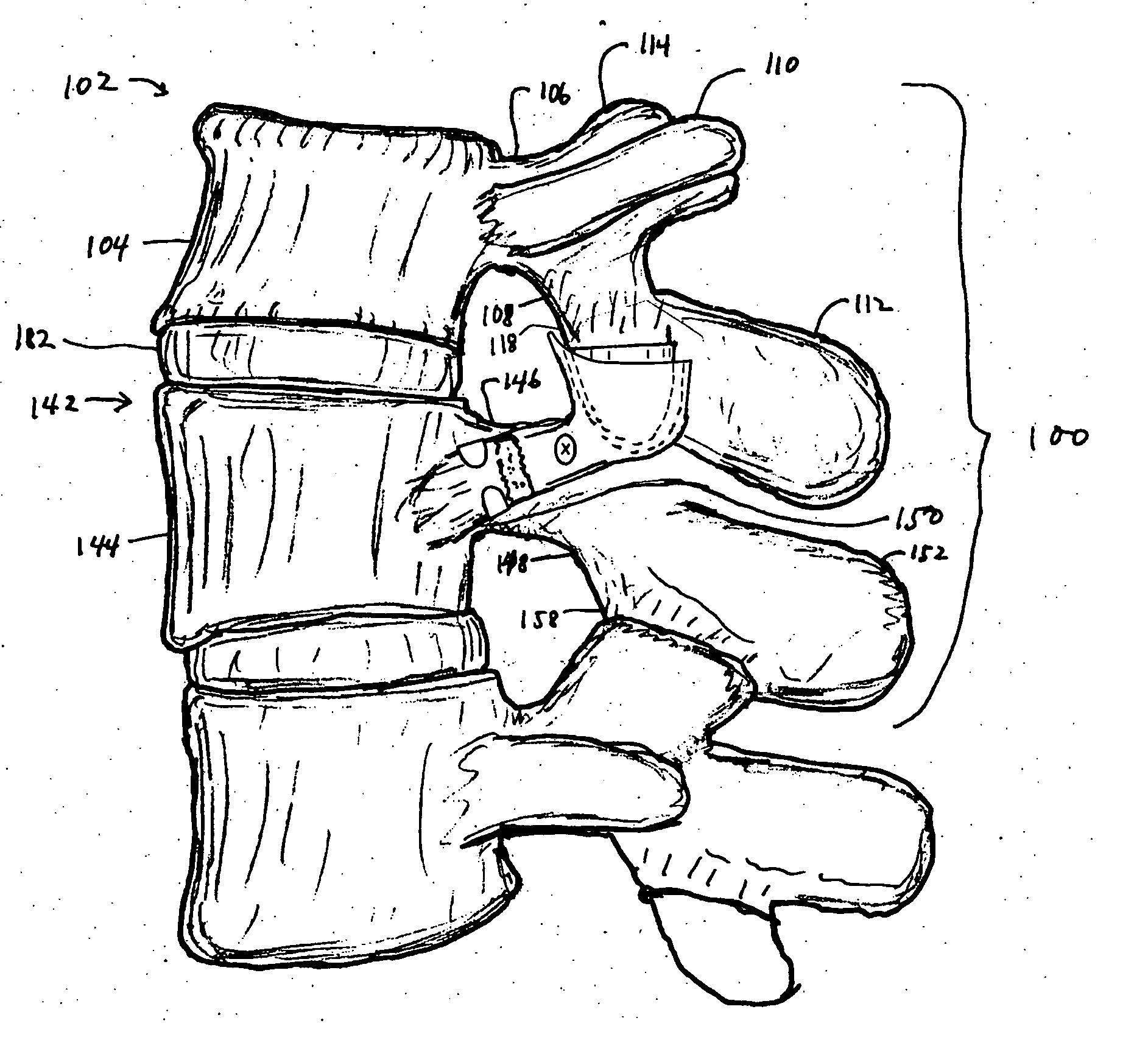
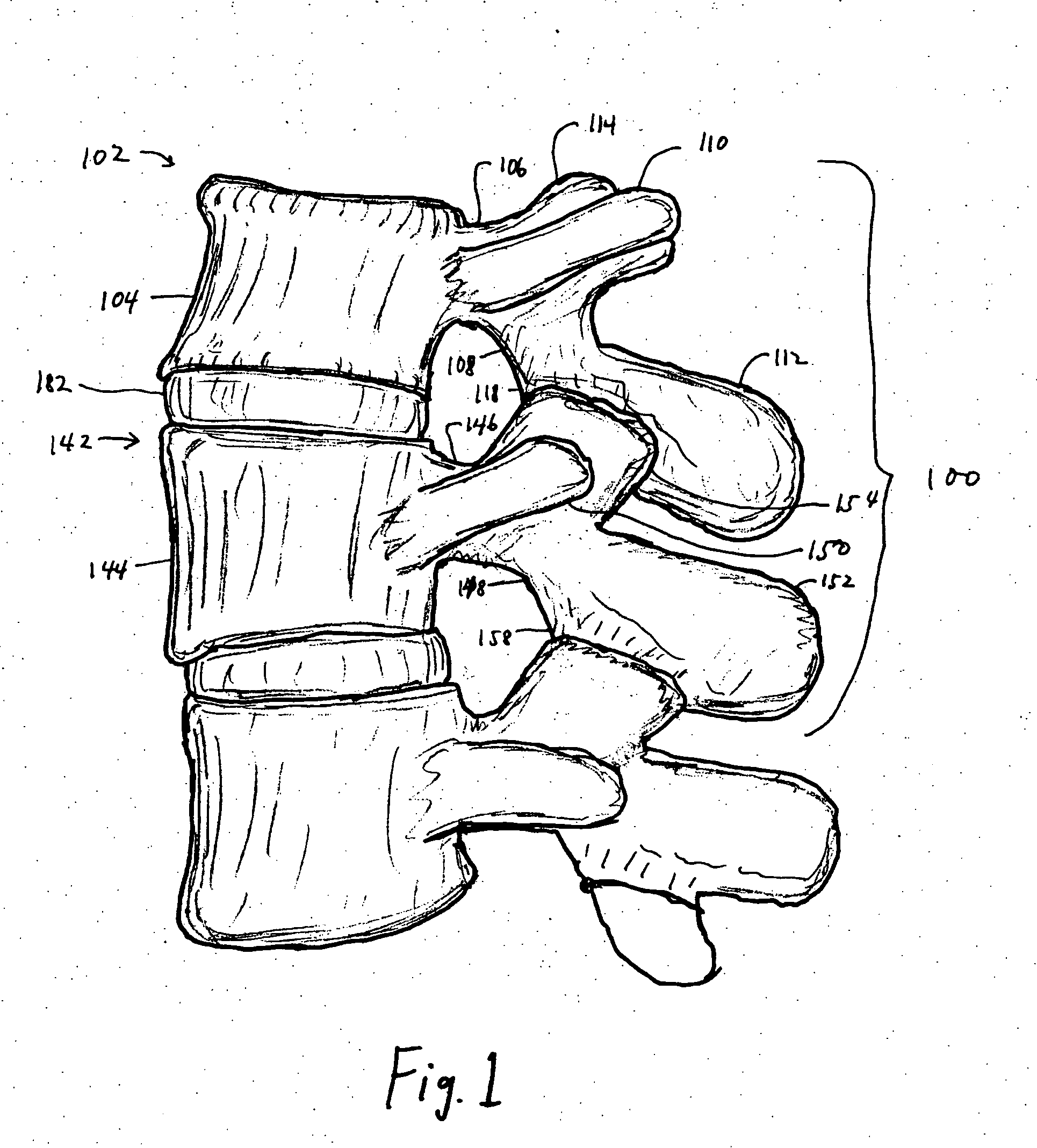
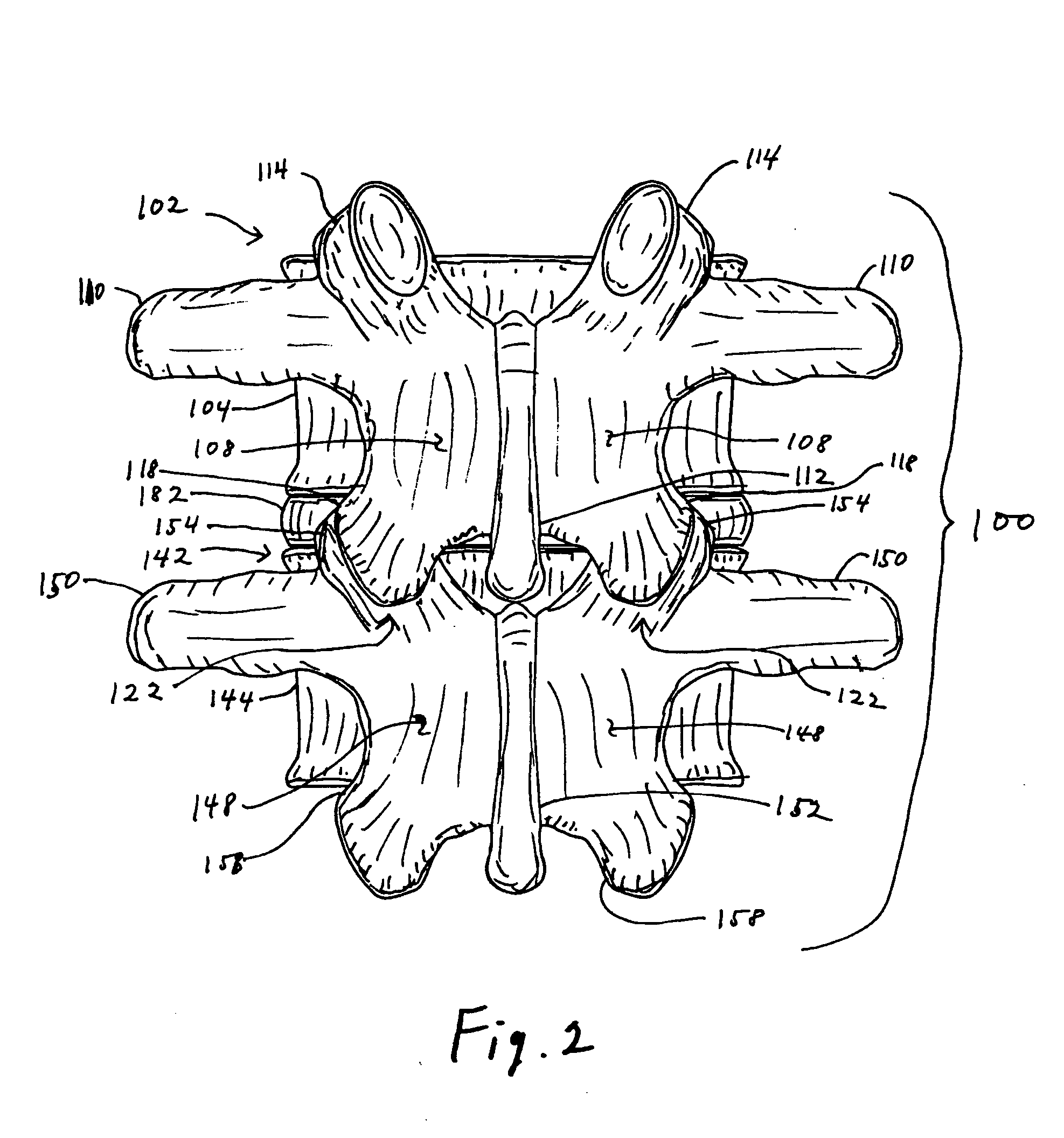
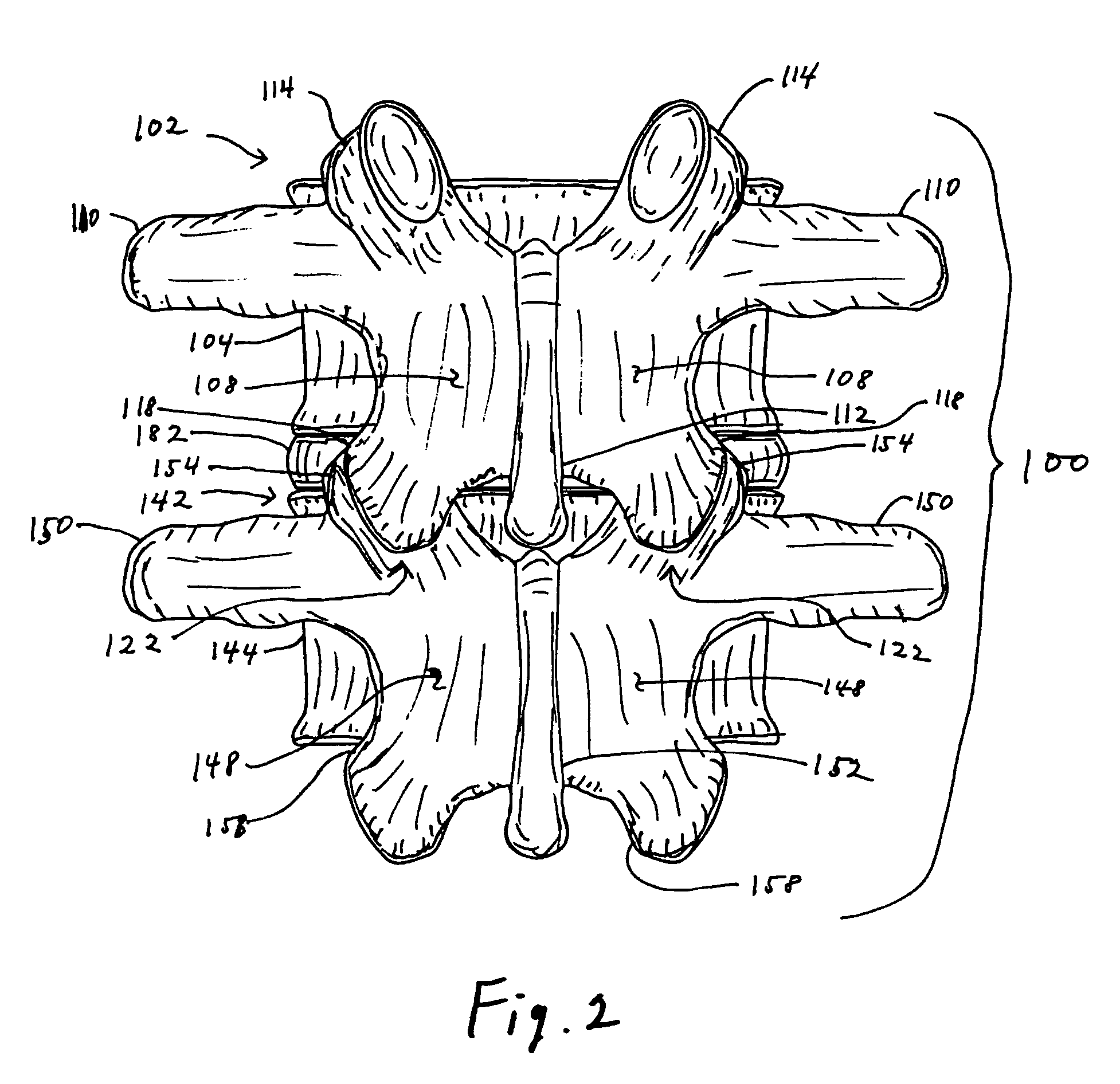
Facet joint prosthesis
A prosthetic implant for replacing a facet joint of a spinal motion segment includes a generally conical superior component adapted to be implanted at a surgically prepared site on a lower articular process of a cephalad vertebra of a spinal motion segment, and a cup-shaped inferior component adapted to be implanted at a surgically prepared site on a superior articular process of a caudad vertebra of the spinal motion segment.
Owner:RE SPINE
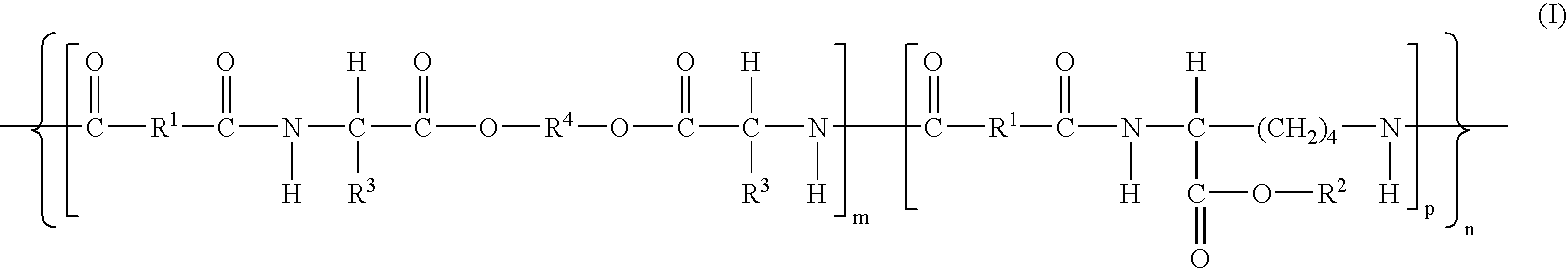
Wound healing polymer compositions and methods for use thereof
The present invention provides bioactive polymer compositions that can be formulated to release a wound healing agent at a controlled rate by adjusting the various components of the composition. The composition can be used in an external wound dressing, as a polymer implant for delivery of the wound healing agent to an internal body site, or as a coating on the surface of an implantable surgical device to deliver wound healing agents that are covalently attached to a biocompatible, biodegradable polymer and / or embedded within a hydrogel. Methods of using the invention bioactive polymer compositions to promote natural healing of wounds, especially chronic wounds, are also provided. Examples of biodegradable copolymer polyesters useful in forming the blood-compatible, hydrophilic layer or coating include copolyester amides, copolyester urethanes, glycolide-lactide copolymers, glycolide-caprolactone copolymers, poly-3-hydroxy butyrate-valerate copolymers, and copolymers of the cyclic diester monomer, 3-(S)[(alkyloxycarbonyl)methyl]-1,4-dioxane-2,5-dione, with L-lactide. The glycolide-lactide copolymers include poly(glycolide-L-lactide) copolymers formed utilizing a monomer mole ratio of glycolic acid to L-lactic acid ranging from 5:95 to 95:5 and preferably a monomer mole ratio of glycolic acid to L-lactic acid ranging from 45:65 to 95:5. The glycolide-caprolactone copolymers include glycolide and ε-caprolactone block copolymer, e.g., Monocryl or Poliglecaprone.
Owner:MEDIVAS LLC
Method and system to determine an optimal tissue compression time to implant a surgical element
A method for determining an optimal compression of tissue to apply a surgical element has the steps of applying a load to the tissue. The method has the step of determining a reactive load applied by the tissue in response to the load. The method also has the step of determining the reactive load per unit time for a predetermined time period and determining a slope of the reactive load per unit time. The method further has the steps of evaluating the slope relative to a predetermined threshold, and signaling when the slope exceeds the predetermined threshold.
Owner:TYCO HEALTHCARE GRP LP
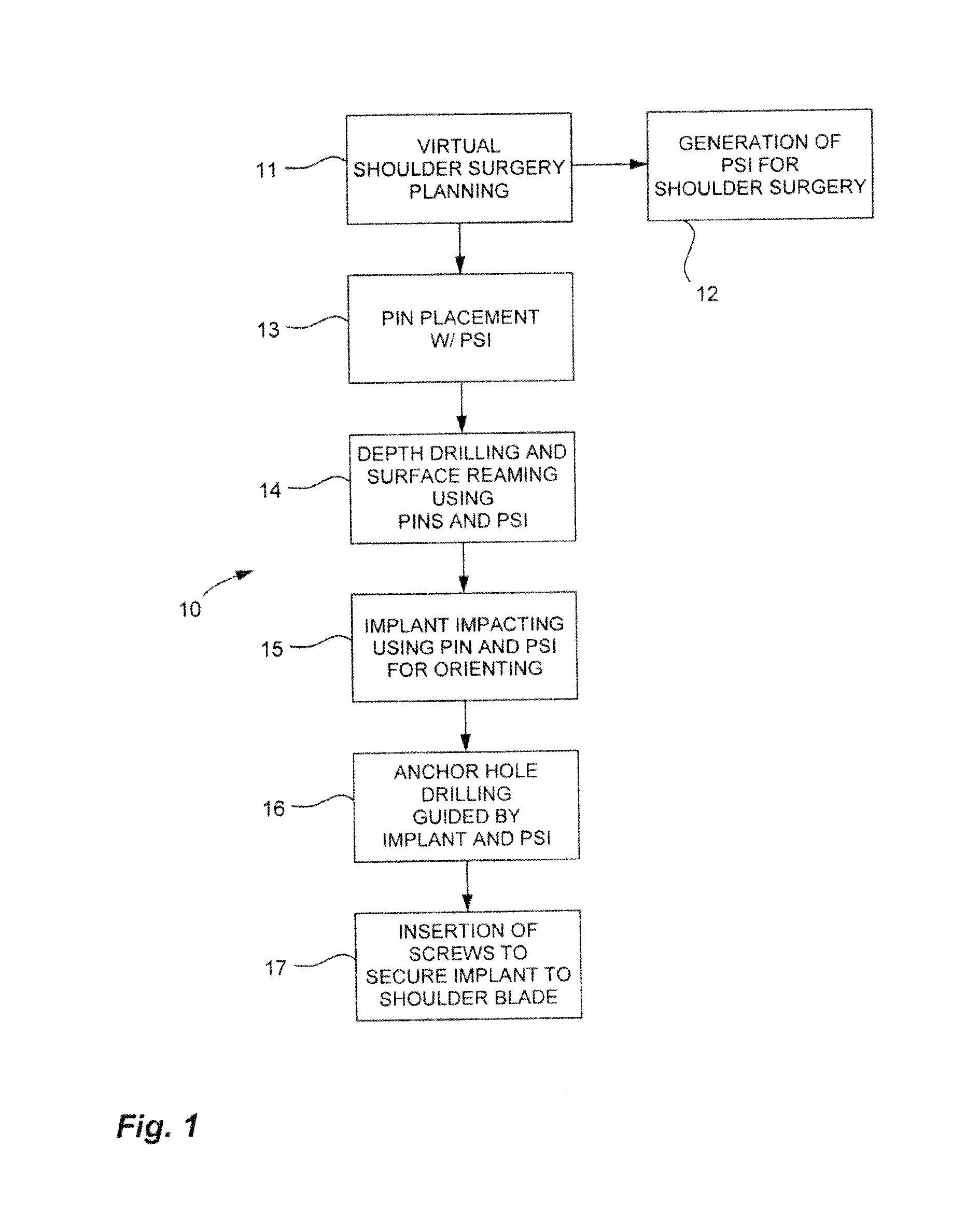
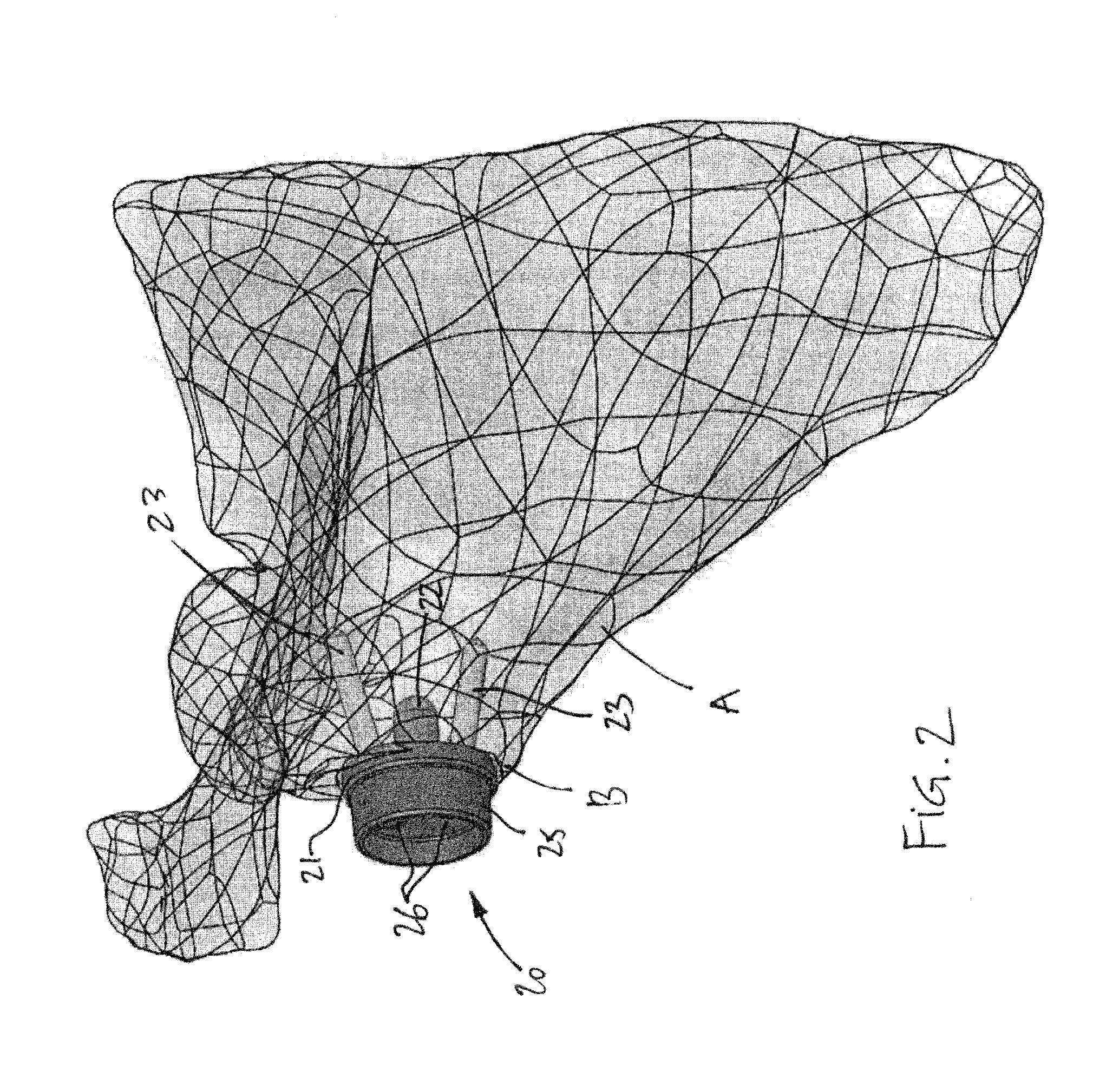
Methods and devices for installing standard and reverse shoulder implants
Surgical procedures, tools and implants are disclosed for both conventional and reverse shoulder implant surgeries. The improved procedures, tools and implants relate to humeral head resurfacing, humeral head resection for standard implants, humeral head resection for reverse shoulder implants, glenoid resurfacing for standard shoulder implants and glenoid resurfacing for reverse shoulder implants. 3D scans and x-rays are used to develop virtual models of the patient anatomy, identify patient specific landmarks for anchoring guide wire installation blocks, templates and drill guides. 3D scans are also used to design patient specific tools and implants for the shoulder implant procedures and to pre-operatively determine the appropriate inclination and retroversion angles.
Owner:SMITH & NEPHEW INC
Custom-fit implant surgery guide and associated milling cutter, method for their production, and their use
InactiveUS7824181B2Ensure total axial stabilityEnsure stabilityDental implantsAdditive manufacturing apparatusMilling cutterEngineering
The invention relates to a custom-fit implantable surgical guide (1) and an associated milling tool (4), which is positioned in straddling on the alveolar ridge (7) of a maxillary or mandible arch (2) and comprises at least one drilling barrel (11) for axially guiding said milling tool (4), wherein said barrel (11) is laterally open and at least one part of the internal surface thereof (17) and at least one part of the external surface of the milling tool (4) interact and axially maintain the entire milling tool (4) with respect to the barrel (11). Said invention makes it possible to carry out high precision osteotomies for lateral insertion dental implants.
Owner:MATERIALISE DENTAL NV
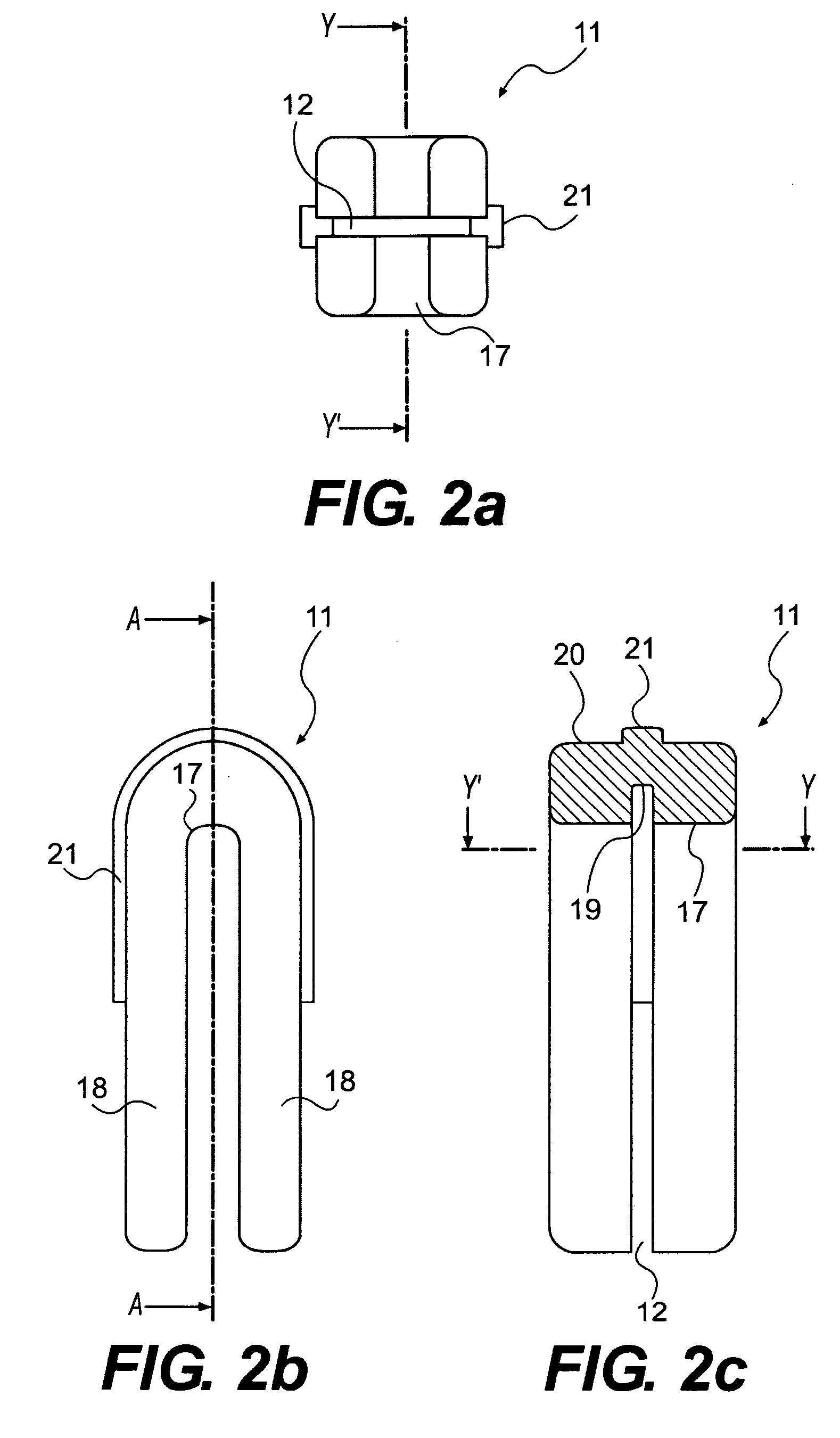
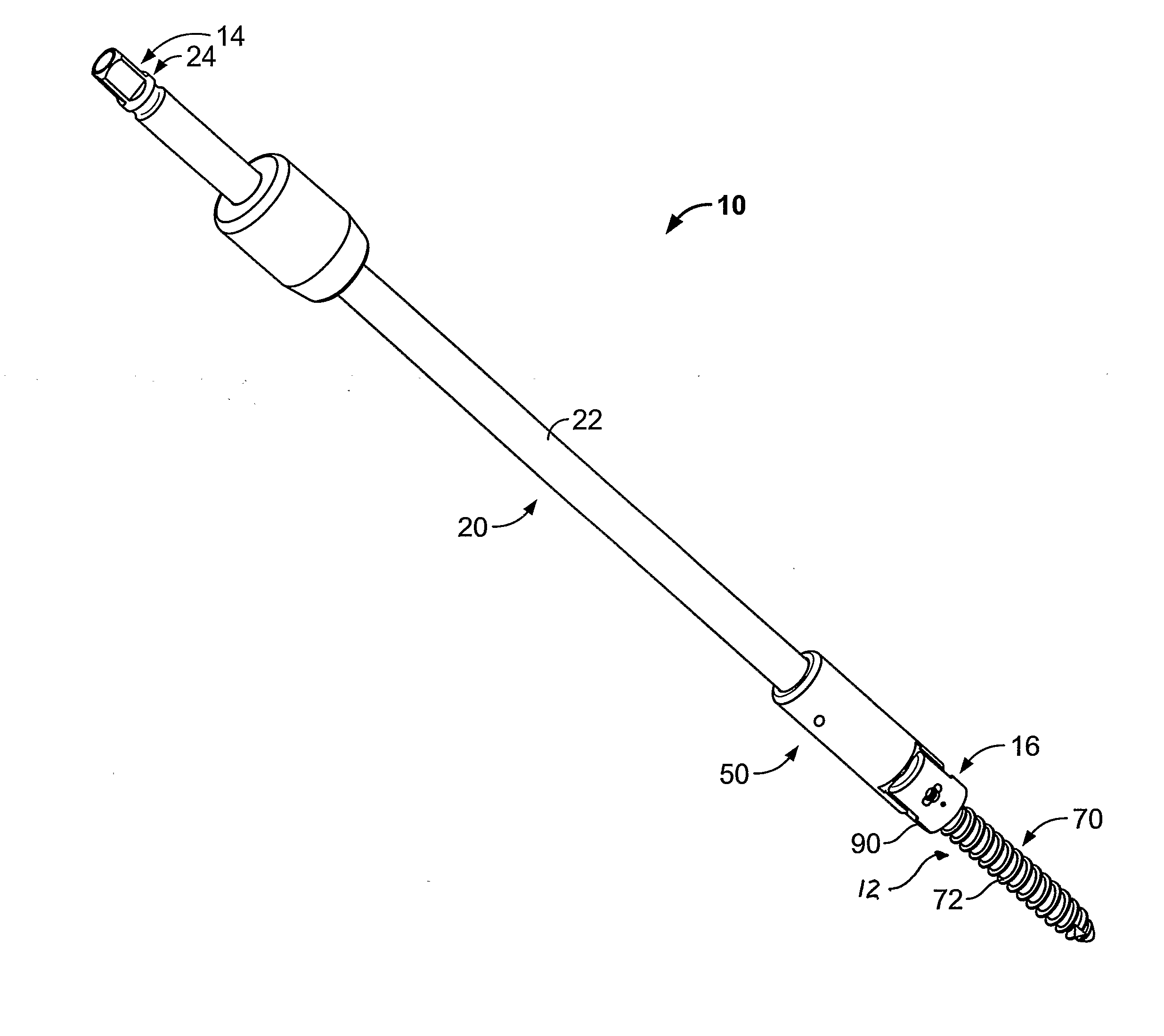
Apparatus and Method for Implantation of Surgical Devices
InactiveUS20090163963A1Avoid threaded connectionsIncrease in sizeInternal osteosythesisSpannersImplantation SiteSurgical device
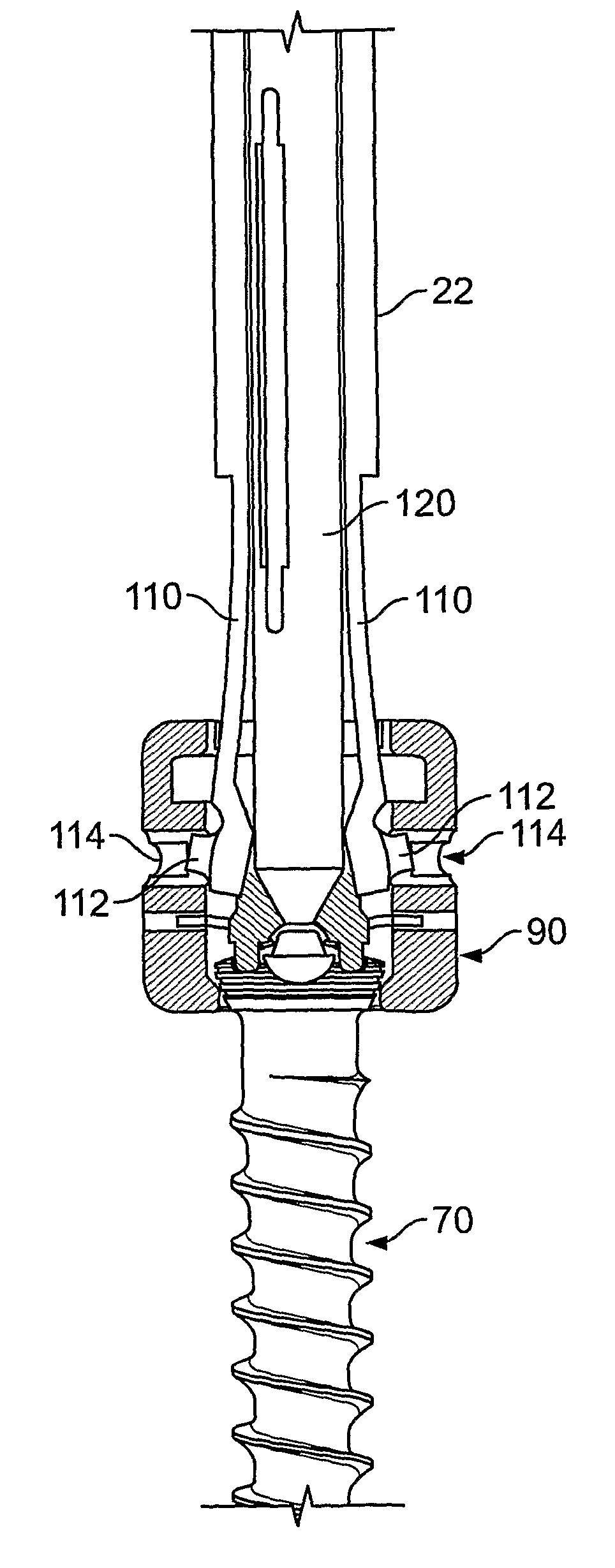
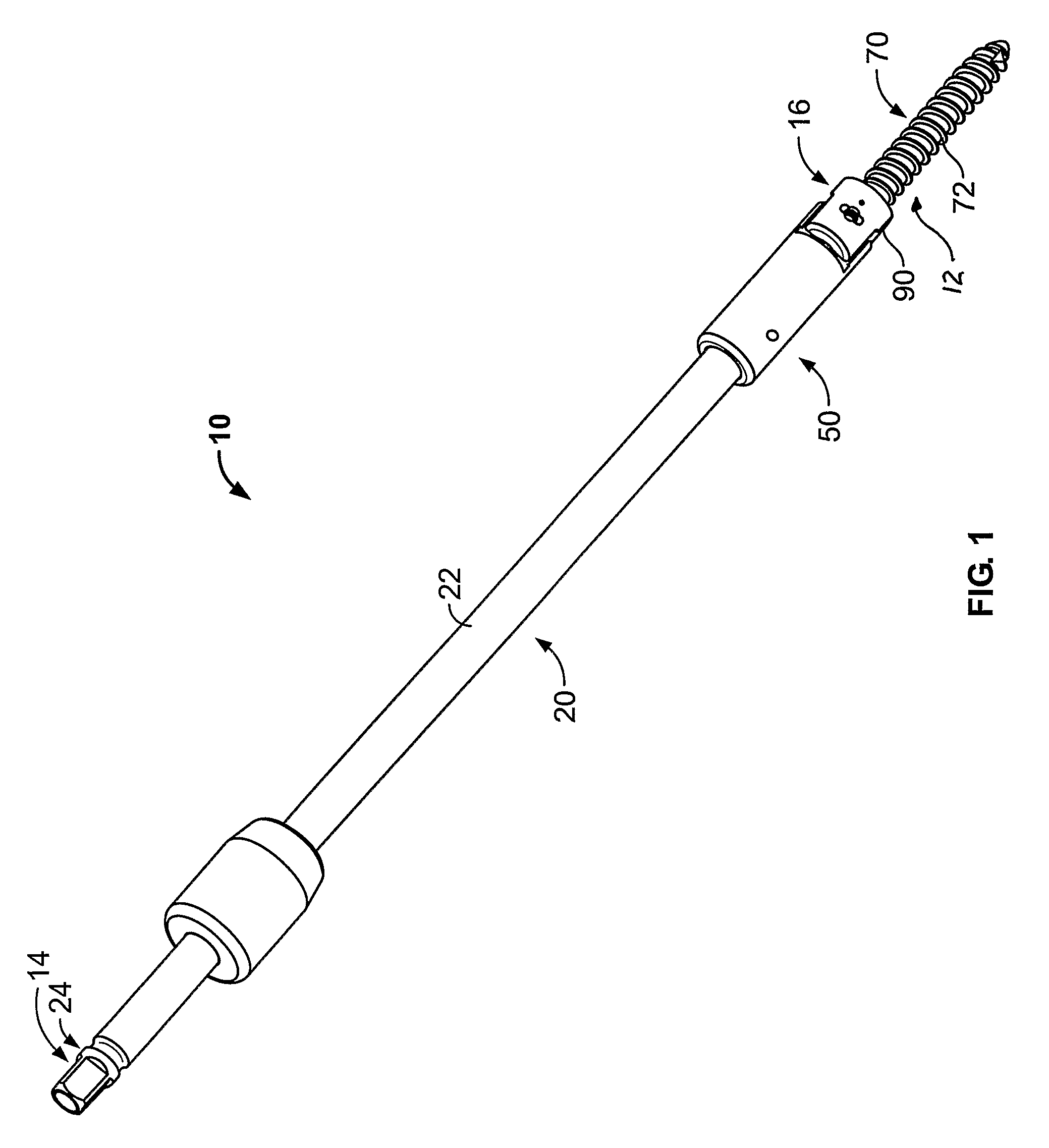
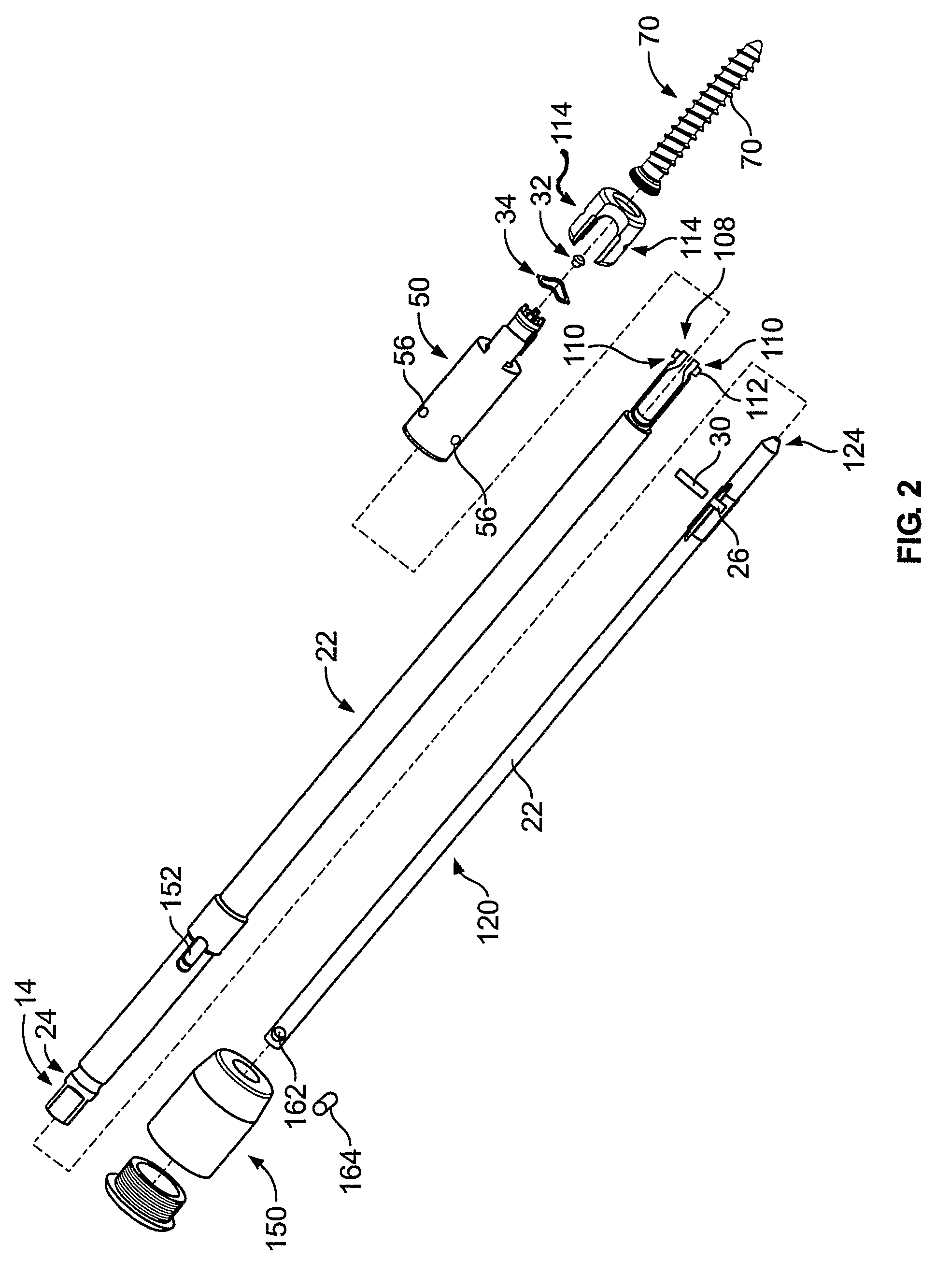
A driver apparatus for implanting spinal rod fixation devices including an anchor member and a coupling member pivotable relative to the anchor member is disclosed. The driver apparatus generally immobilizes the anchor member and coupling member relative to each other and to the driver apparatus during insertion to minimize interference between the coupling member and tissues surrounding the implantation site, as well as to minimize the clearance required for implantation. The driver apparatus includes a driver portion engageable with the anchor member for effecting seating of the anchor member, such as by threadably driving the anchor member in the bone. The driver portion is closely fit between portions such as upstanding walls of the coupling member to prevent relative rotation therebetween. The driver apparatus further includes locking portions shiftable to secure the coupling member with the driver apparatus when the driver is engaged with the anchor member
Owner:PIONEER SURGICAL TECH INC
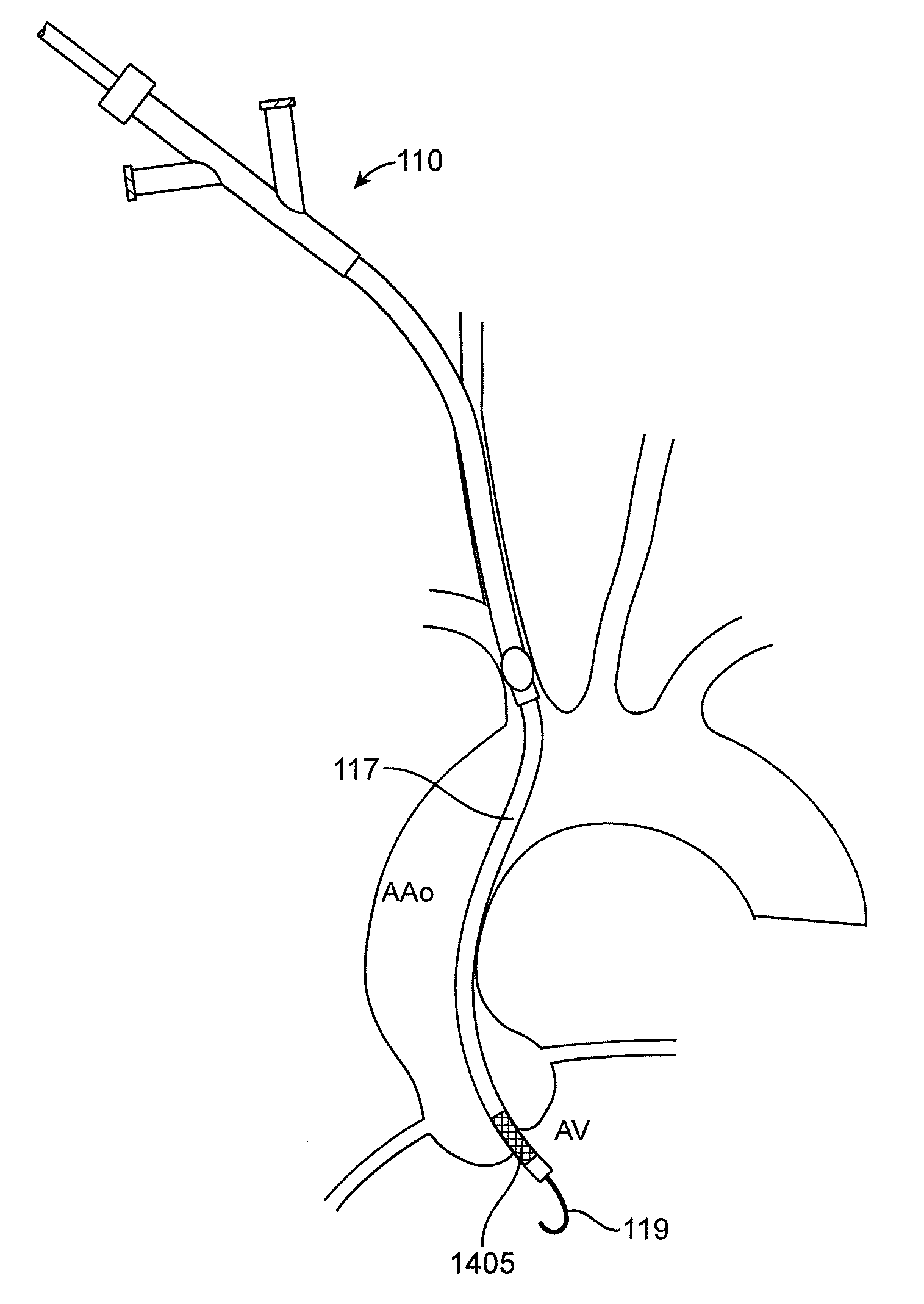
Systems and methods for transcatheter aortic valve treatment
ActiveUS20110213459A1Shorter and straight access pathEasy to placeSuture equipmentsStentsTRANSCATHETER AORTIC VALVE IMPLANTCatheter
Devices and methods are configured to allow transcervical or subclavian access via the common carotid artery to the native aortic valve, and implantation of a prosthetic aortic valve into the heart. The devices and methods also provide means for embolic protection during such an endovascular aortic valve implantation procedure.
Owner:SILK ROAD MEDICAL
Wound care polymer compositions and methods for use thereof
The present invention provides wound healing or wound care polymer compositions that can be formulated to release a wound healing agent at a controlled rate by adjusting the various components of the composition. The compositiona can be used in an external wound dressing, as a polymer implant for delivery of the wound healing agent to an internal body site, or as a coating on the surface of an implantable surgical device to deliver wound healing agents that are dispersed in a biodegradable polymer or hydrogel, or both. Methods of using the invention bioactive polymer compositions to deliver wound healing agents that promote natural healing of wounds, especially chronic wounds, are also provided.
Owner:MEDIVAS LLC
Surgical guide for dental implant and methods therefor
A surgical guide assembly is provided for positioning a drill bit during a dental implant procedure. The guide assembly includes a mounting member configured to mount to one or more teeth adjacent to an edentulous area, a base connected to the mounting member and dimensioned to extend over the edentulous area, a translation member adjustable with respect to the base, and a rotation member adjustable with respect to the translation member. The rotation member includes an aperture configured to receive a radiographic marker or a drill. The translation and rotation members may be configured to adjust one of the mesio-distal (MD) rotational alignment and the buccal-lingual (BL) rotational alignment of the radiographic marker inserted in the aperture while holding the other of the MD and BL rotational alignment mechanically fixed. Also, the translation and rotation members may be configured to adjust the other of the MD and BL translational alignment of the radiographic marker while holding said one of the MD and BL translational alignment mechanically fixed. A method of using the dental implant positioning assembly is also disclosed.
Owner:STUMPEL LAMBERT J
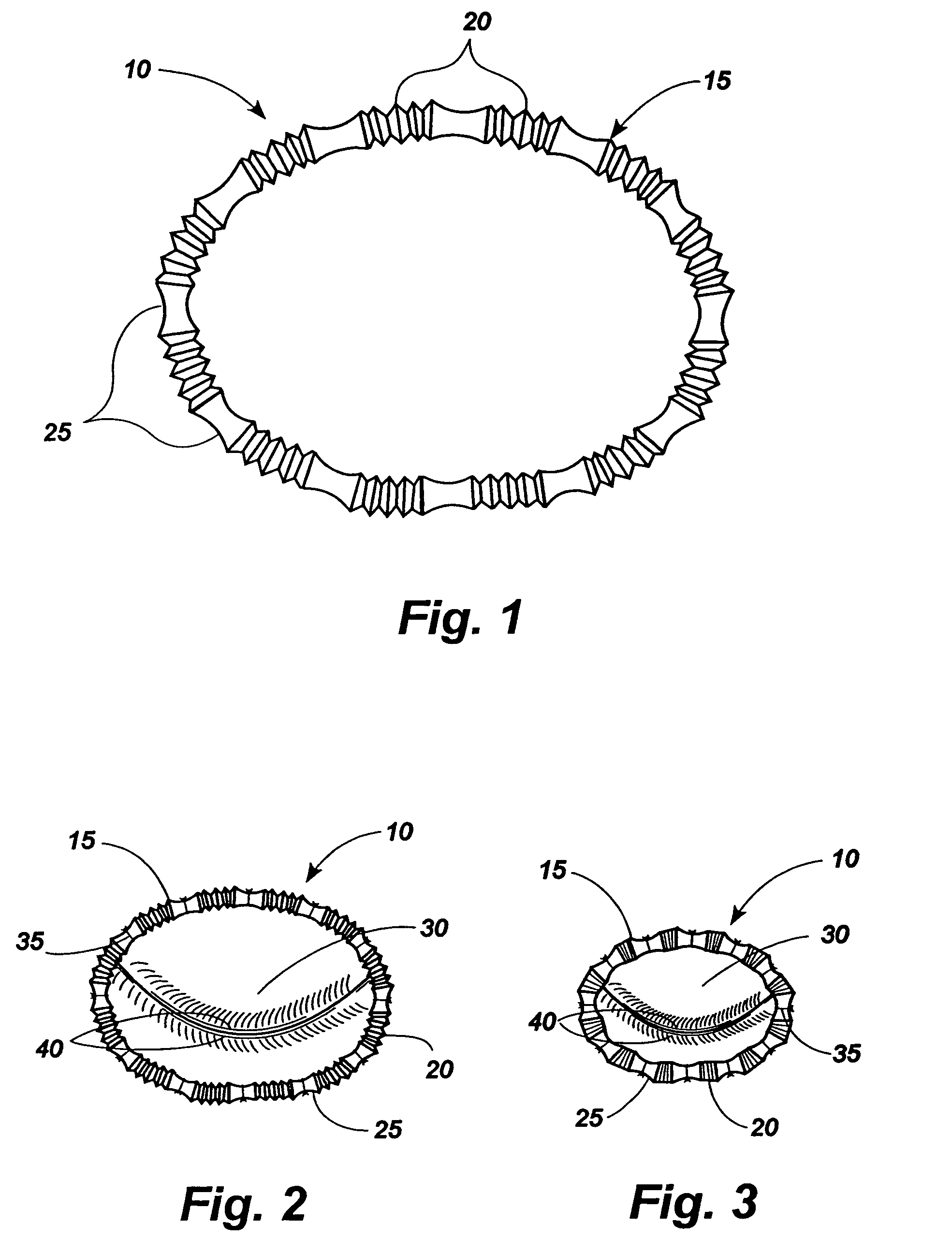
Method for implanting an adjustable band
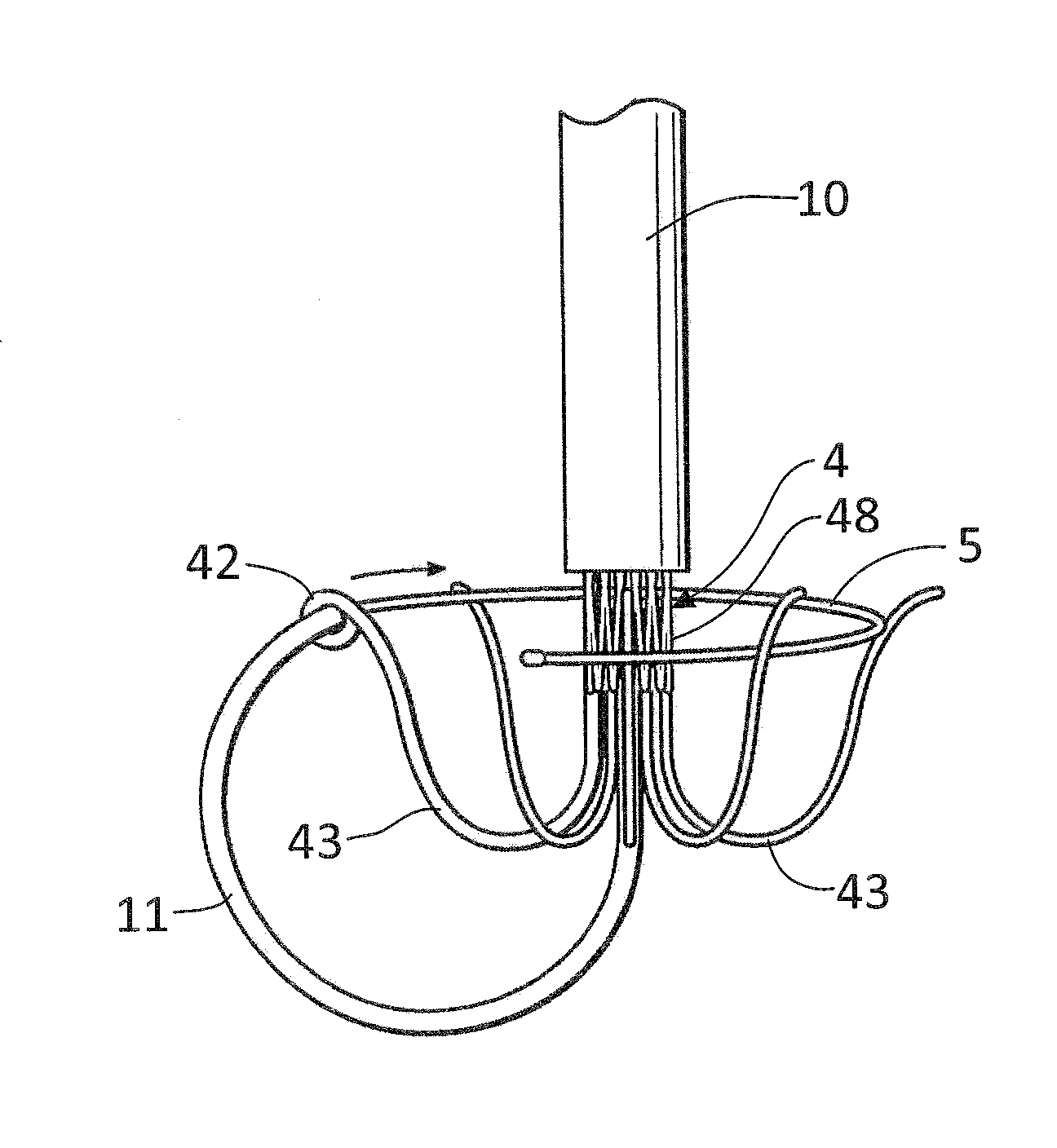
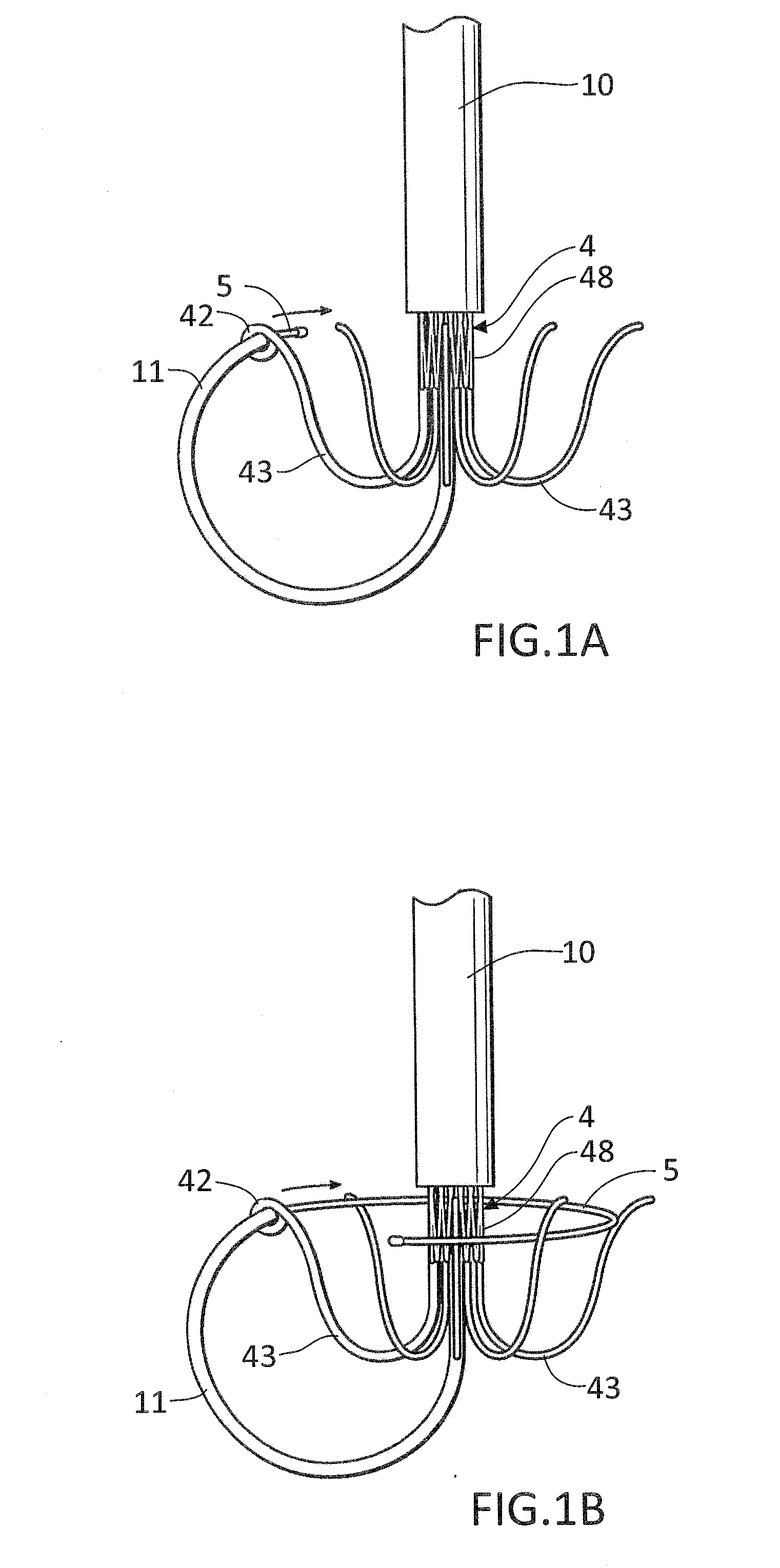
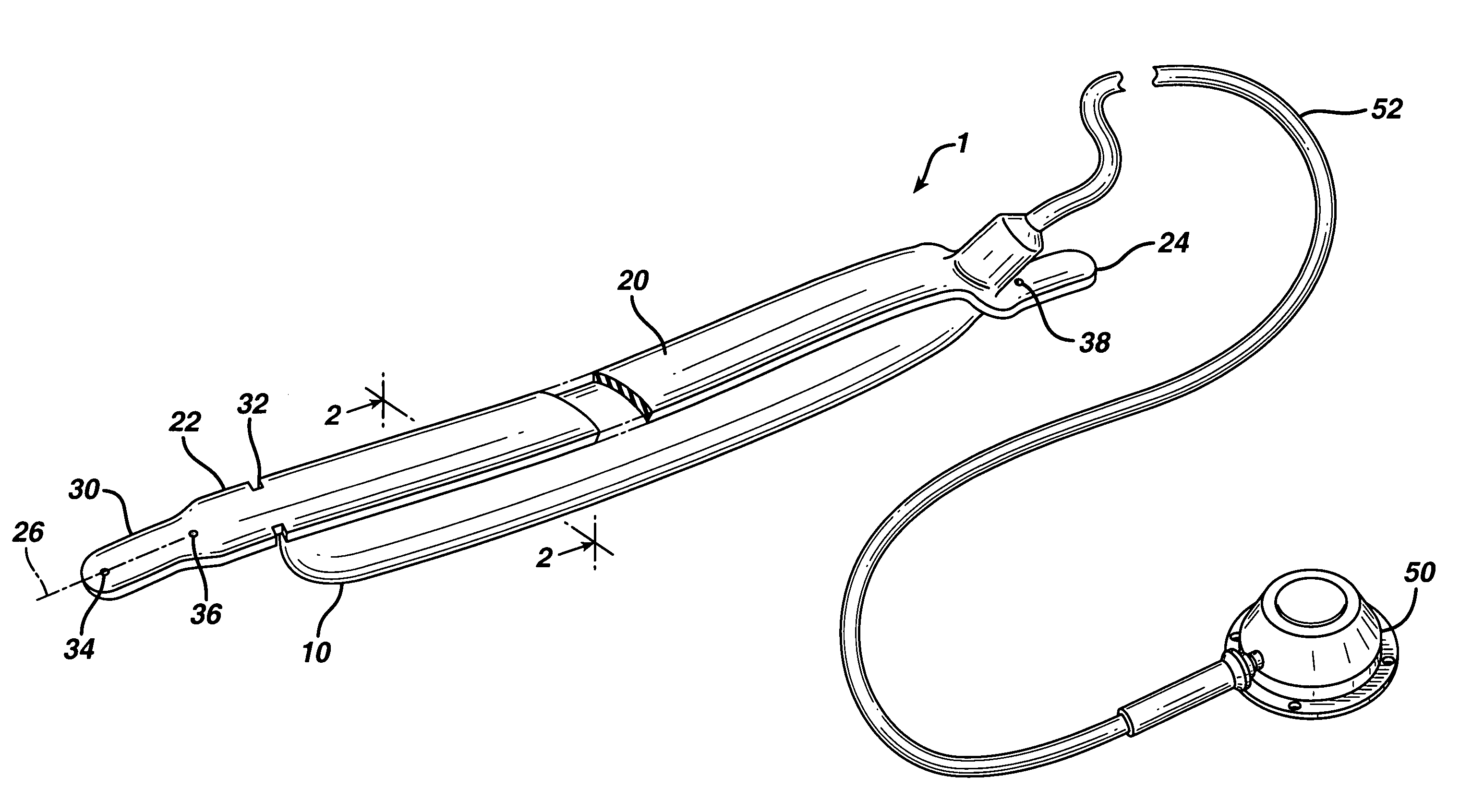
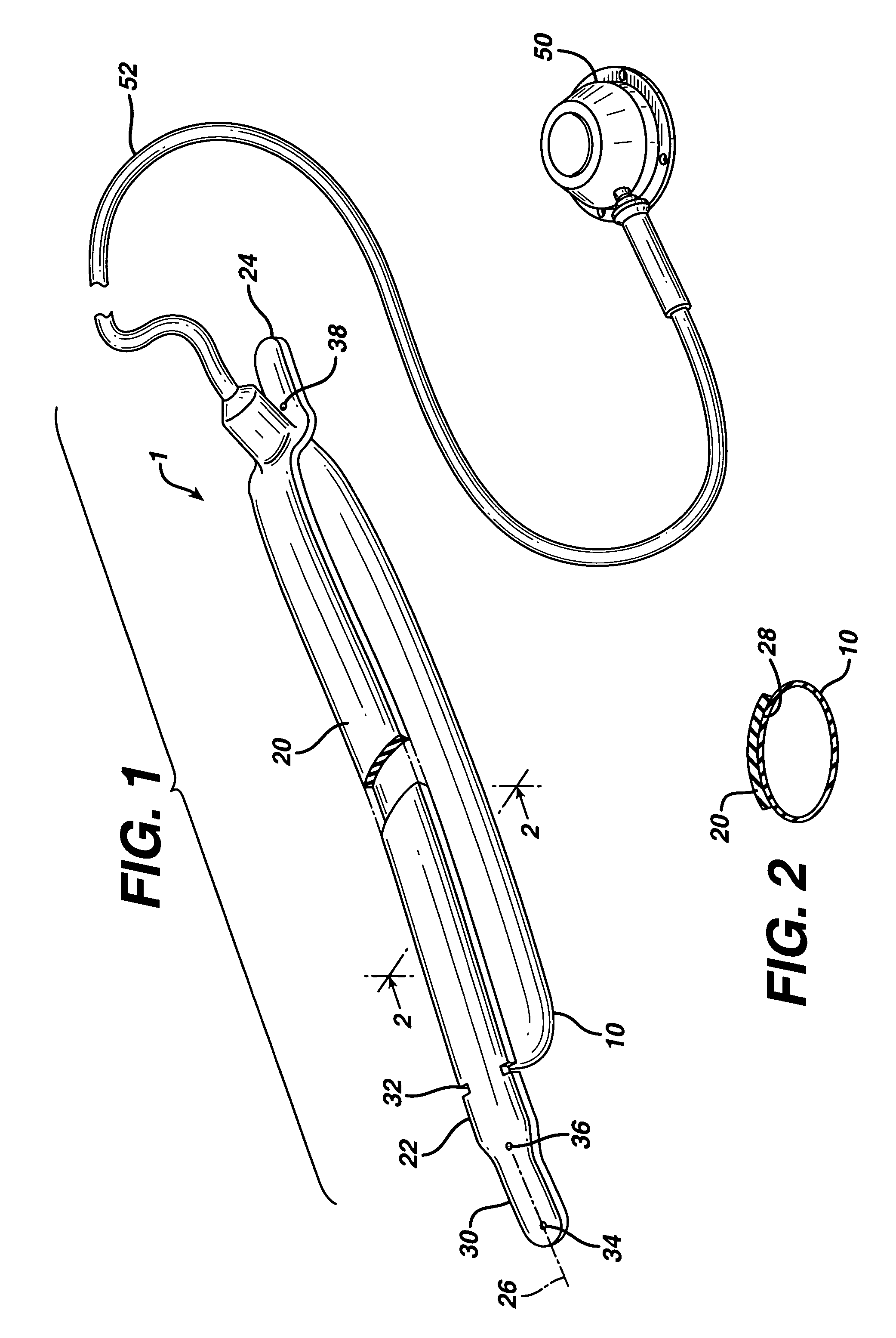
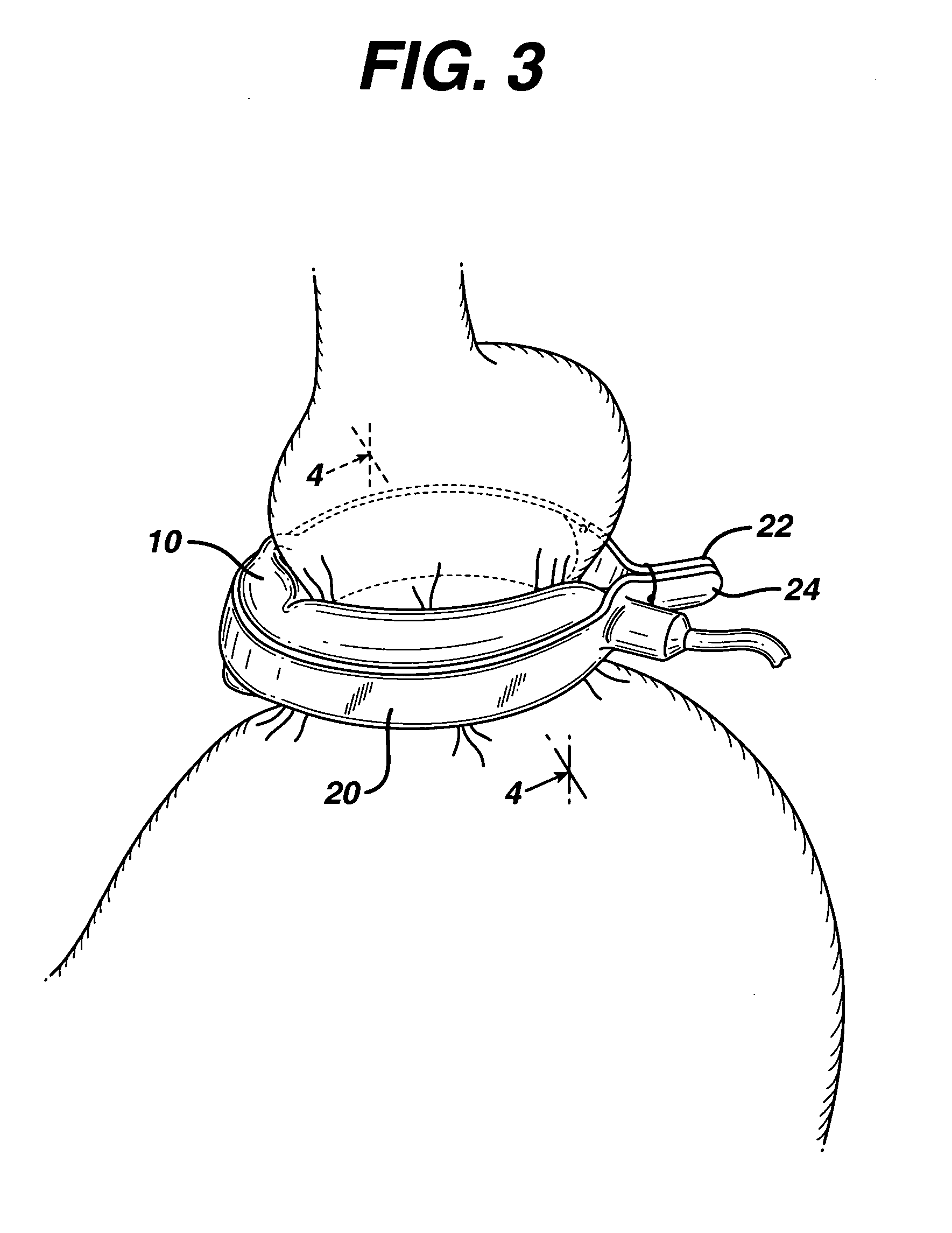
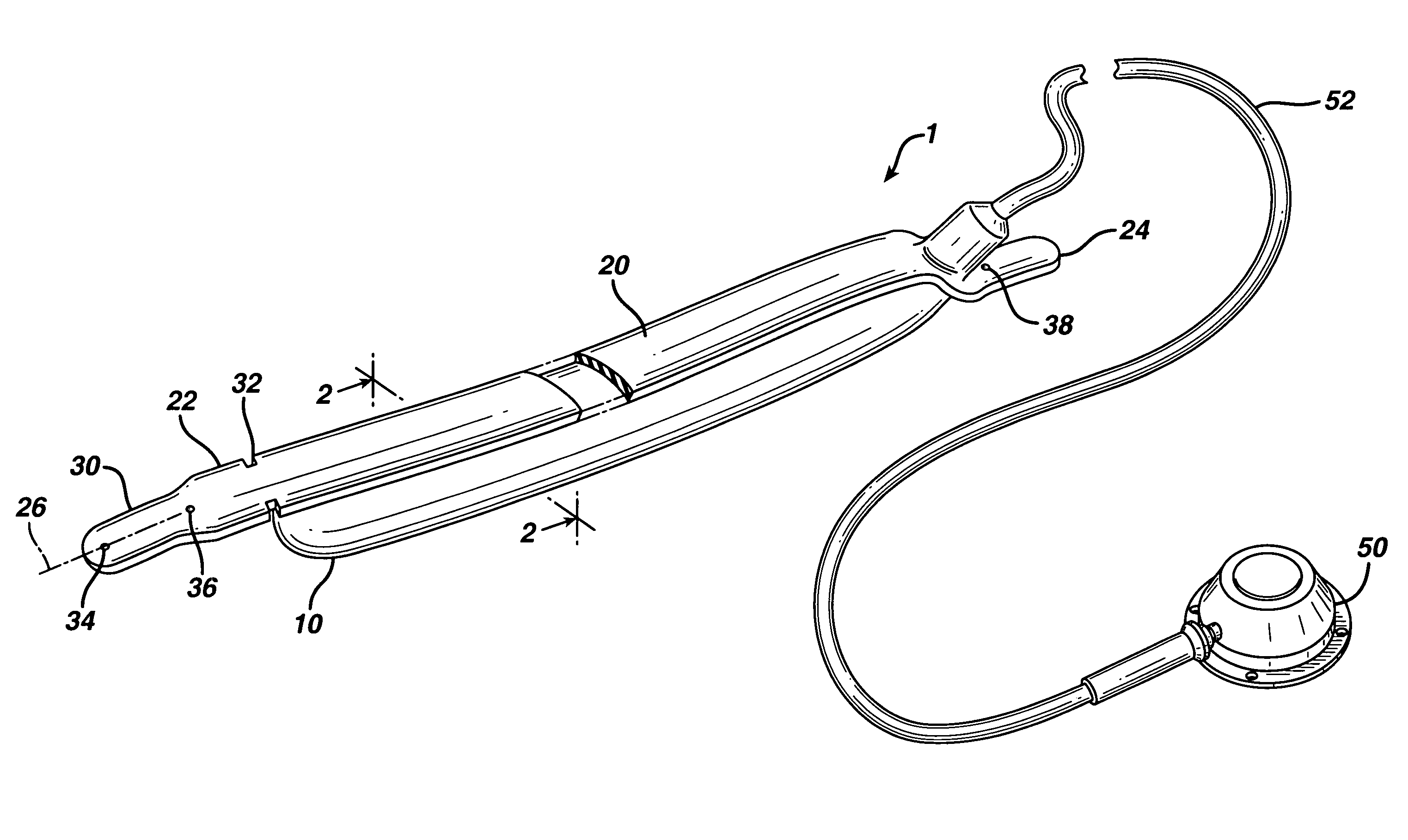
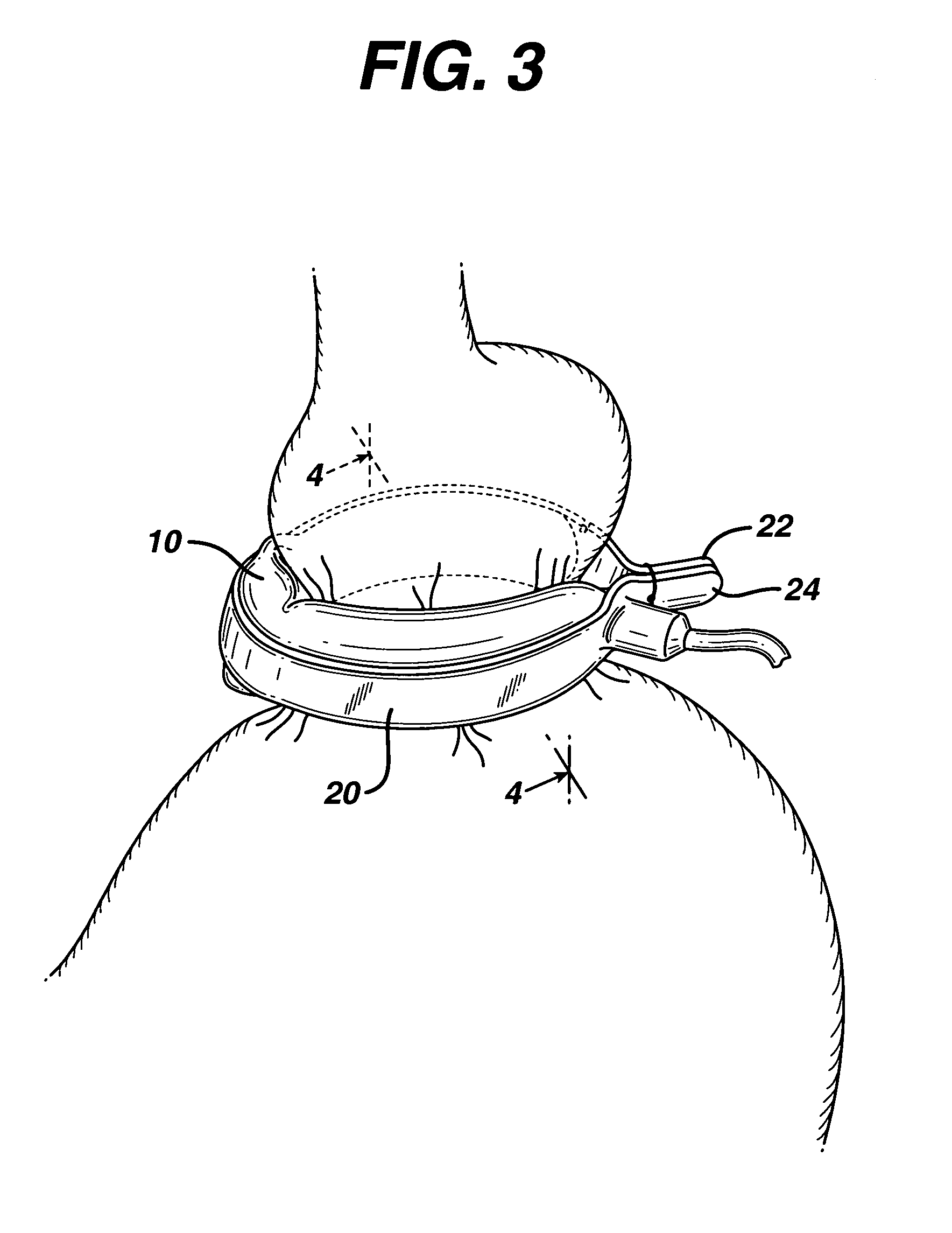
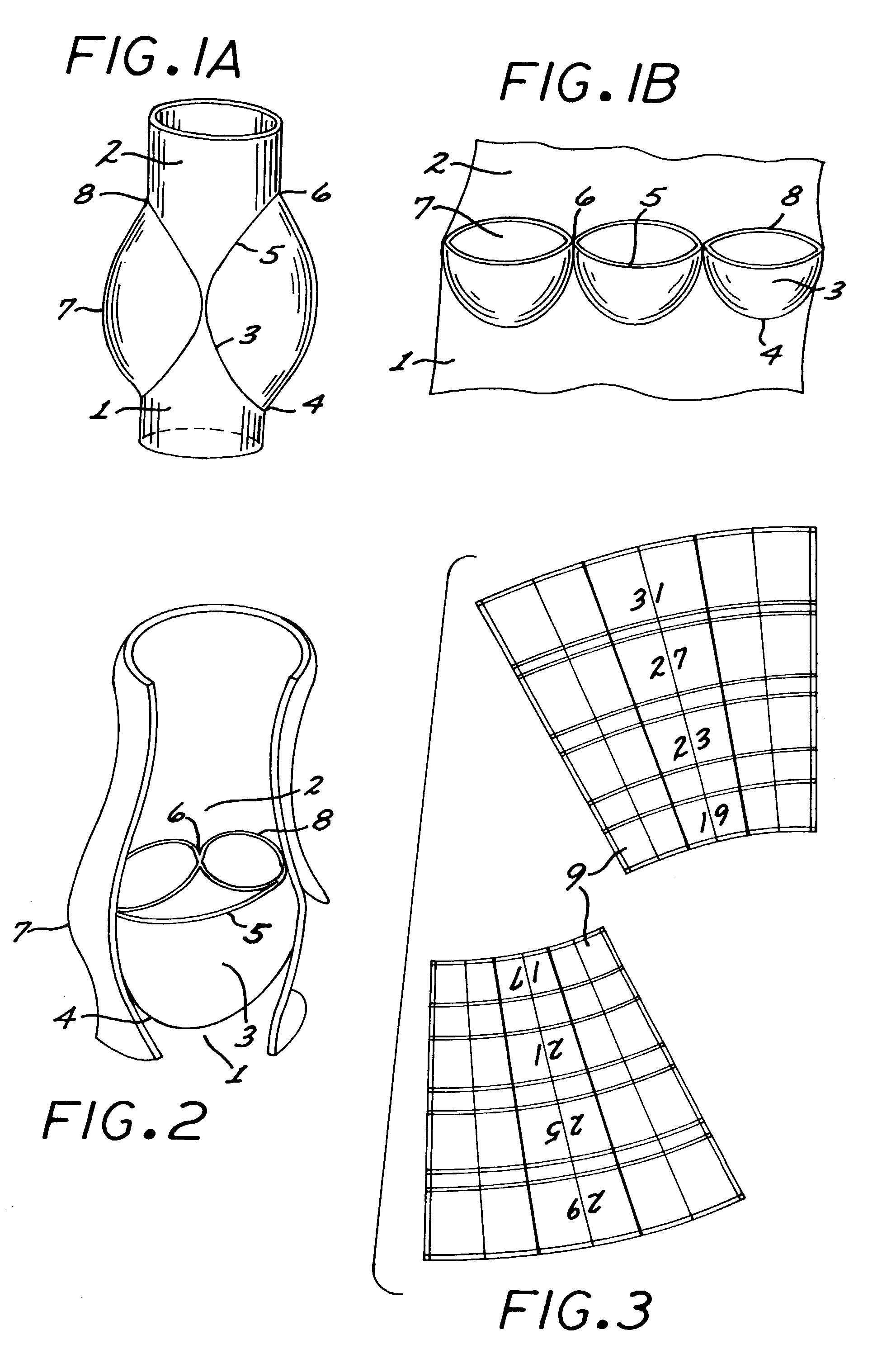
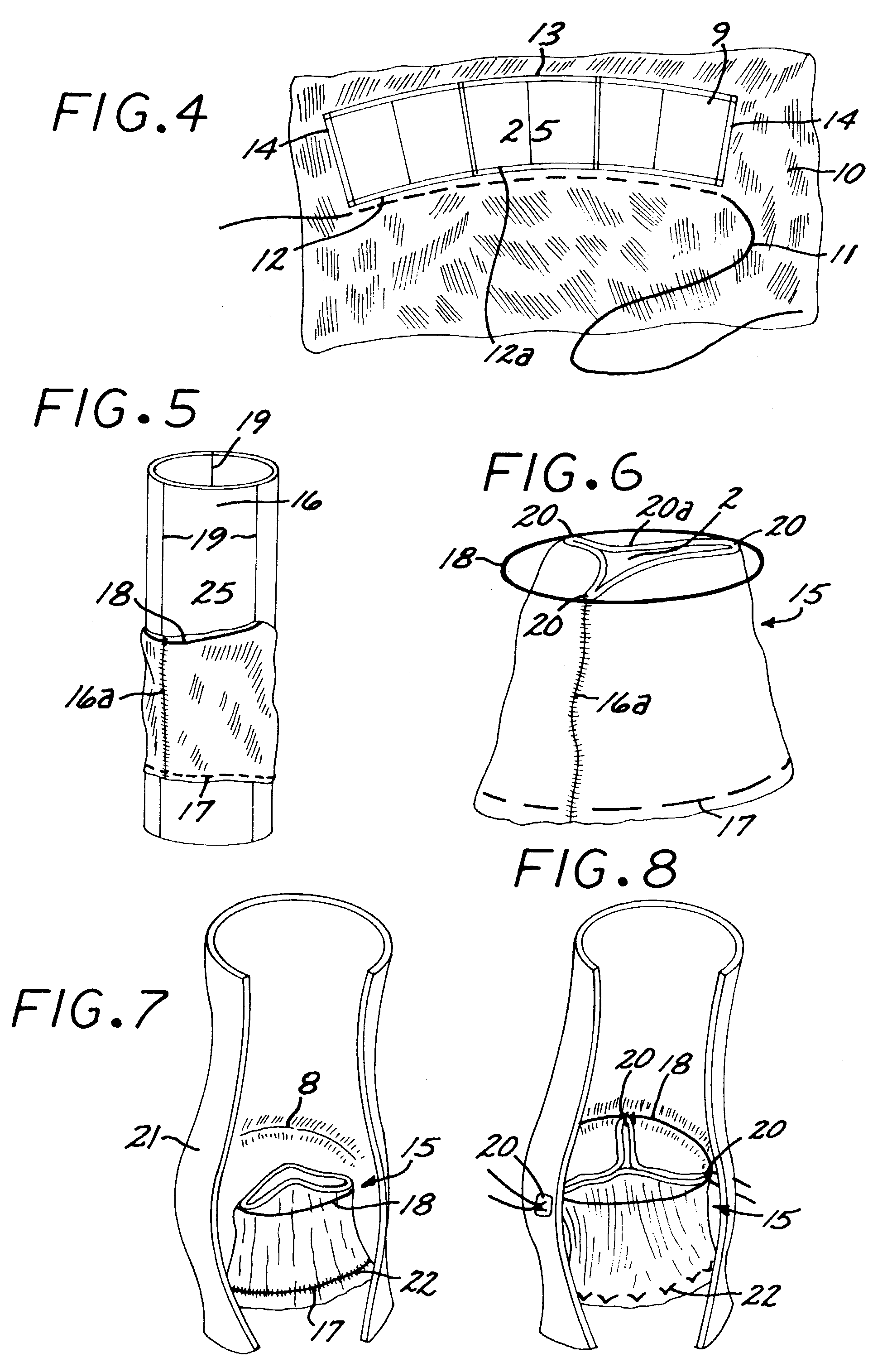
In accordance with the present invention, there is provided A method for implanting surgical device. The method involves providing a surgical device comprising an elongated flexible inflatable portion, an elongated flexible and substantially inextensible band portion having a distal end and a proximal end, and an inflatable portion. The band portion is attached to the inflatable portion along an inner face thereof. The band portion has a concave cross section when taken perpendicular to its longitudinal axis. The method involves deforming the band, so that it has a substantially flat cross section when taken perpendicular to the longitudinal axis, by encircling the band around body tissue.
Owner:ETHICON ENDO SURGERY INC
Surgically implantable adjustable band having a flat profile when implanted
An implantable surgical device having a deployed shape and an undeployed shape. The device includes an elongated flexible inflatable balloon portion and an elongated flexible and substantially inextensible band portion. The band portion has a distal end, a proximal end and a longitudinal axis therebetween. The band portion is attached to the balloon portion along an inner face thereof. When the device is in its undeployed shape at least a portion of the band portion has a concave cross section taken perpendicular to the longitudinal axis.
Owner:ETHICON ENDO SURGERY INC
Facet joint prosthesis
A prosthetic implant for replacing a facet joint of a spinal motion segment includes a generally conical superior component adapted to be implanted at a surgically prepared site on a lower articular process of a cephalad vertebra of a spinal motion segment, and a cup-shaped inferior component adapted to be implanted at a surgically prepared site on a superior articular process of a caudad vertebra of the spinal motion segment.
Owner:RE SPINE
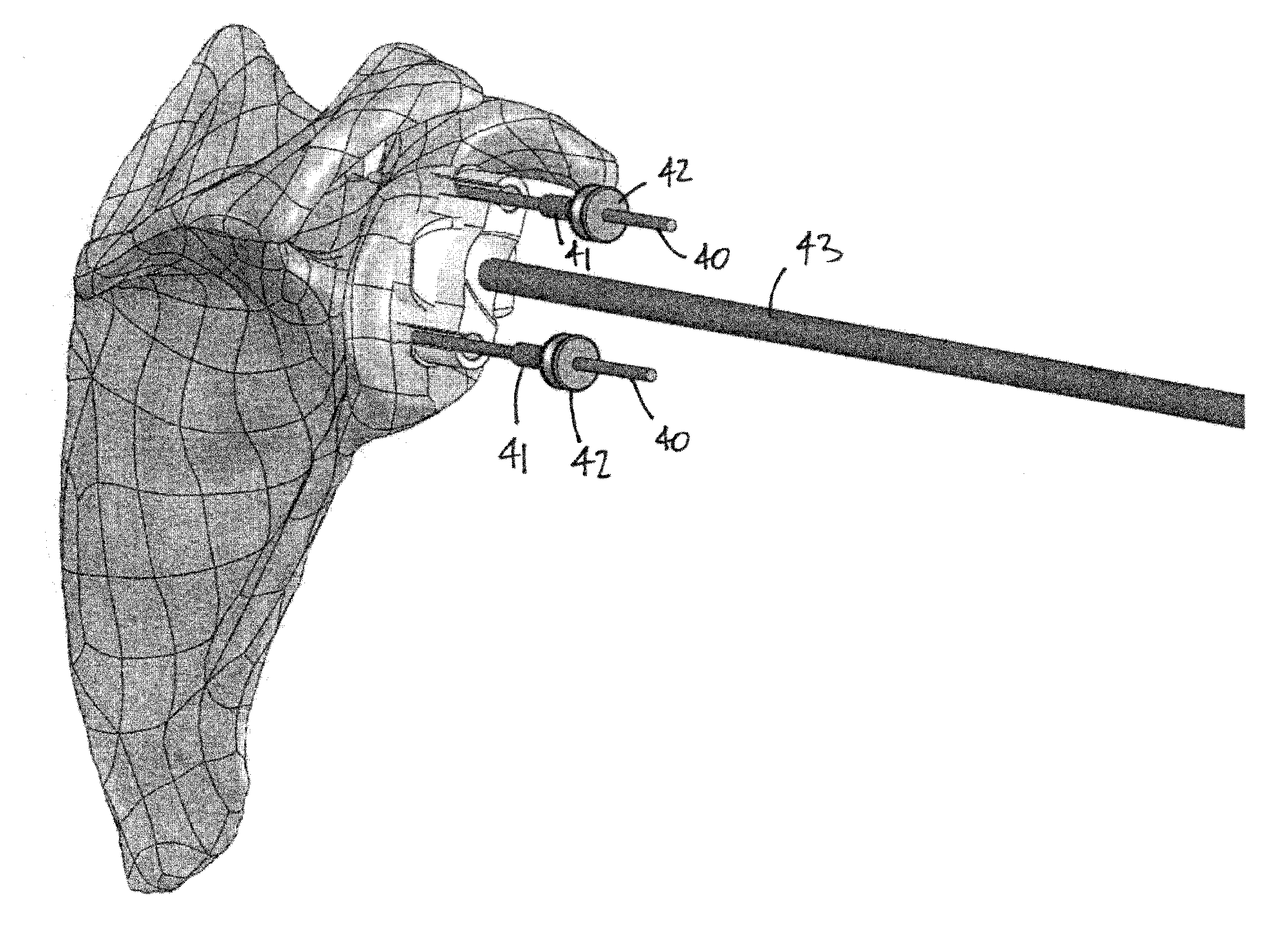
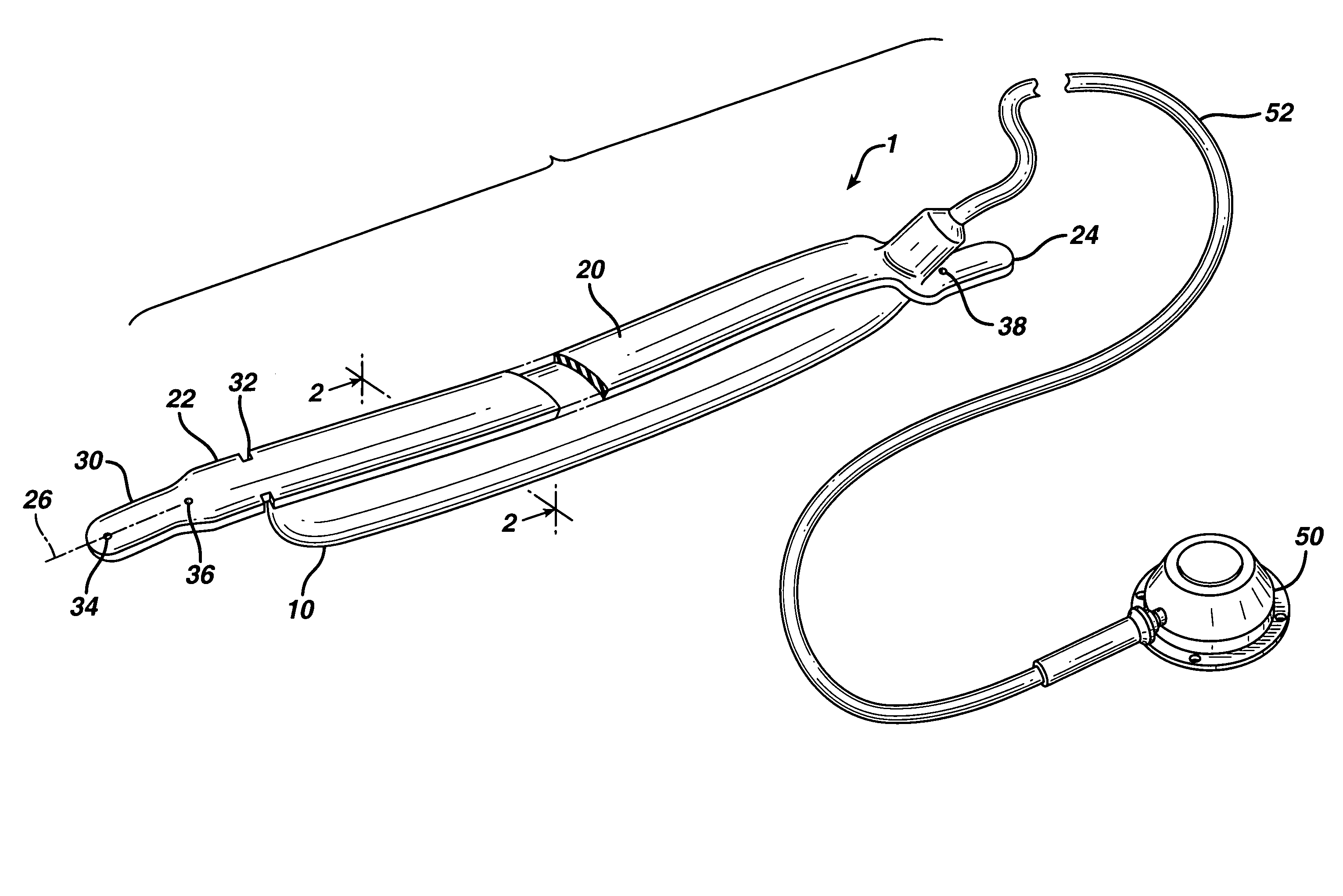
Glenoid implant surgery using patient specific instrumentation
ActiveUS20150073424A1Joint implantsComputer-aided planning/modellingDrill guideBiomedical engineering
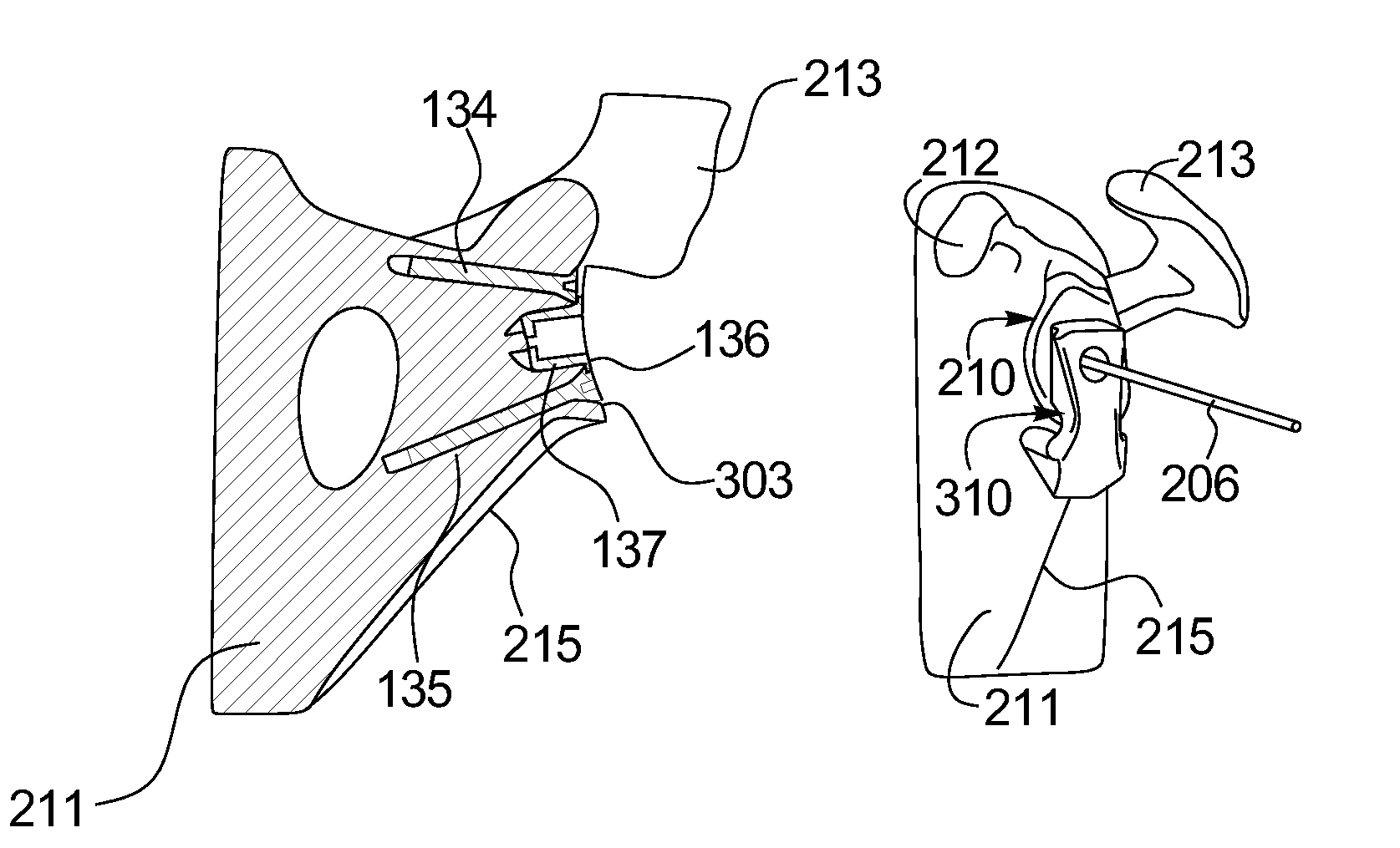
A pin placement instrument for placing a pin in a bone comprises an anatomical interface with a hook-like portion being opened in a lateral direction of the instrument to receive a bone therein in a planned position. A drill guide is connected to the anatomical interface and defining at least one guide slot in a longitudinal direction of the instrument. The guide slot has a lateral opening over its full length in the drill guide to allow lateral withdrawal of the instrument in said lateral direction with the pin placed in the bone passing through the lateral opening. A bushing is removably placed in said guide slot via said longitudinal direction in a planned fit, the bushing defining a throughbore aligned with the guide slot and adapted to receive the pin extending in said longitudinal direction when the bushing is in the guide slot for pin placement.
Owner:ZIMMER INC
Method for implanting an adjustable band
In accordance with the present invention, there is provided A method for implanting surgical device. The method involves providing a surgical device comprising an elongated flexible inflatable portion, an elongated flexible and substantially inextensible band portion having a distal end and a proximal end, and an inflatable portion. The band portion is attached to the inflatable portion along an inner face thereof. The band portion has a concave cross section when taken perpendicular to its longitudinal axis. The method involves deforming the band, so that it has a substantially flat cross section when taken perpendicular to the longitudinal axis, by encircling the band around body tissue.
Owner:ETHICON ENDO SURGERY INC
Intravascular device and method for axially stretching blood vessels
Intravascular devices and methods are provided for forming a vascular graft by axially distending a blood vessel to induce growth. These devices advantageously can be implanted via a catheter, thereby eliminating the need for a more invasive implantation procedure when the stretching is to be done in vivo. Preferably, the device for axially distending a blood vessel to induce growth of the vessel includes an intravascular stretching mechanism attachable directly to an interior lumen portion of the blood vessel, and a means for operating the stretching mechanism to cause the vessel to distend axially. The stretching mechanism can include a pair of wires or stents that engage the blood vessel wall. Components of the stretching mechanism can include a shape memory material.
Owner:GEORGIA TECH RES CORP
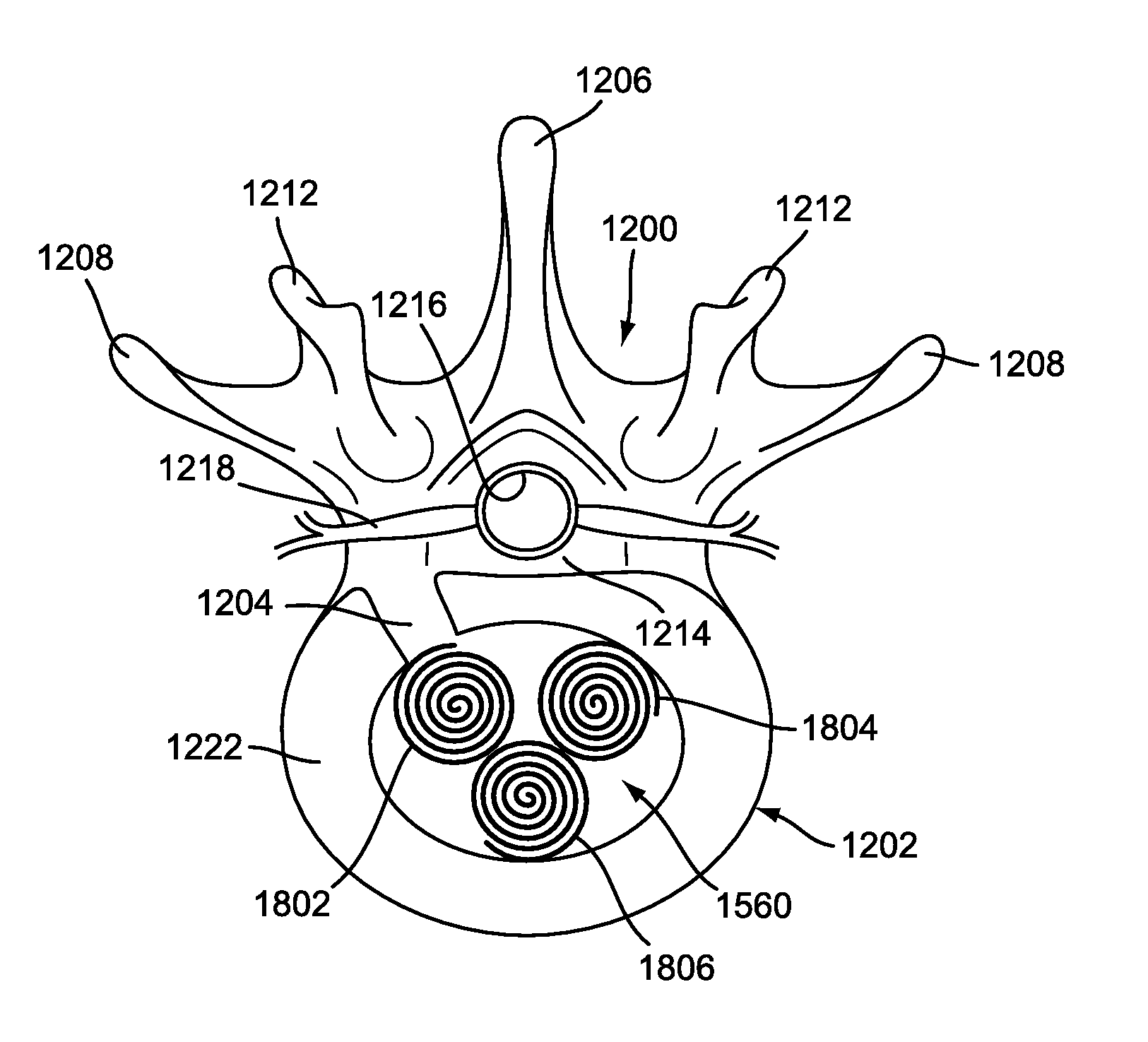
Disk Fusion Implant
An implant strip is disclosed. In some cases, the prosthesis can take the form of an implant strip that may be implanted through the use of a surgical procedure that minimizes incision sizes and may be considered less invasive than typical spinal implant procedures. The implant strip includes provisions for implantation, including teeth, spacing provisions and various shapes.
Owner:JMEA CORP
Systems and methods for transcatheter aortic valve treatment
ActiveUS8545552B2Shorter and straight access pathEasy to placeSuture equipmentsStentsTRANSCATHETER AORTIC VALVE IMPLANTBlood vessel
Owner:SILK ROAD MEDICAL
Sheet or Tubular Structure Consisting of Elastic Biocompatible Material and its Use
InactiveUS20090182425A1Flexibility and ease of use and point-of-care individualizationHigh selectivitySurgeryPharmaceutical delivery mechanismBiomedical engineeringBiologically active substances
The present invention relates to a sheet or tubular structure consisting of biocompatible material, which is elastic and comprises at least one biologically active substance in at least one region and to a sheet or tubular structure, which comprises at least one biologically active substance release-modifying agent in at least one region as well as an implant covered at least partially by the sheet or tubular structure of the invention. The present invention further provides a method and an apparatus for producing, and preferably customizing and / or optimizing, the sheet or tubular structure of the invention. The sheet or tubular structure of the invention can be customized / optimized before implantation in the operating room.
Owner:CHARITE UNIVS MEDIZIN BERLIN
Manufacturing method for jaw defect individual restoration
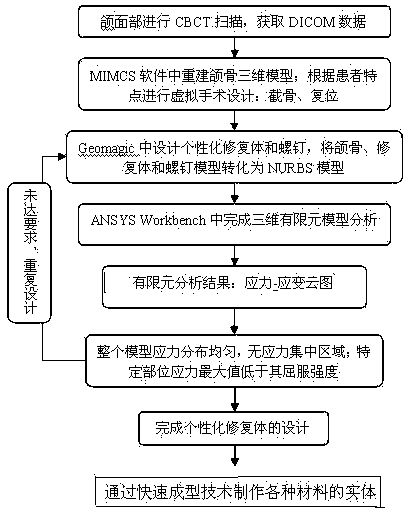
ActiveCN103919631AHigh breaking strengthIncrease stressBone implantSurgeryBiomechanicsComputer-aided
The invention relates to the fields of computer-aided technology and maxillofacial plastic medical apparatus and instruments, in particular to an optimal design and manufacturing method for a jaw defect individual restoration. The optimal design and manufacturing method is characterized by comprising the following steps: conducting CBCT scanning on the maxillofacial part of a patient, obtaining DICOM data, guiding the data into three-dimensional modeling software-MIMICS (Materialise, Belgium) to reconstruct a jaw three-dimensional model, designing the individual restoration, designing models of a plurality of virtual screw according to the size of retention screws supposed to be adopted, then moving the models to the appropriate positions of the jaw to simulate the situation to the screw retention restoration, obtaining Von Mises stress and a strain nephogram, and manufacturing entities of various materials through the fast forming technology. The jaw defect individual restoration made through the manufacturing method for the jaw defect individual restoration has good performance in biomechanical properties including breaking strength, stress-strain distribution, the service life and the like. So far, a patient with upper and lower jaw defects accepting an implanting operation is observed for two years, and no bad complications of fracture, exposure and the like happen.
Owner:SICHUAN UNIV
Multilayer composite vascular access graft
ActiveUS7297158B2Improve performanceDesirable characteristicStentsMedical devicesMedicineBlood vessel
A multilayer composite vascular access graft and a method of constructing such a graft are disclosed. The mcVAG has improved performance characteristics, which include desirable handling characteristics such as ease of suturing, kink resistance and the ability to serve as a cannulation route soon after the implant procedure.
Owner:TC1 LLC
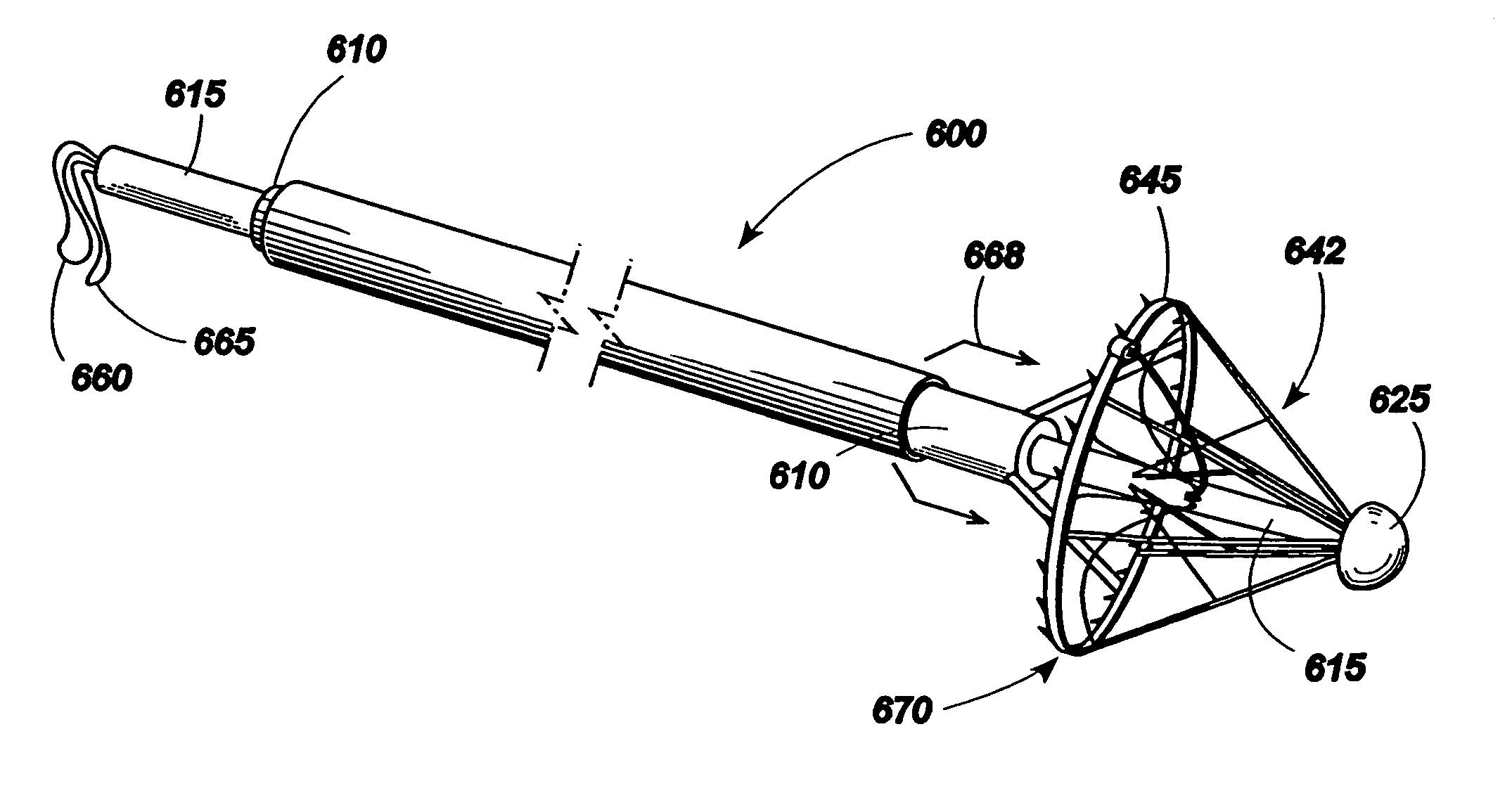
Reed valve for implantation into mammalian blood vessels and heart with optional temporary or permanent support
A multi-leaflet valve adapted to serve as a prosthesis for diseased native valve of a mammal is constructed of biologic membrane or of biocompatible synthetic membrane. The valve has the shape of a truncated cone that has an inflow and an outflow orifice with leaflets forming the outflow orifice and forming a plurality of commissures. A first flexible stent is removably affixed in a substantially circular fashion around the truncated cone in proximity of the inflow orifice, and a second flexible stent is removably affixed at the location of the commissures to form a circle around the truncated cone in proximity of the outflow orifice. The stents maintain the shape of the valve during the surgical implantation procedure. Each stent independently can be left in the valve or can be removed during the implantation procedure based upon the judgement of the cardiac surgeon performing the implantation procedure. A holder designed to maintain the geometry of the valve during implantation to a mammal is also disclosed.
Owner:THE INT HEART INST OF MONTANA FOUND
Apparatus and method for implantation of surgical devices
InactiveUS8343165B2Minimized in sizeMinimize damageInternal osteosythesisSpannersImplantation SiteSurgical device
A driver apparatus for implanting spinal rod fixation devices including an anchor member and a coupling member pivotable relative to the anchor member is disclosed. The driver apparatus generally immobilizes the anchor member and coupling member relative to each other and to the driver apparatus during insertion to minimize interference between the coupling member and tissues surrounding the implantation site, as well as to minimize the clearance required for implantation. The driver apparatus includes a driver portion engageable with the anchor member for effecting seating of the anchor member, such as by threadably driving the anchor member in the bone. The driver portion is closely fit between portions such as upstanding walls of the coupling member to prevent relative rotation therebetween. The driver apparatus further includes locking portions shiftable to secure the coupling member with the driver apparatus when the driver is engaged with the anchor member.
Owner:PIONEER SURGICAL TECH INC
Methods and devices for installing standard and reverse shoulder implants
Surgical procedures, tools and implants are disclosed for both conventional and reverse shoulder implant surgeries. The improved procedures, tools and implants relate to humeral head resurfacing, humeral head resection for standard implants, humeral head resection for reverse shoulder implants, glenoid resurfacing for standard shoulder implants and glenoid resurfacing for reverse shoulder implants. 3D scans and x-rays are used to develop virtual models of the patient anatomy, identify patient specific landmarks for anchoring guide wire installation blocks, templates and drill guides. 3D scans are also used to design patient specific tools and implants for the shoulder implant procedures and to pre-operatively determine the appropriate inclination and retroversion angles.
Owner:SMITH & NEPHEW INC
Disc Implant
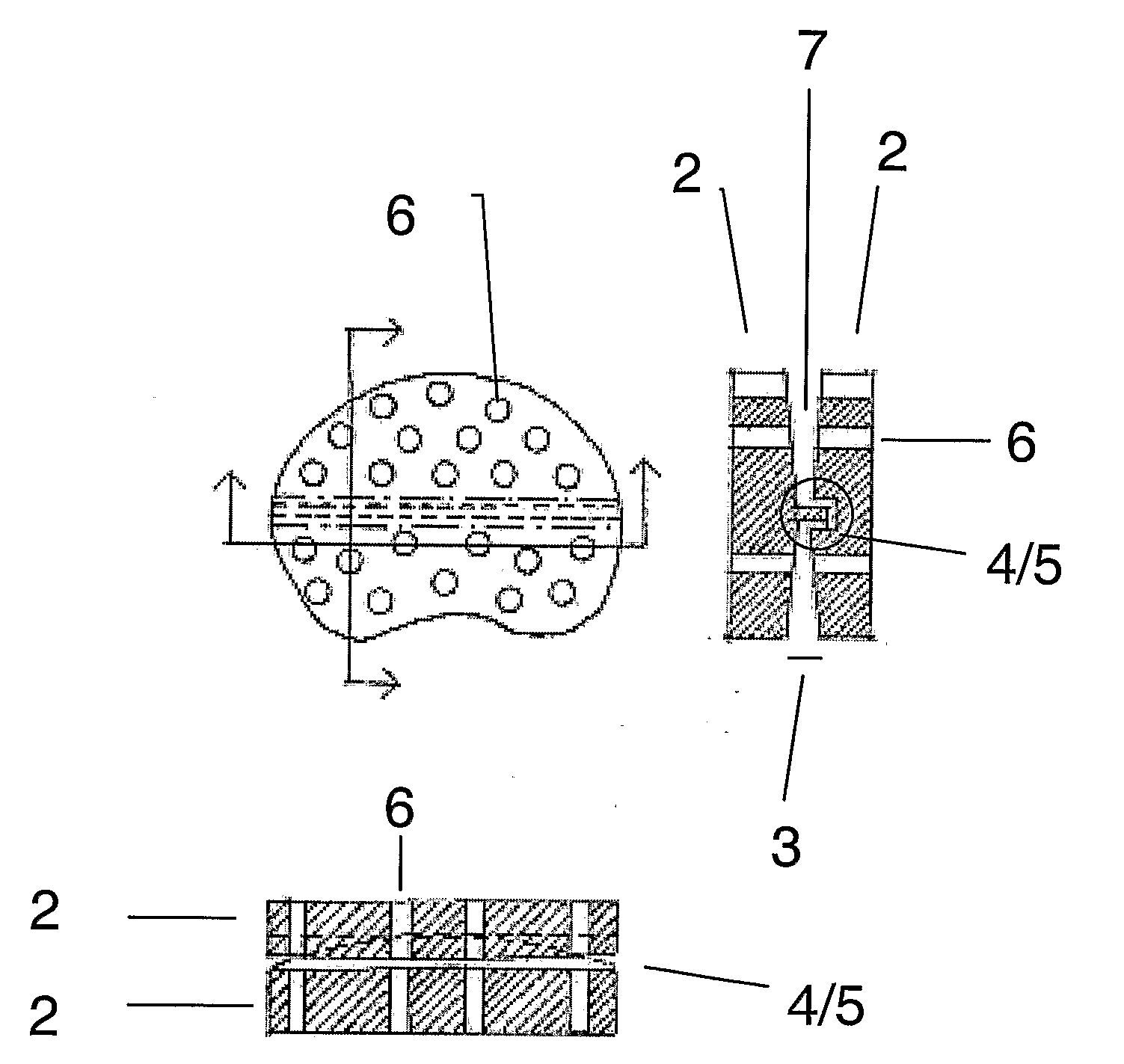
A problem with total disc implant surgery appears to be the positioning of the implant which if not correct may lead to pain and eventually new surgery. The present invention relates to an improved disc implant (1) for total disc replacement, comprising two inter-vertebral elements (2) which are flexibly connected via coupling means (4,5). Following surgery, the relative movability of said two inter-vertebral elements is decreased overtime, as bone ingrowth occurring around the implant and specifically through osseointegrative sections gradually degrease the movability of the elements relative to each other Following, the relative movability of the implant elements is replaced by fixation of the elements. The fixation has flowingly occurred in a position affected by the movement of the patient, and is thereby more acceptable to the patient.
Owner:FBCDEVICE
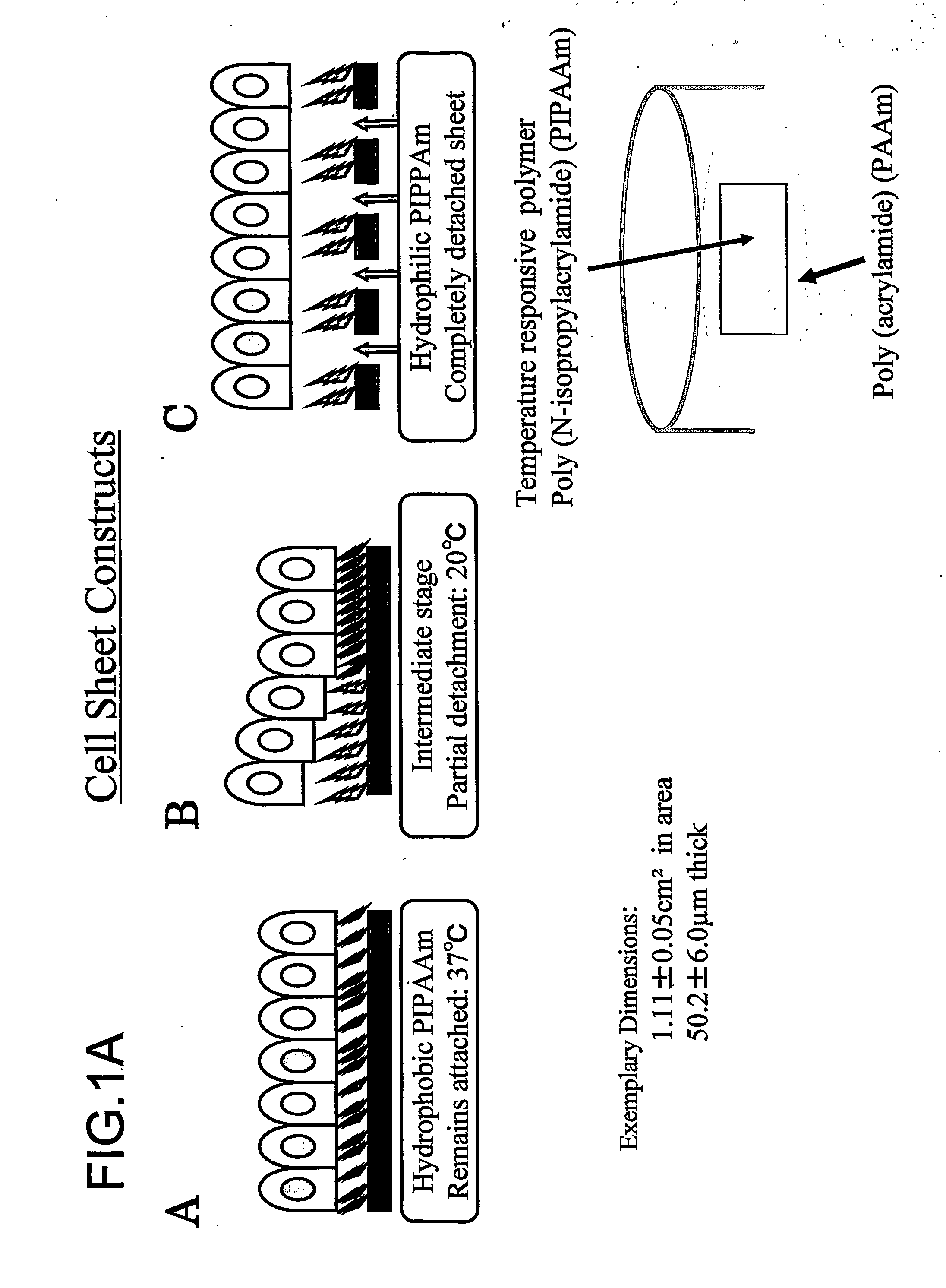
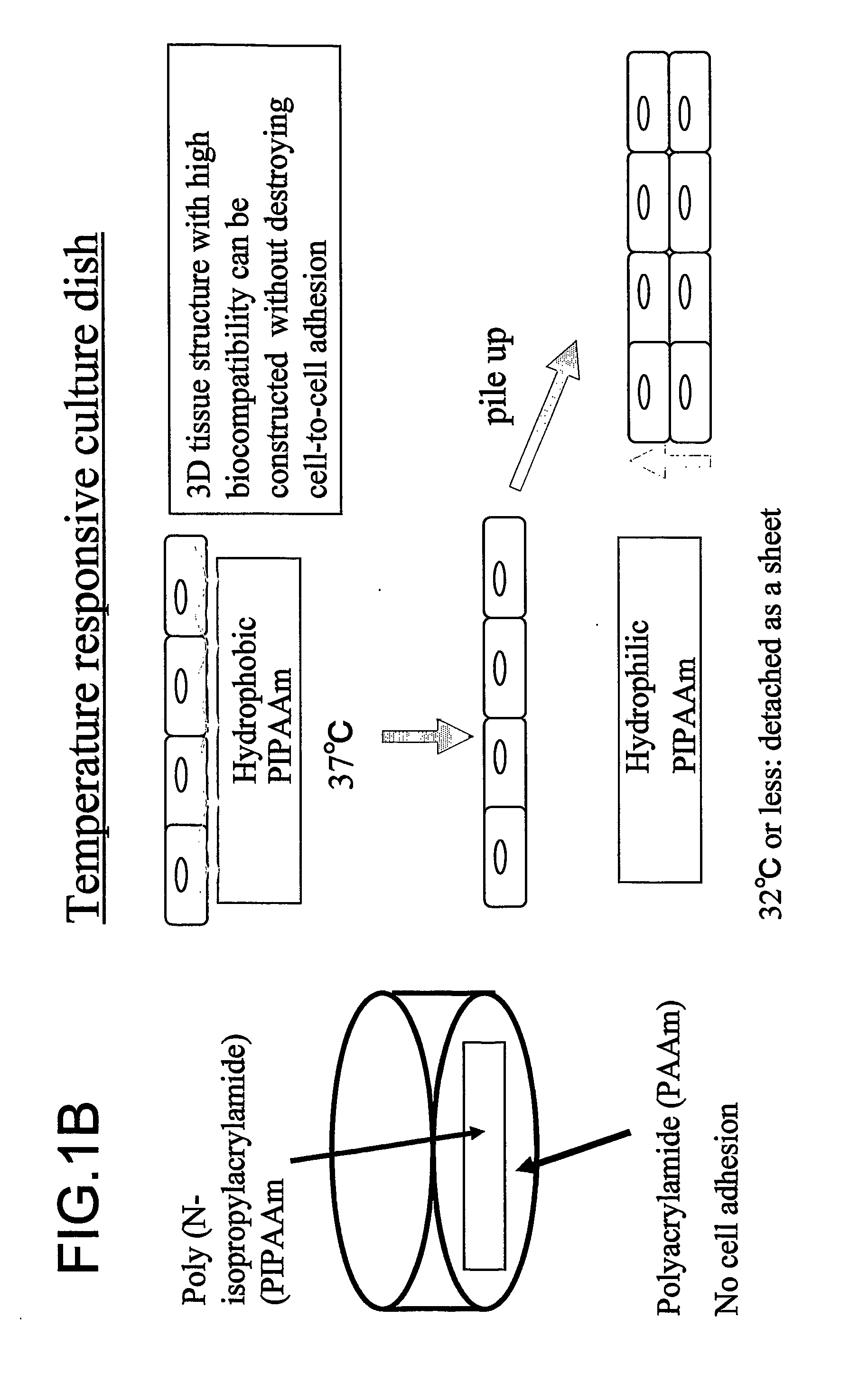
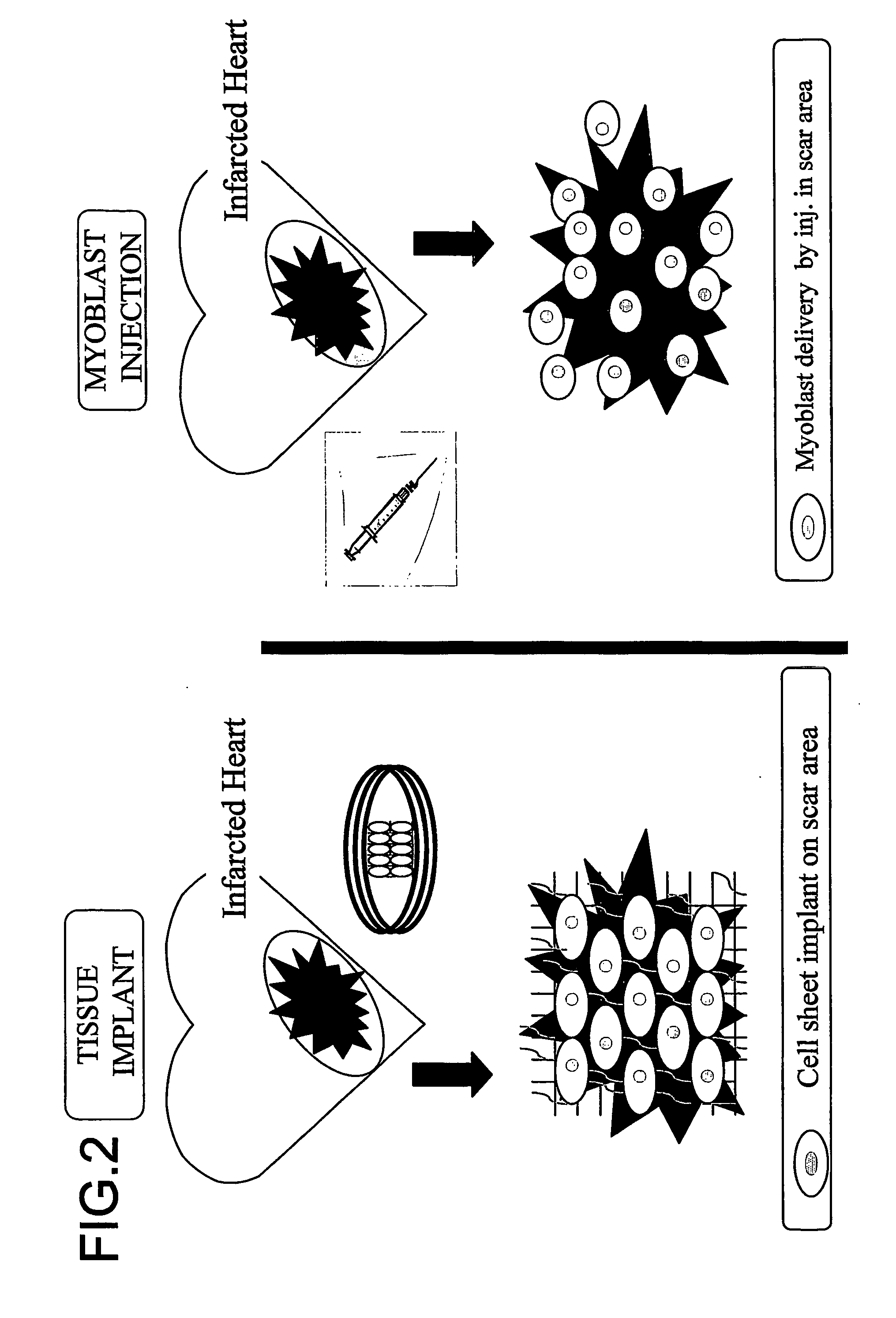
Three-dimentional tissue structure
InactiveUS20070092492A1Easy to identifySmall sizeBiocideMammal material medical ingredientsCulture cellCardiac muscle
The present invention provides a prosthetic tissue or sheet capable of withstanding implantation operations, which can be used in actual operation and can be produced by culture. The present invention also provides a novel therapy which can substitute for cell therapy. Particularly, the present invention provides a method for producing a prosthetic tissue comprising a cell derived from a part other than myocardium and capable of withstanding implantation operation. The above-described objects of the present invention were partially achieved by finding that by culturing cells under specific culture conditions, the cells are unexpectedly organized into a tissue, and the resultant prosthetic tissue is capable of being detached from culture dishes. The present invention also provides a three-dimensional structure applicable to heart, comprising a cell derived from a part other than the myocardium of an adult.
Owner:CELLSEED
Method of making a surgical template used for a computer-guided dental implant surgery
InactiveUS20100316974A1Excellent in implant-guiding effectData efficientDental implantsTeeth fillingComputed tomographySurgical template
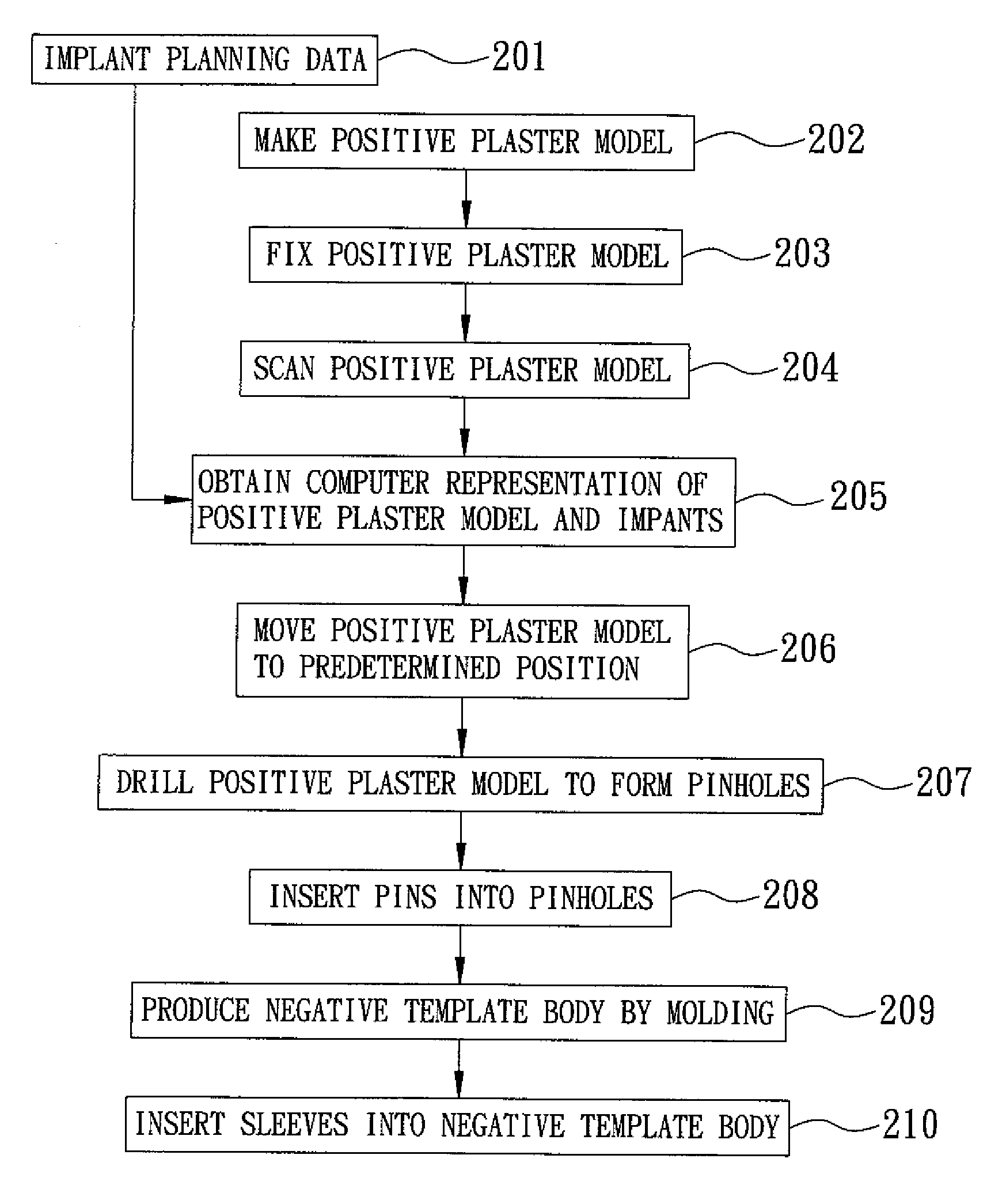
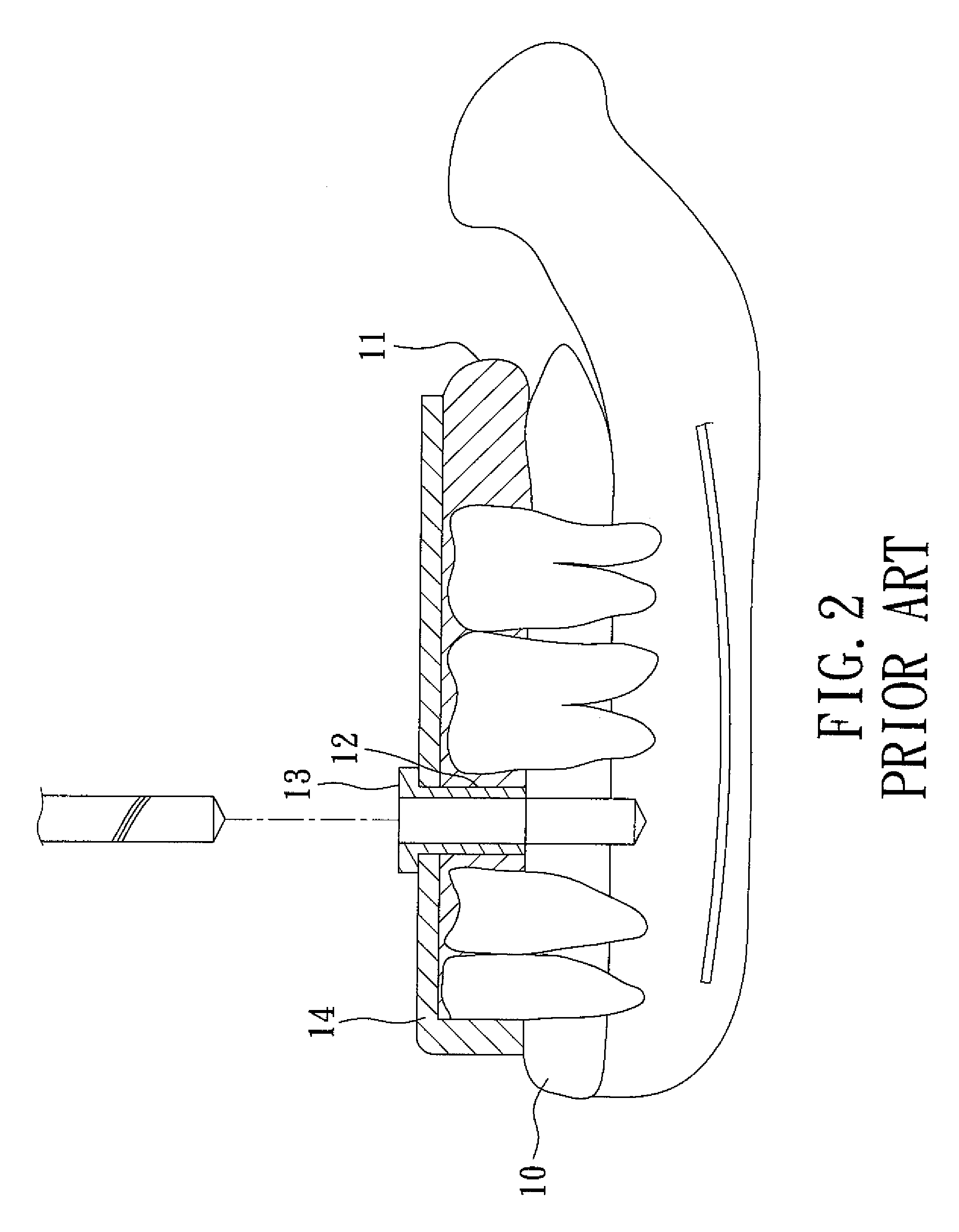
A method of making a surgical template comprises: producing a 3-D geometrical image by a CT scanning performed on a patient's jaw and establishing corresponding implant planning data to obtain a 3-D first digital image, making a positive plaster model of the patient's jaw, scanning the plaster model to obtain a 3-D second digital image, overlapping the second digital image on the first digital image to obtain a computer representation of the plaster model and at least one implant to be mounted according to the implant planning data, drilling the plaster model to form at least one pinhole according to the implant planning data, inserting a pin into the pinhole, producing a negative template body from an assembly of the plaster model and the pin with a thermoplastic dental material so that the negative template body has at least one implant guide hole and constitutes the surgical template.
Owner:POU BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com