Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
30 results about "Degenerative disc" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
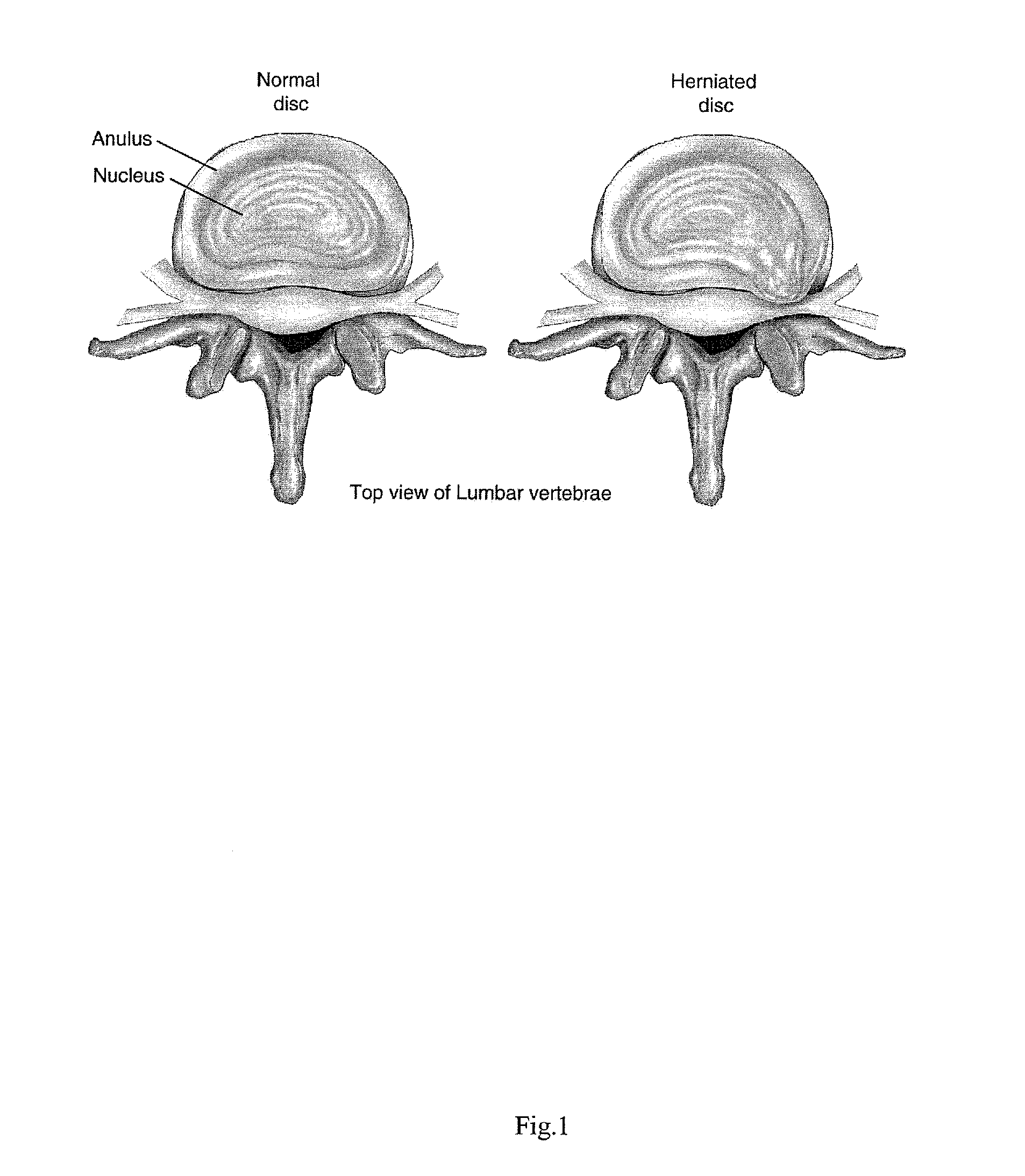
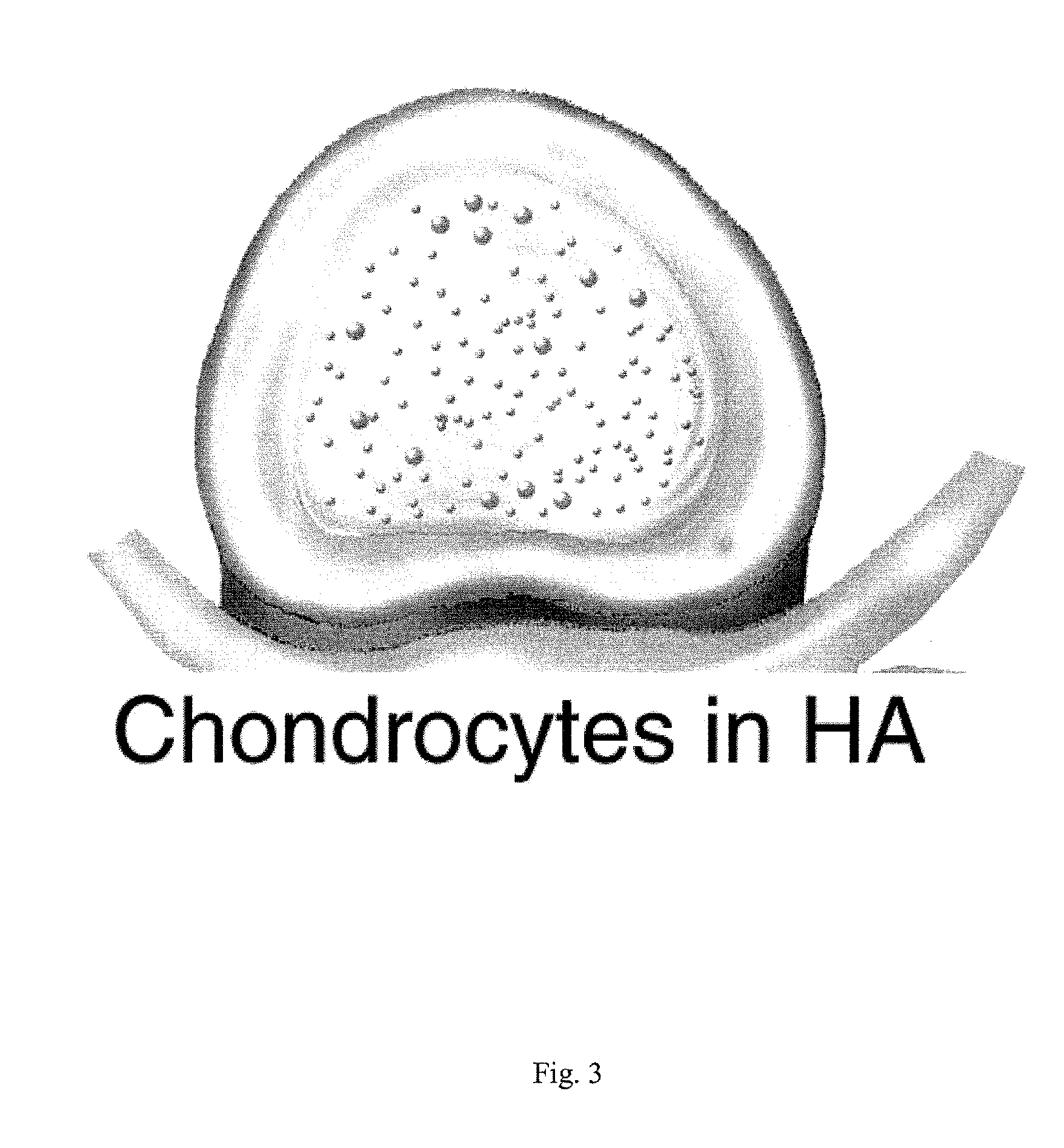
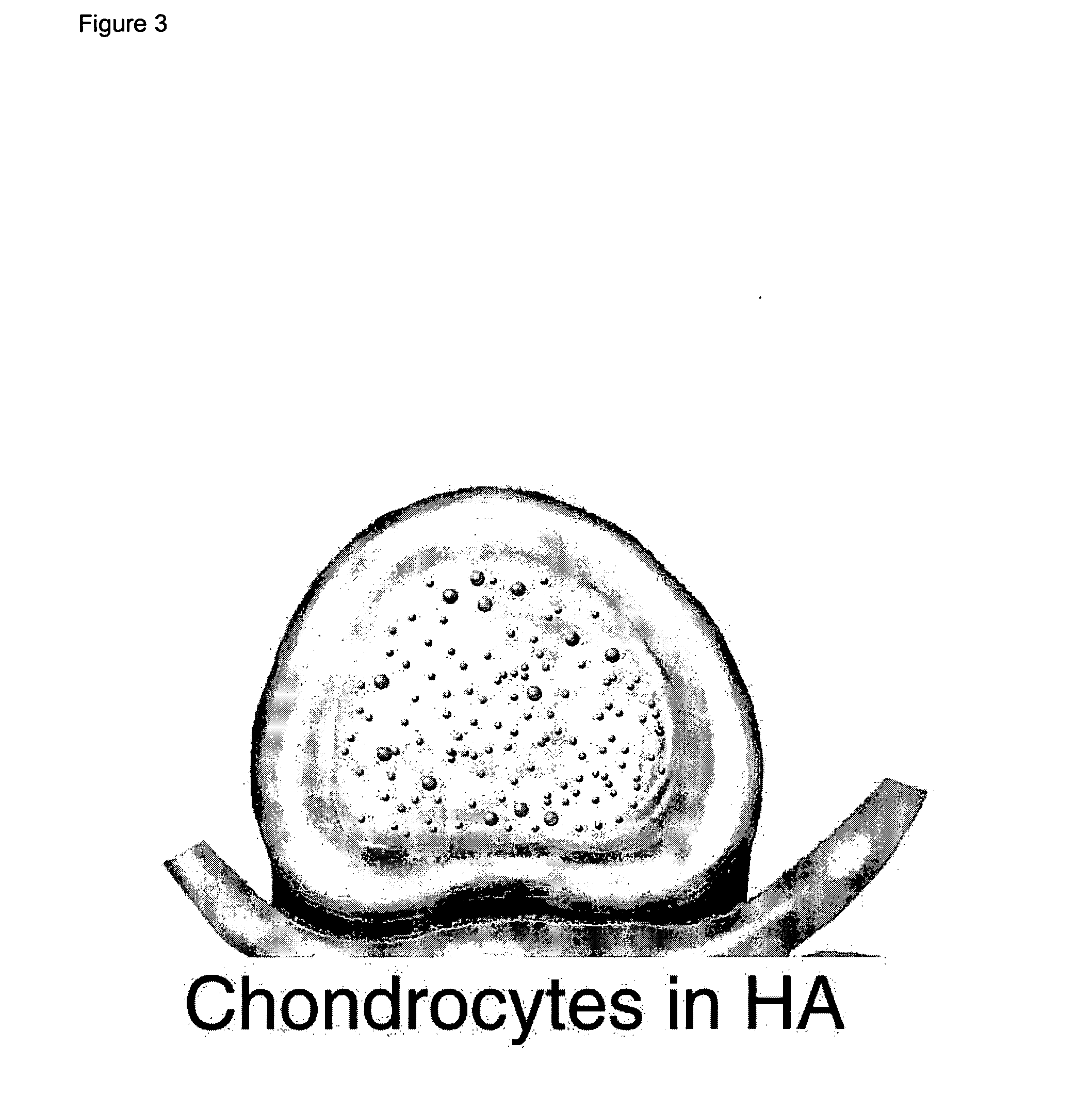
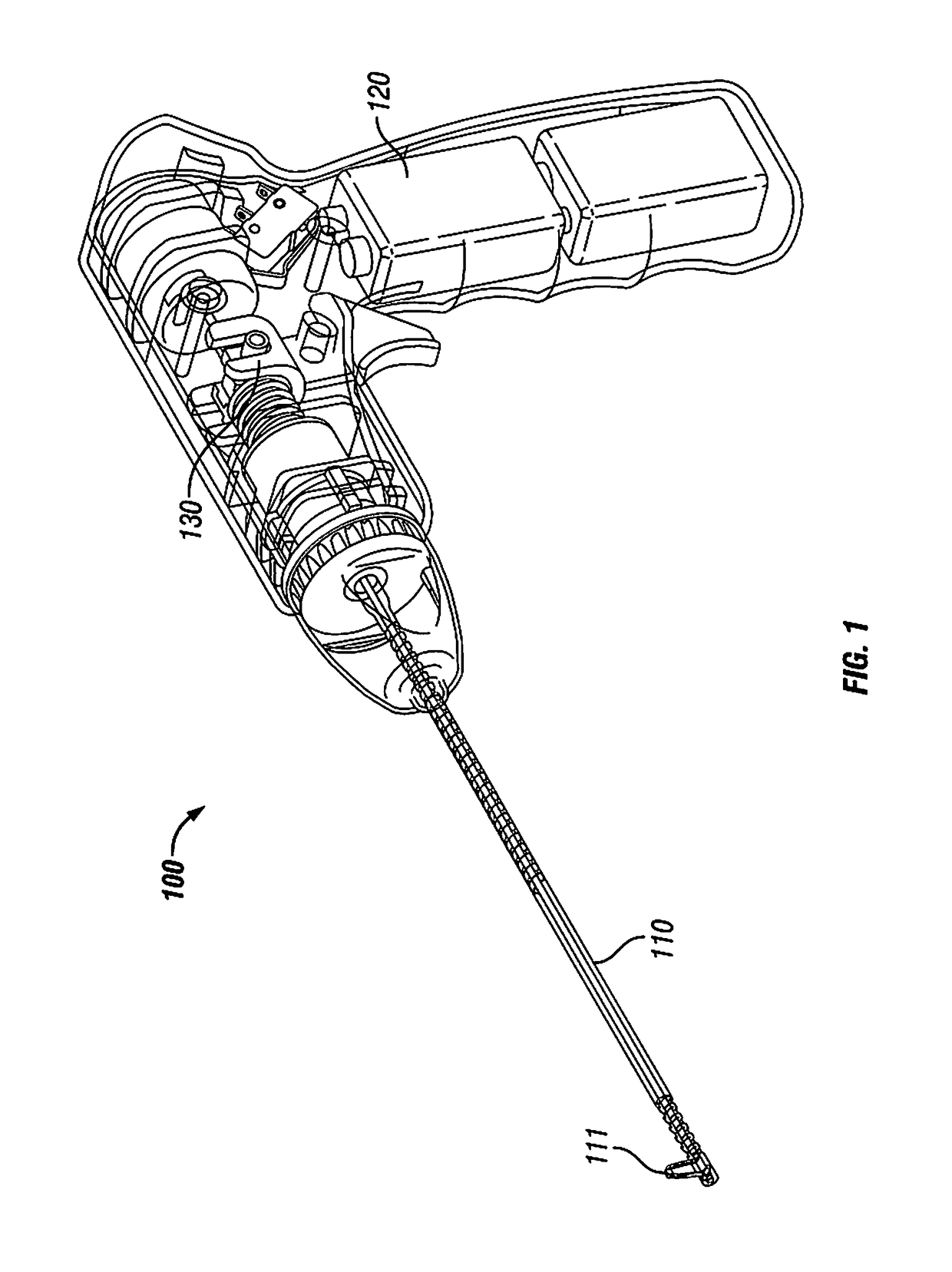
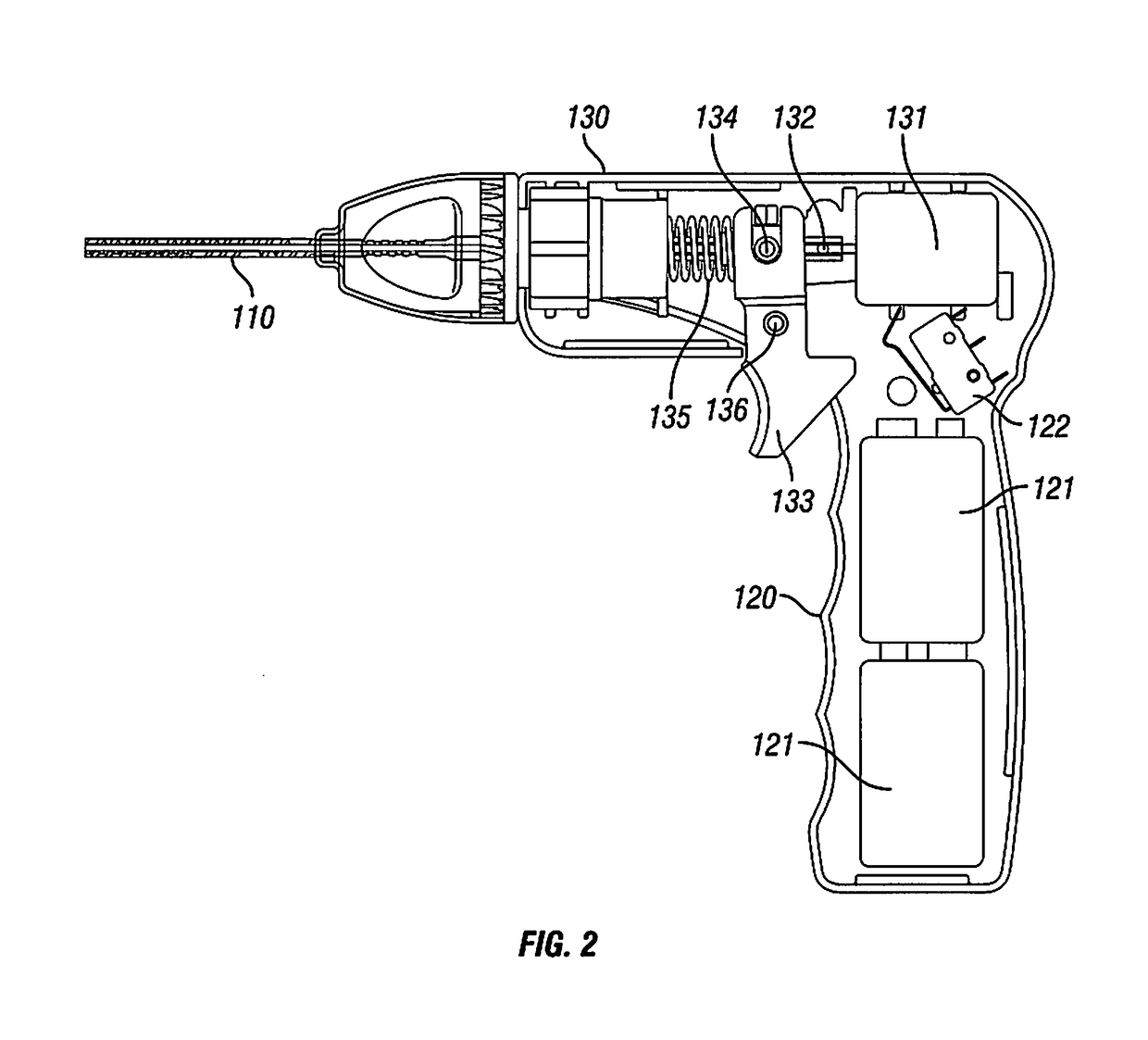
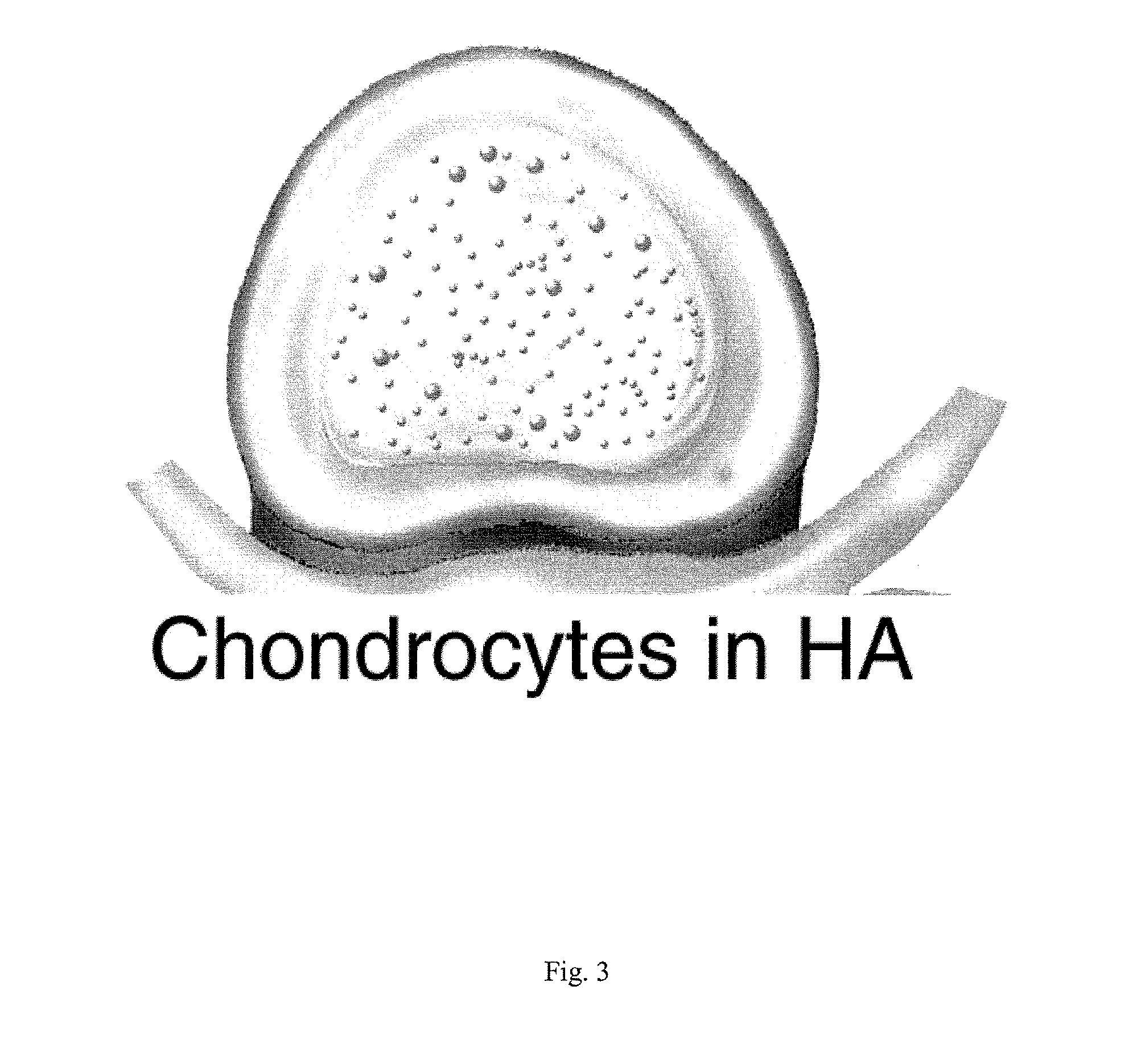
Intervertebral Disc Repair, Methods and Devices Therefor
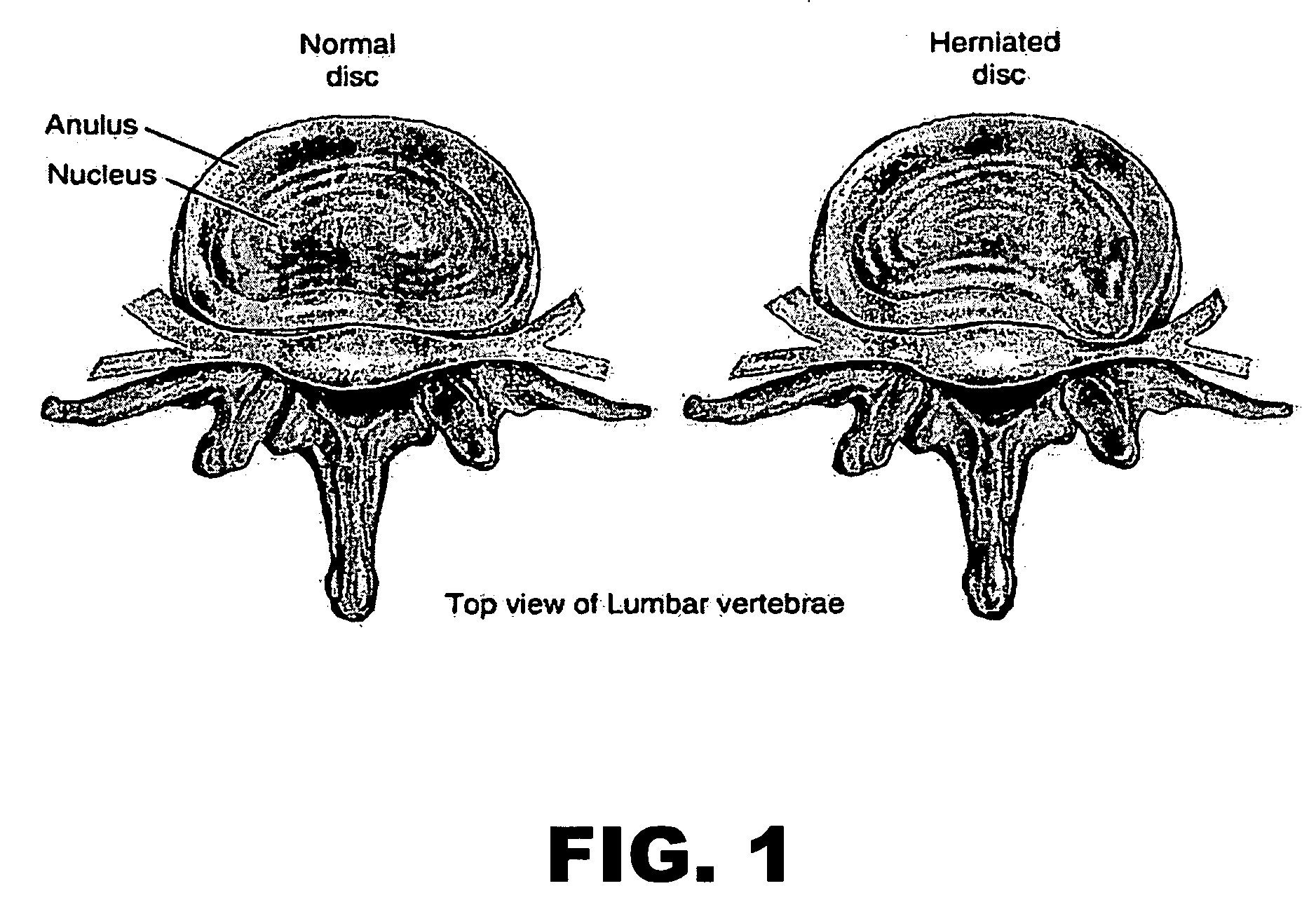
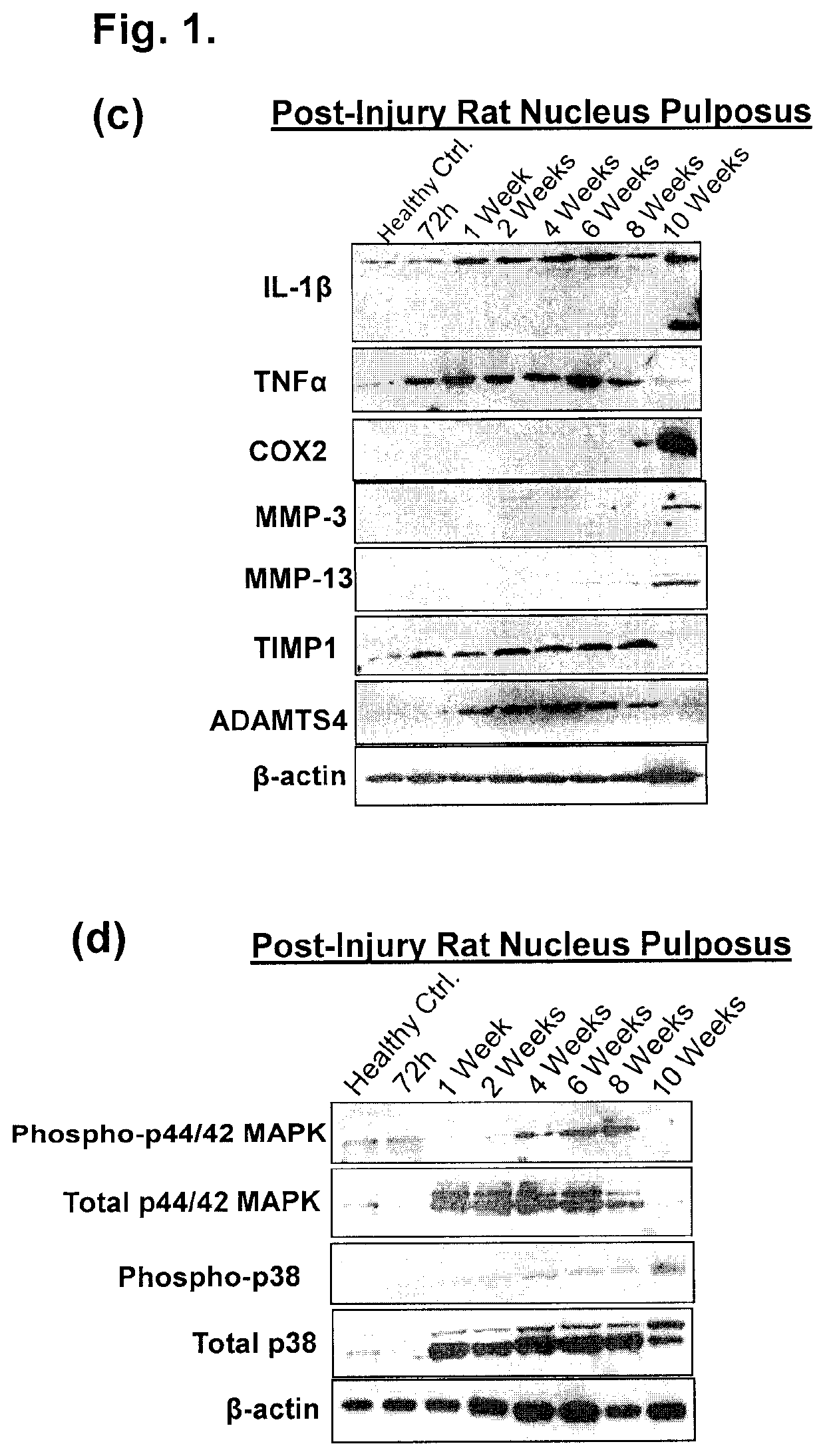
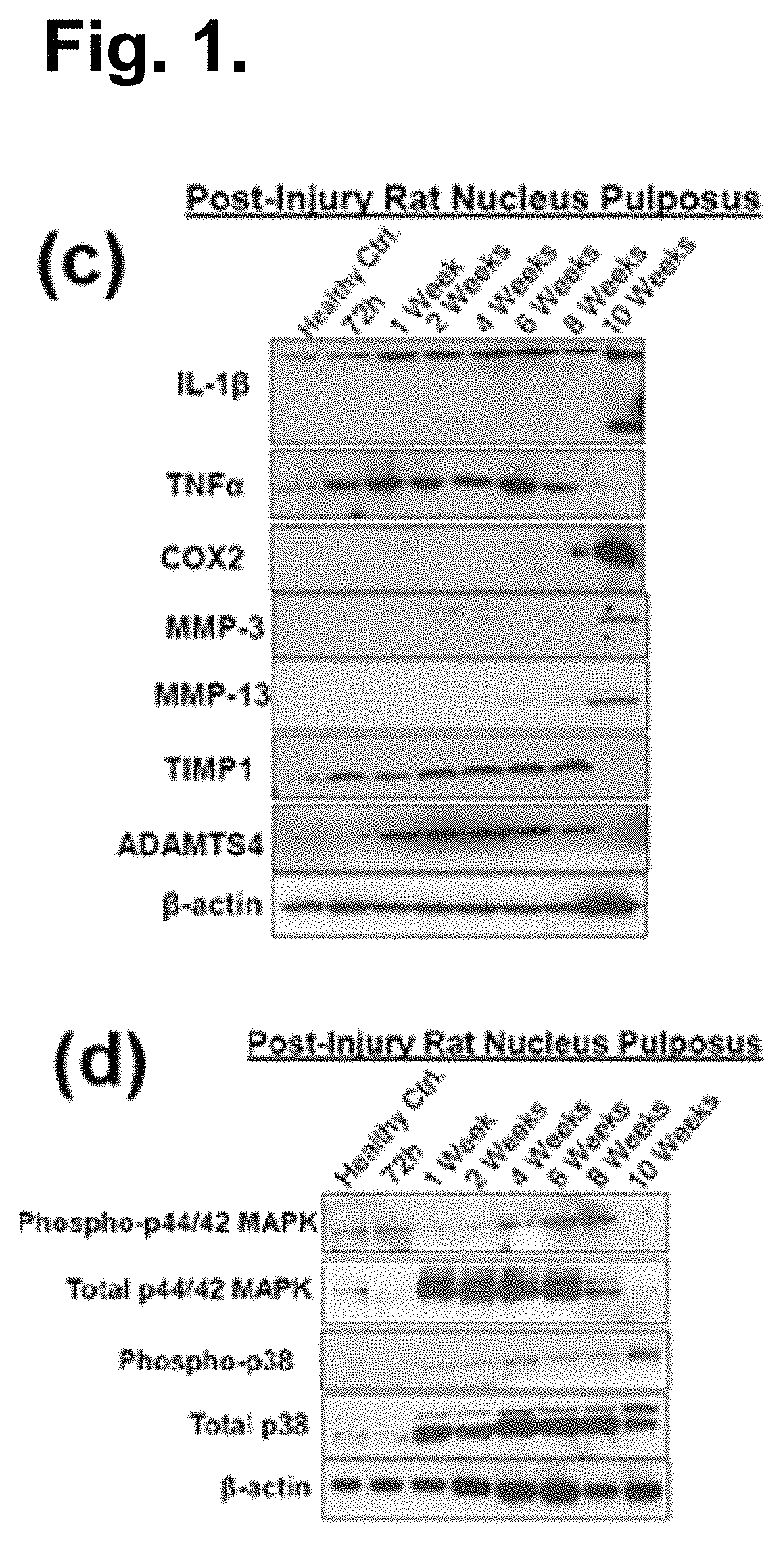
The present application discloses compositions, methods and devices for treatment of a degenerative intervertebral disc. A composition can comprise chondrocytes expressing type II collagen. These chondrocytes can be obtained from human cadavers up to about two weeks following death, and can be grown in vitro. The compositions can further comprise one or more biocompatible molecules. Treatment of a degenerative disc can comprise injecting or implanting a composition comprising the chondrocytes into a degenerative disc through an aperture or incision. If the aperture or incision is closed with a suture or a glue after introduction of the chondrocytes, the closure can withstand over 400 N of compression force.
Owner:VELOCITY FINANCIAL GRP INC ITS SUCCESSORS & ASSIGNS
Intervertebral disc repair, methods and devices therefor
ActiveUS20050196387A1High molecular weightBiocidePeptide/protein ingredientsHuman cadaverType II collagen
The present application discloses compositions, methods and devices for treatment of a degenerative intervertebral disc. A composition can comprise chondrocytes expressing type II collagen. These chondrocytes can be obtained from human cadavers up to about two weeks following death, and can be grown in vitro. The compositions can further comprise one or more biocompatible molecules. Treatment of a degenerative disc can comprise injecting or implanting a composition comprising the chondrocytes into a degenerative disc.
Owner:VELOCITY FINANCIAL GRP INC ITS SUCCESSORS & ASSIGNS
Degenerative disc regeneration techniques
InactiveUS20070093905A1Reducing TNF-a productionLowering IL-1 productionSpinal implantsTissue regenerationIntervertebral diskDegenerative disc disease
In the repair / regenerate of the intervertebral disc, all or a portion of the nucleus pulposus or annulus fibrosus is excised and treated for reinsertion into the disc or adjacent or alternate level disc. Alternatively certain bioactive agents may be injected into the degenerative disc without excising disc material.
Owner:DEPUY SPINE INC (US) +1
Mixed porous structure interbody fusion cage and preparation method thereof
InactiveCN102440852AGood mechanical compatibilityGood bone conductionSpinal implantsFreeze-dryingReticular formation
Disclosed are a mixed porous structure interbody fusion cage and a preparation method thereof. The interbody fusion cage comprises a porous metal support and porous structure filling bodies, the porous metal support is a three-dimensional net-shaped structure, a plurality of holes are arranged in the porous metal support, and the porous structure filling bodies are fully filled in the holes. The preparation method includes steps that the metal rapid forming technology is directly combined with the freeze drying technology, the porous metal support is manufactured via a structural design and the direct metal rapid forming technology, then uniformly mixed polymer gel or polymer / biological ceramic compound gel is poured in the porous metal support to realize freeze treatment, so that the porous structure filling bodies with the micropore feature are formed after freeze drying, and the mixed porous structure interbody fusion cage is obtained. Mechanical compatibility is good, contact area between the mixed porous structure interbody fusion cage and natural centrum is further increased, instant stability is good, fusion rate is improved, and the mixed porous structure interbody fusion cage and the preparation method thereof can be used for treating clinical degenerative disc diseases.
Owner:SHANGHAI JIAO TONG UNIV
Intervertebral Disc Repair, Methods and Devices Therefor
The present application discloses compositions, methods and devices for treatment of a degenerative intervertebral disc. A composition can comprise chondrocytes expressing type II collagen. These chondrocytes can be obtained from human cadavers up to about two weeks following death, and can be grown in vitro. The compositions can further comprise one or more biocompatible molecules. Treatment of a degenerative disc can comprise injecting or implanting a composition comprising the chondrocytes into a degenerative disc.
Owner:VELOCITY FINANCIAL GRP INC ITS SUCCESSORS & ASSIGNS
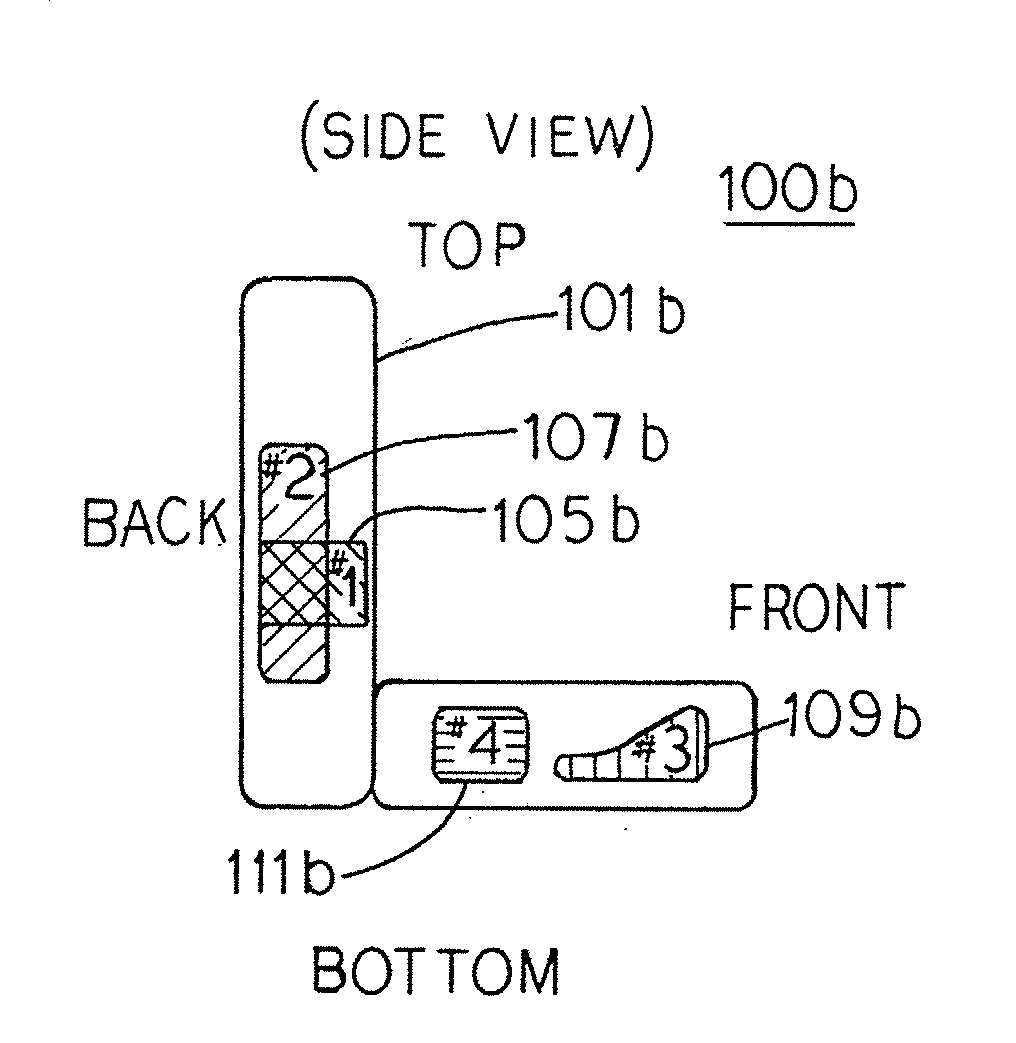
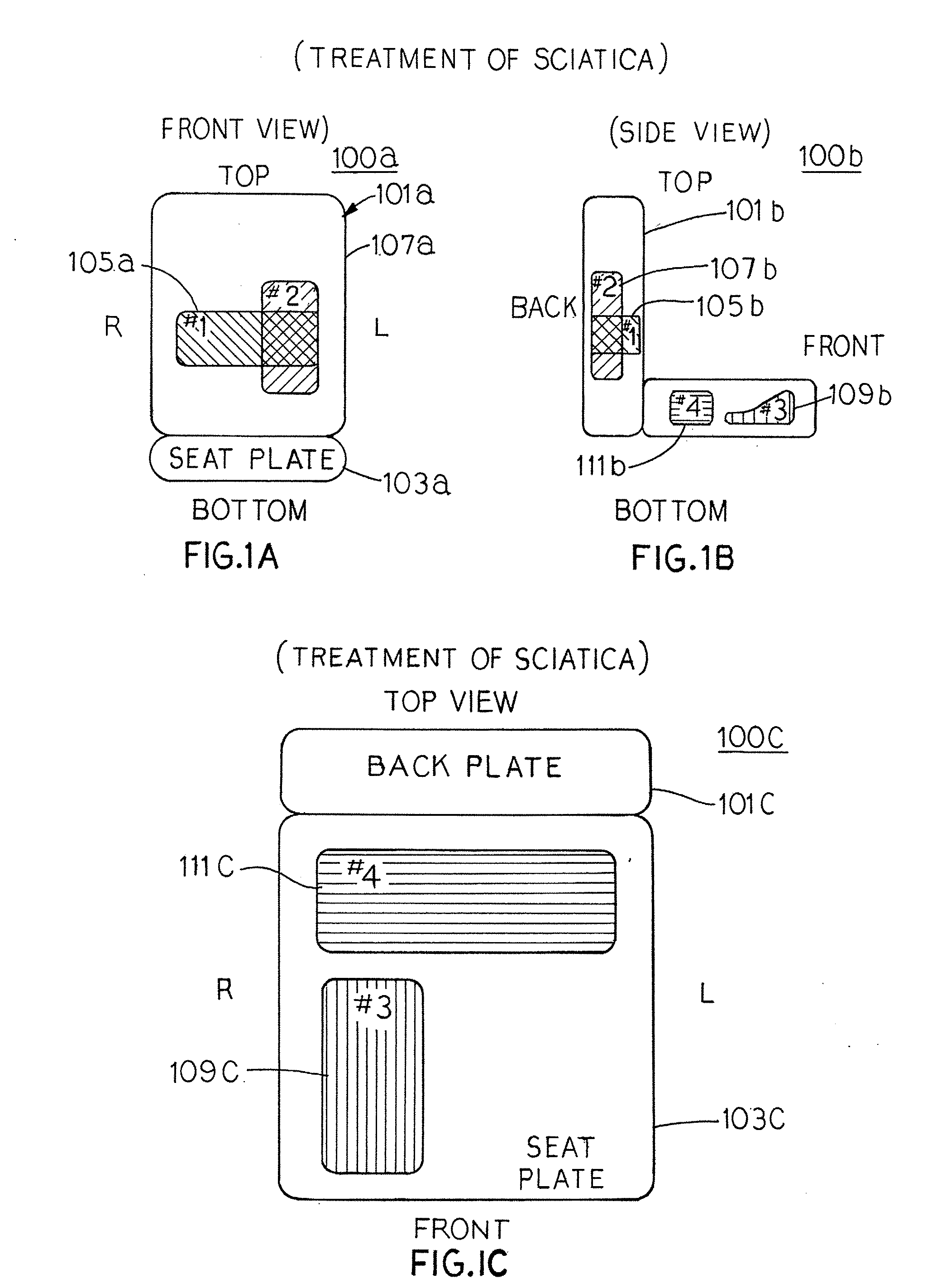
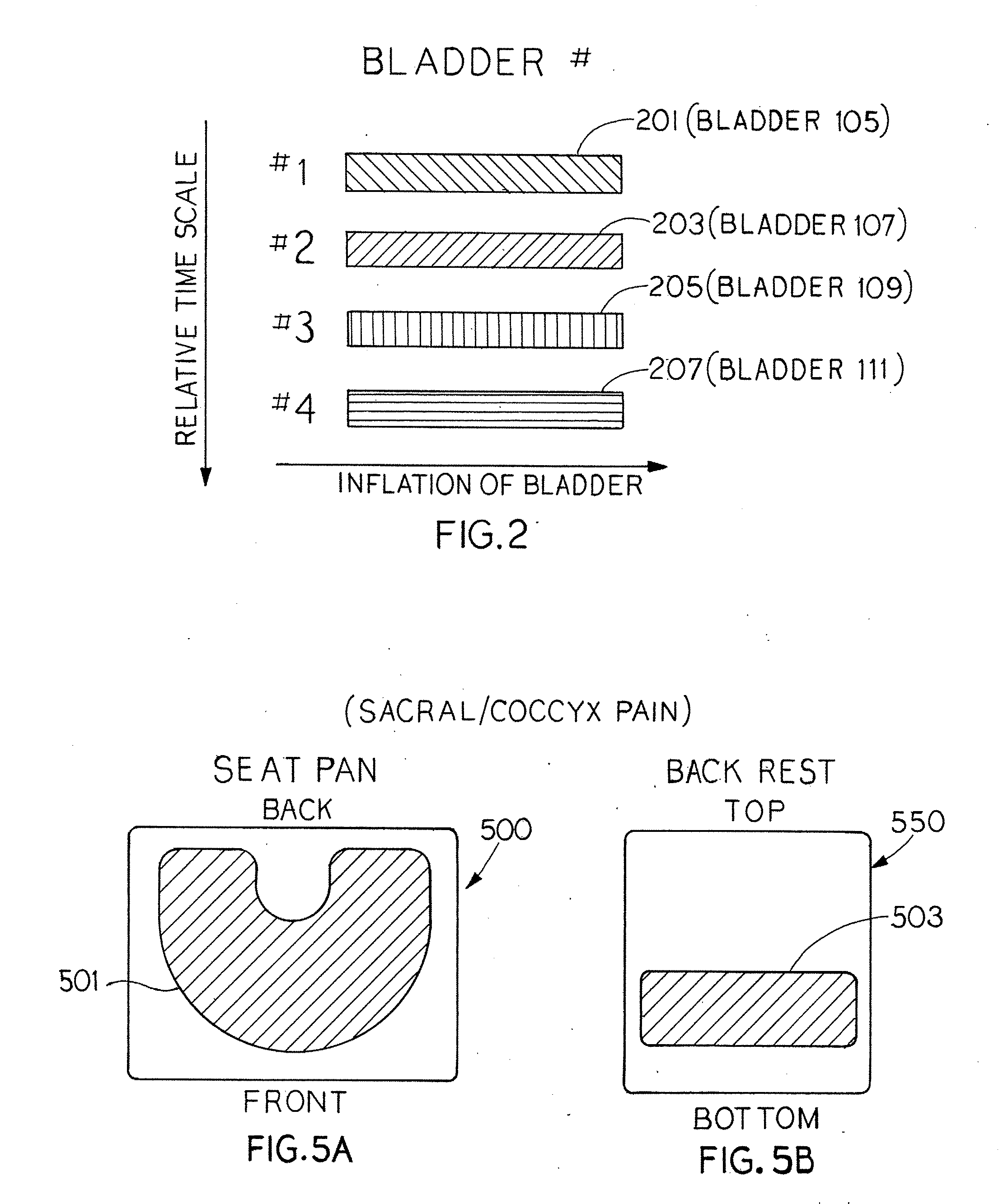
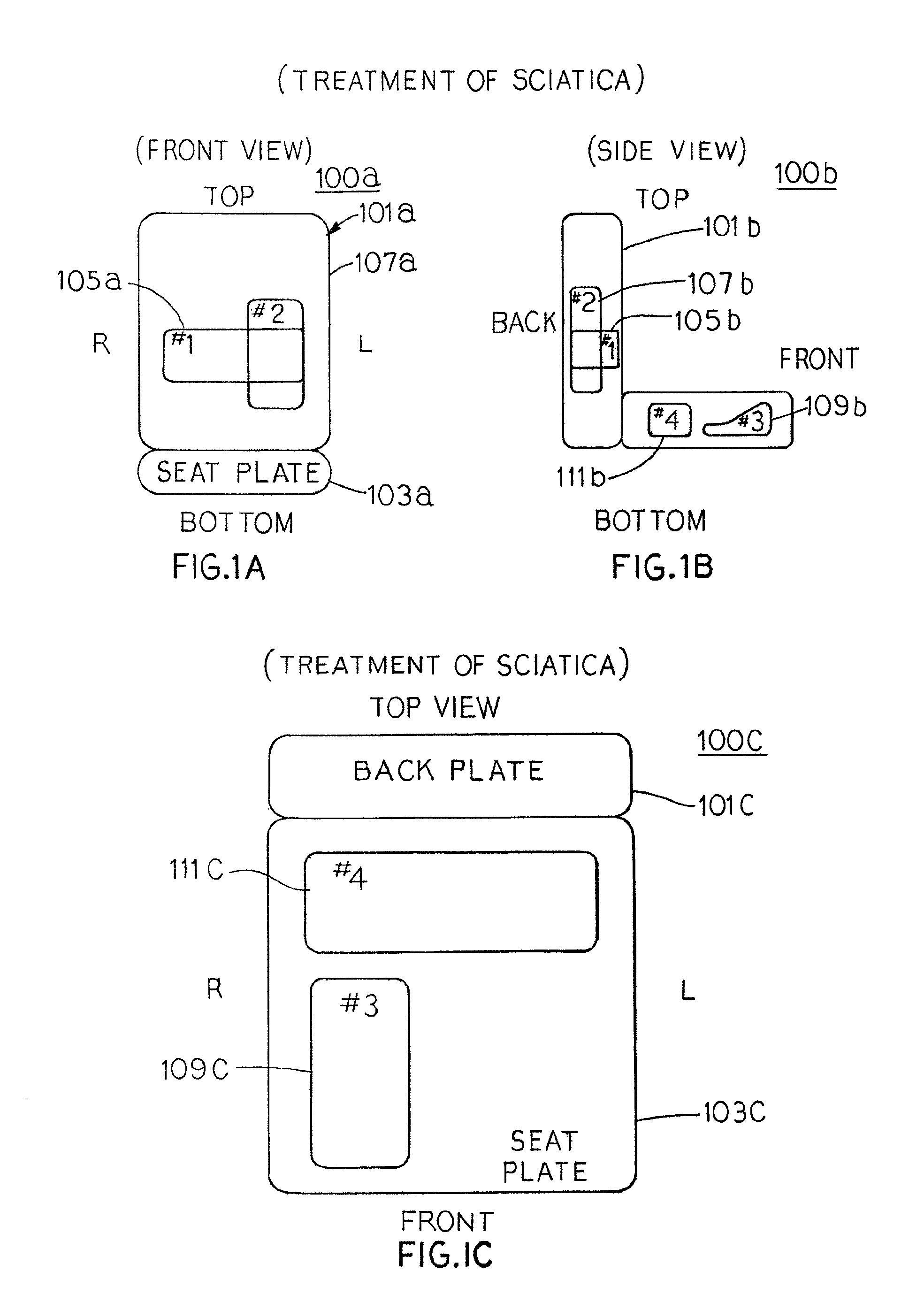
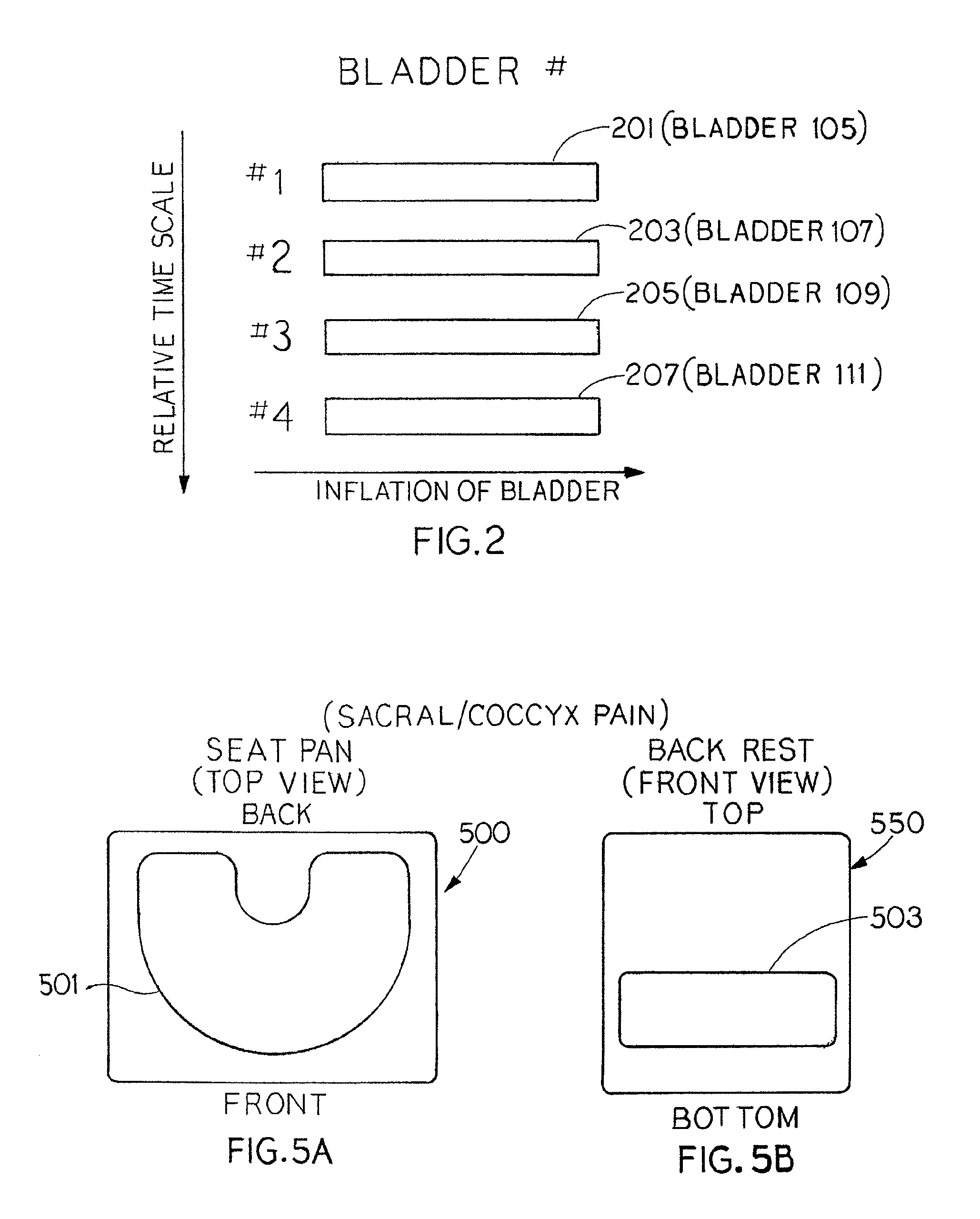
Inflation and deflation of an encased bladder system
The present invention provides seating apparatuses that may reduce an effect of an ailment such as sciatica, joint illness, degenerative disc condition, hip bursitis, tendinitis, or sacral / coccyx pain. A seating apparatus includes a back portion and a seating portion, each portion having at least one encased bladder that is inflated by a pumping component in a timed sequence. The bladders may be differently shaped, asymmetrically situated with respect to a center axis, and spatially overlapping. The bladders are inflated and deflated in accordance with a predetermined time sequence and are independently controlled. The seating apparatus may contain a set of encased bladders that may be configured for different aliments. A user selects a treatment for one of the ailments through a remote control unit. Consequently, a subset of bladders is configured so that the selected subset of bladders is inflated and deflated.
Owner:MORRISON CORINA
Injectible bodily prosthetics employing methacrylic copolymer gels
InactiveUS7183369B1Improve stabilityPharmaceutical delivery mechanismTissue regenerationCosmetic proceduresEther
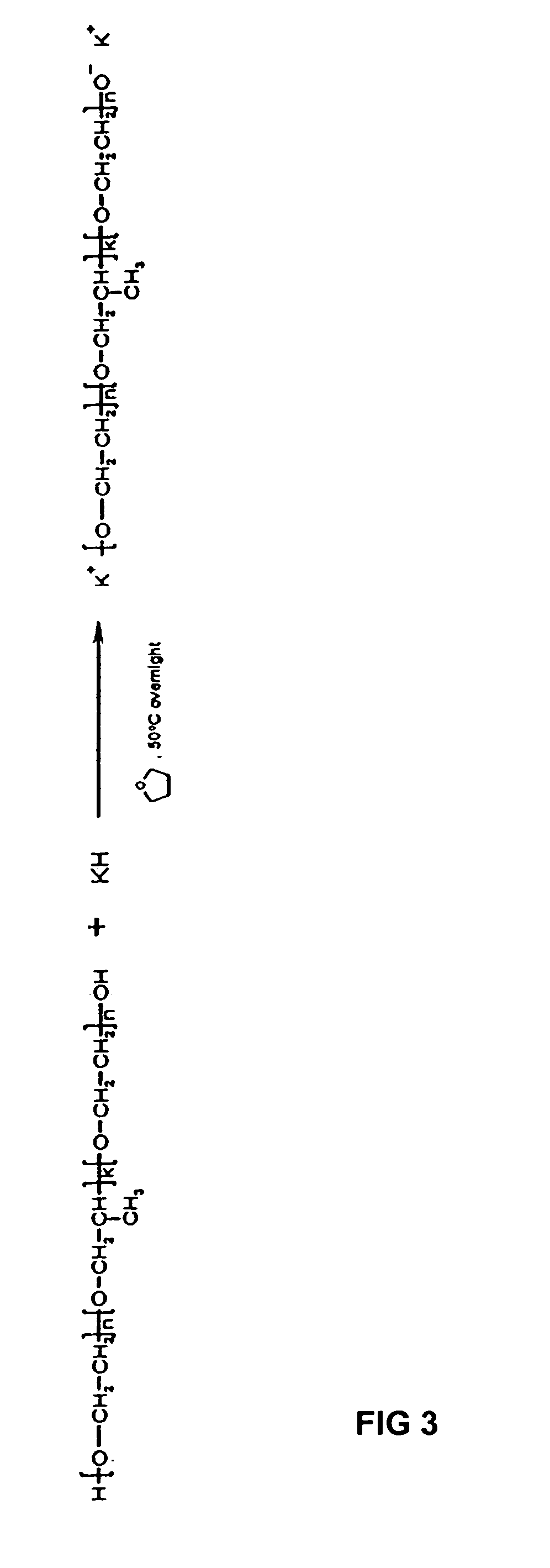
The present invention provides novel block copolymers as structural supplements for injectible bodily prosthetics employed in medical or cosmetic procedures. The invention also includes the use of such block copolymers as nucleus pulposus replacement materials for the treatment of degenerative disc disorders and spinal injuries. The copolymers are constructed by polymerization of a tertiary amine methacrylate with either a (poly(ethylene oxide)-b-poly(propylene oxide)-b-poly(ethylene oxide) polymer, such as the commercially available Pluronic® polymers, or a poly(ethylene glycol) methyl ether polymer.
Owner:IOWA STATE UNIV RES FOUND
Spinal disk regenerative composition and method of manufacture and use
ActiveUS20160015753A1High viscosityImprove grinding efficiencyPowder deliveryInternal osteosythesisRoom temperatureLactate ringer
Owner:VIVEX BIOLOGICS GRP INC
Implants for treatment of symptomatic or degenerated intervertebral discs
Devices for the treatment of intervertebral discs are described. The devices, when implanted into the nucleus pulposus of an intervertebral disc, provide for the controlled release of one or more active agents into the disc. The active agent can be a chemonucleolytic agent such as chymopapain. The device can also comprise one or more binders. The device can be an elongate solid body having a tapered or rounded insertion end. Alternatively, the device can include a plurality of particles. For devices containing multiple active agents, the configuration of the device be chosen to provide for the sequential or simultaneous release of each of the active agents. The elongate solid body can include a sheath comprising a first active agent and a core comprising a second active agent.
Owner:WARSAW ORTHOPEDIC INC
Devices and methods for the preparation of intervertebral discs
ActiveUS9848890B2Easy to operateExcision instrumentsEndoscopic cutting instrumentsLamina terminalisPower tool
A power tool for removing an intervertebral disc and preparing vertebral endplates is described. The power tool may include a cutting element, and the height of the cutting element may be adjustable. The cutting element may be a braided cable and may include one or more beads to enhance the effectiveness of the cable. The cutting element may have a minimum height requirement, which may not be satisfied in patients with a collapsed disc due to degenerative disc disorder. For these patients, also described are bone tamps for increasing the intervertebral distance and providing access to tissues distal to the tamp. One type of bone tamp features an inflatable balloon with an inner lumen. Another type of bone tamp includes an expanding distal end and an inner cannula. Also described is a manual expander scraper tool that is compatible with both types of bone tamp.
Owner:GLOBUS MEDICAL INC
Methods for the treatment of degenerative disc diseases by human birth tissue material composition
ActiveUS9993506B1Pharmaceutical delivery mechanismMammal material medical ingredientsDiseaseTissue material
Methods for treating degenerative disc disease by administering a human birth tissue material composition are provided. The method includes the step of administering a human birth tissue material composition onto or into at least one intervertebral disc or intervertebral space in need of treatment.
Owner:BIODLOGICS
Inflation and deflation of an encased bladder system
The present invention provides seating apparatuses that may reduce an effect of an ailment such as sciatica, joint illness, degenerative disc condition, hip bursitis, tendinitis, or sacral / coccyx pain. A seating apparatus includes a back portion and a seating portion, each portion having at least one encased bladder that is inflated by a pumping component in a timed sequence. The bladders may be differently shaped, asymmetrically situated with respect to a center axis, and spatially overlapping. The bladders are inflated and deflated in accordance with a predetermined time sequence and are independently controlled. The seating apparatus may contain a set of encased bladders that may be configured for different aliments. A user selects a treatment for one of the ailments through a remote control unit. Consequently, a subset of bladders is configured so that the selected subset of bladders is inflated and deflated.
Owner:MORRISON CORINA
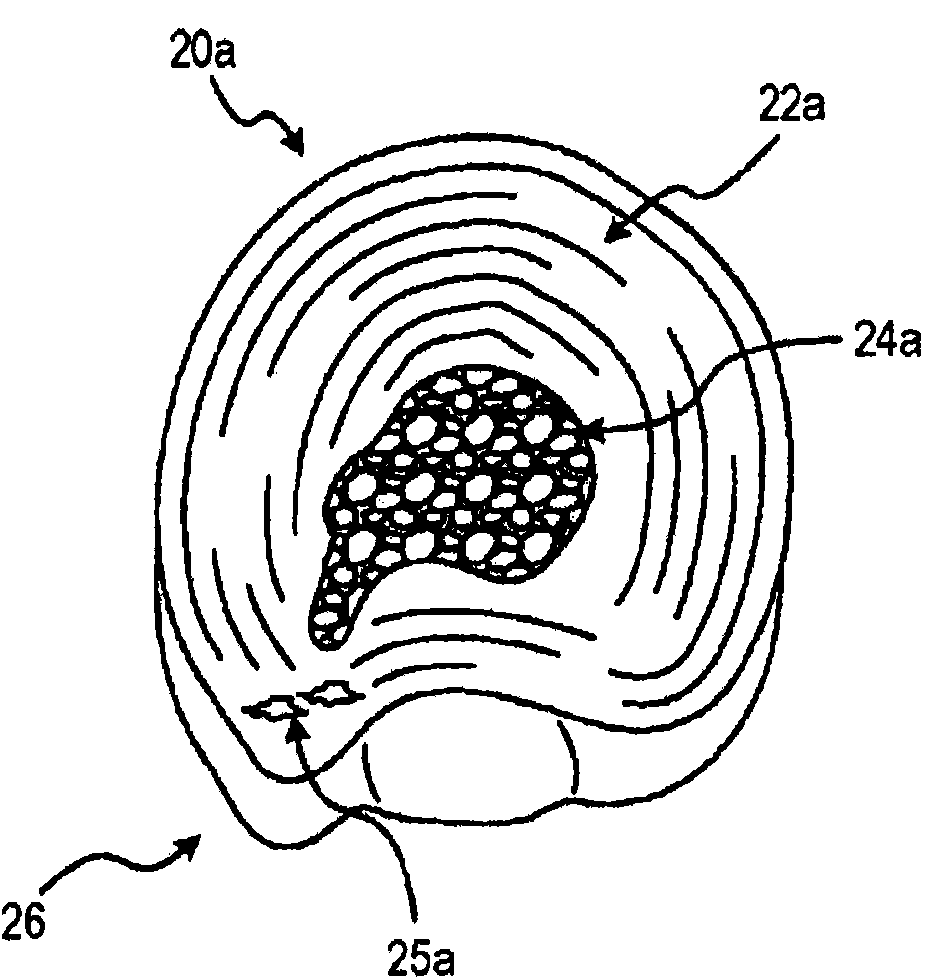
Restorative device
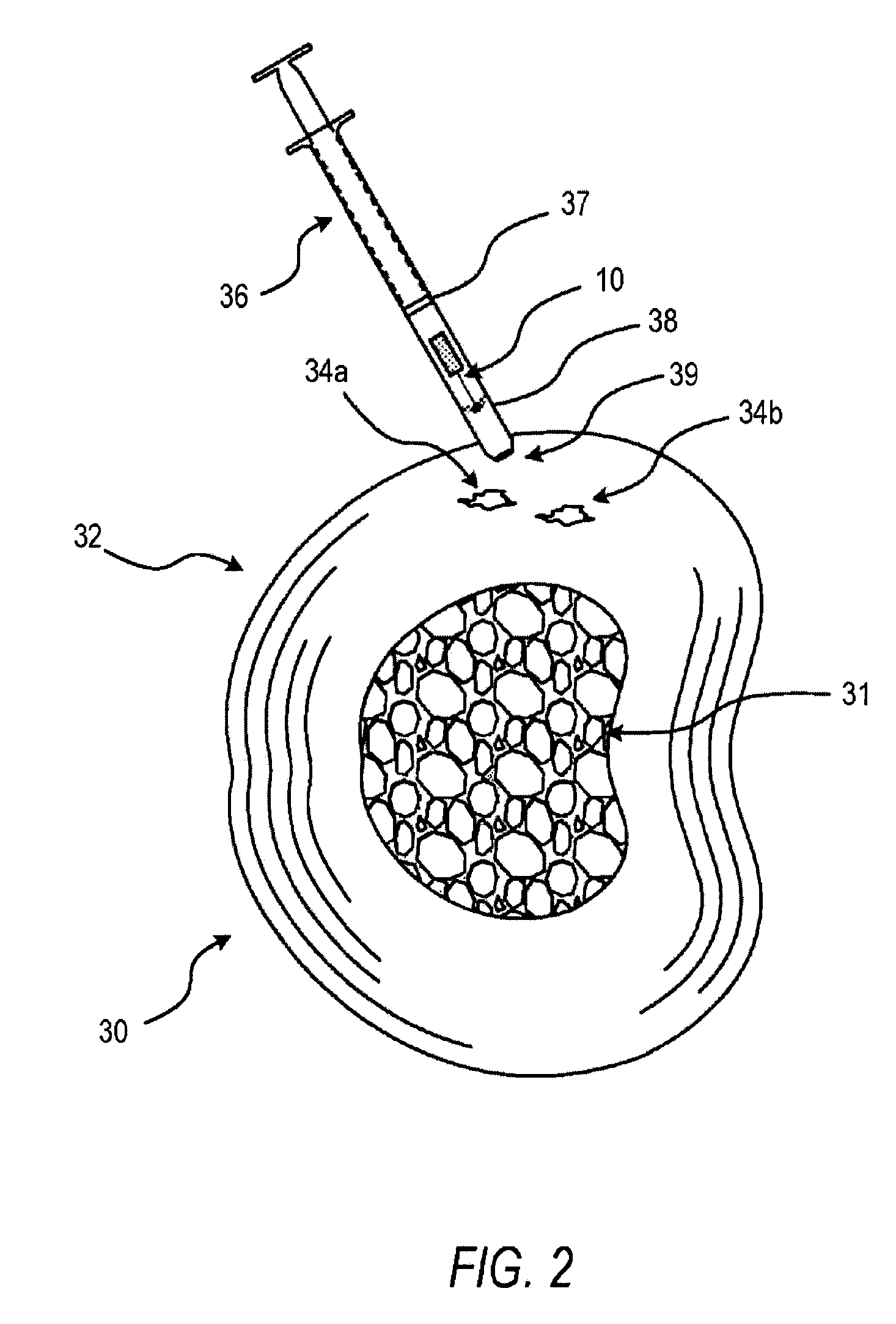
The present invention provides a disc restoration system for relieving symptoms of a degenerative disc, comprising: a) a percutaneously insertable expandable element (88) adapted to be (i) inserted in between two adjacent vertebraes of the spinal cord; and, (ii) expanded to form a scaffold; said scaffold provides mechanical support to said two adjacent vertebraes so as to restore said degenerative disc to approximately the dimensions of a normal disc; b) an injectable filler having a first flowable state, and a second non-flowable set state; the filler is configured for being introduced, in said first flowable state, into a confined volume formed by the expandable element and the tissues, following the full expansion of said expandable element in said disc; and, following introduction of said filler, said filler is adapted to set into said second non-flowable state; such that following setting of said filler into said second state said disc is internally supported by said set filler.
Owner:CHI2GEL LTD
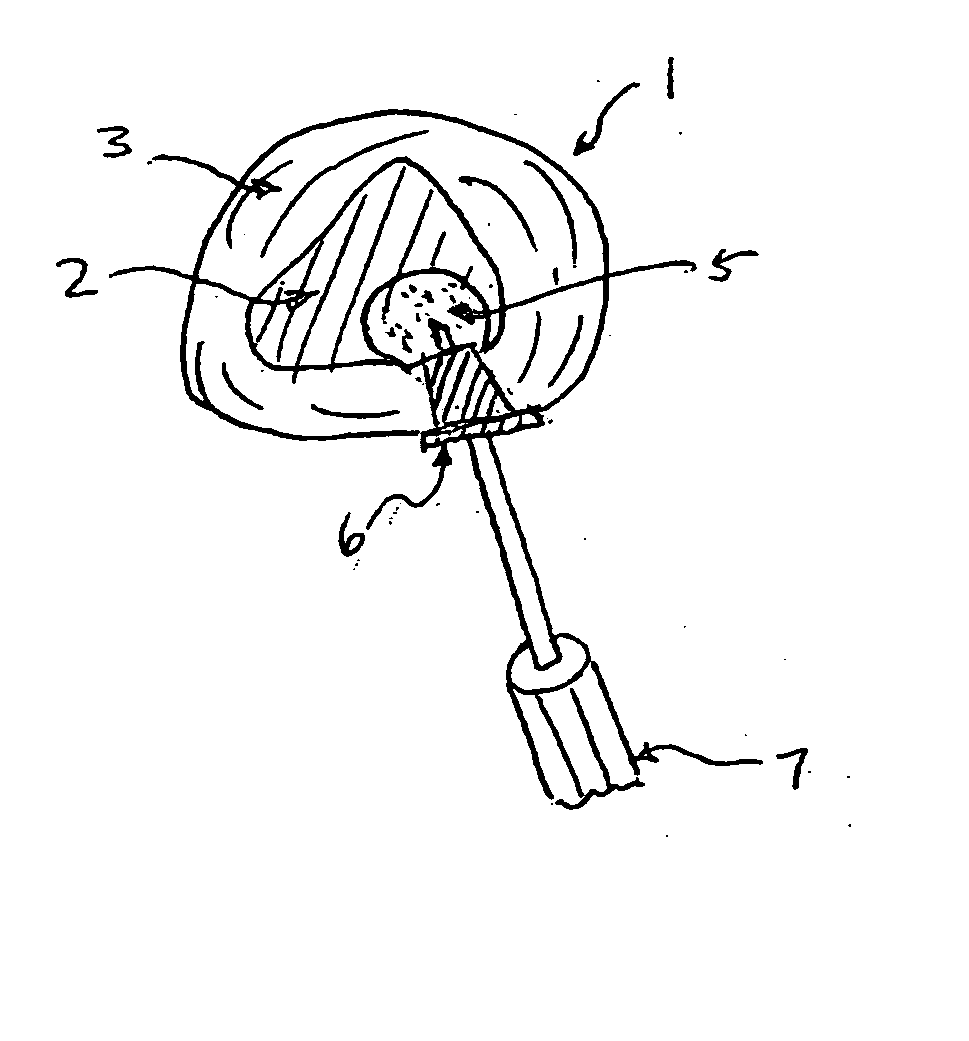
Spinal disc regenerative composition and method of manufacture and use
ActiveUS9655929B2High viscosityImprove grinding efficiencyPowder deliveryInternal osteosythesisSaline waterRoom temperature
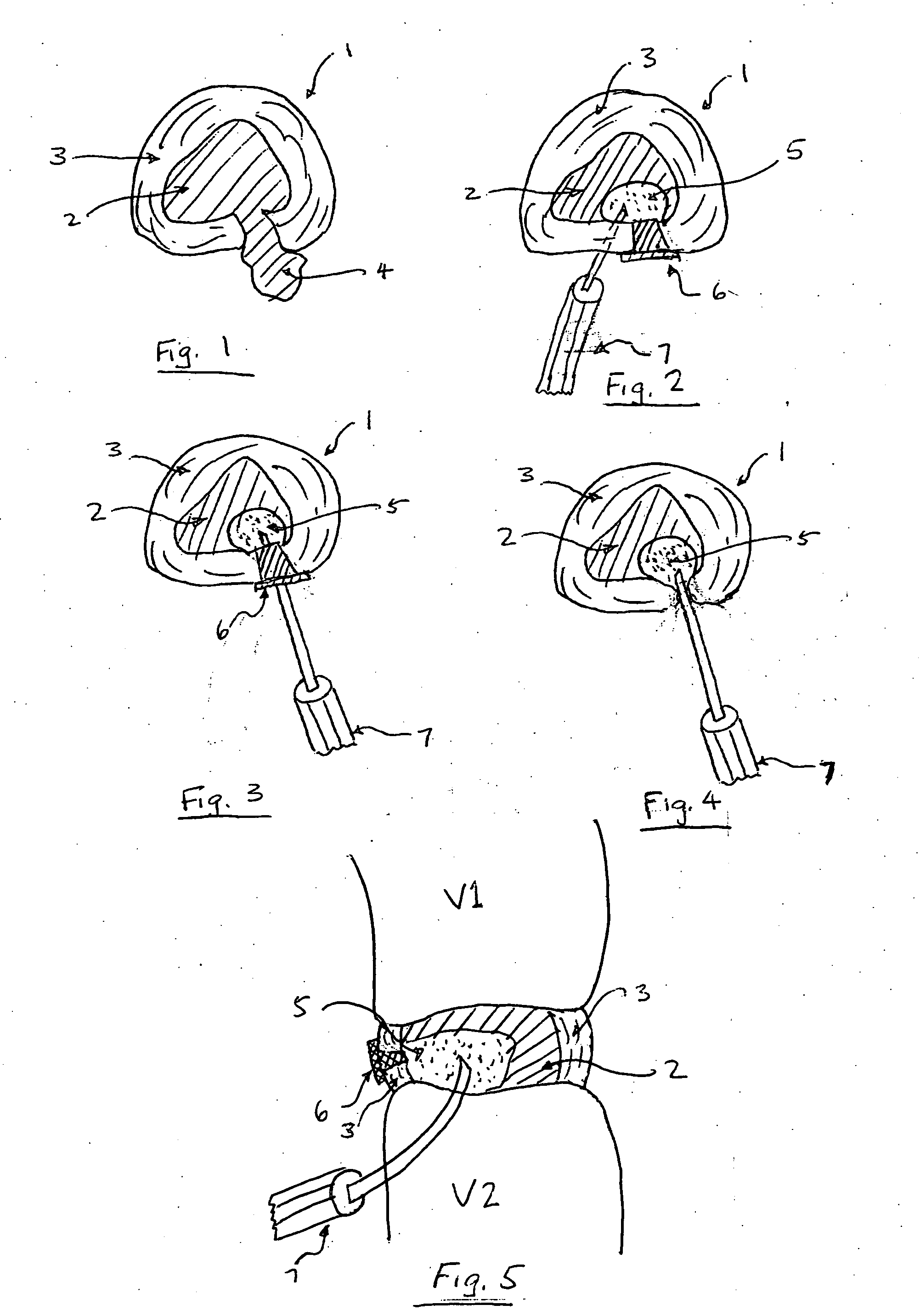
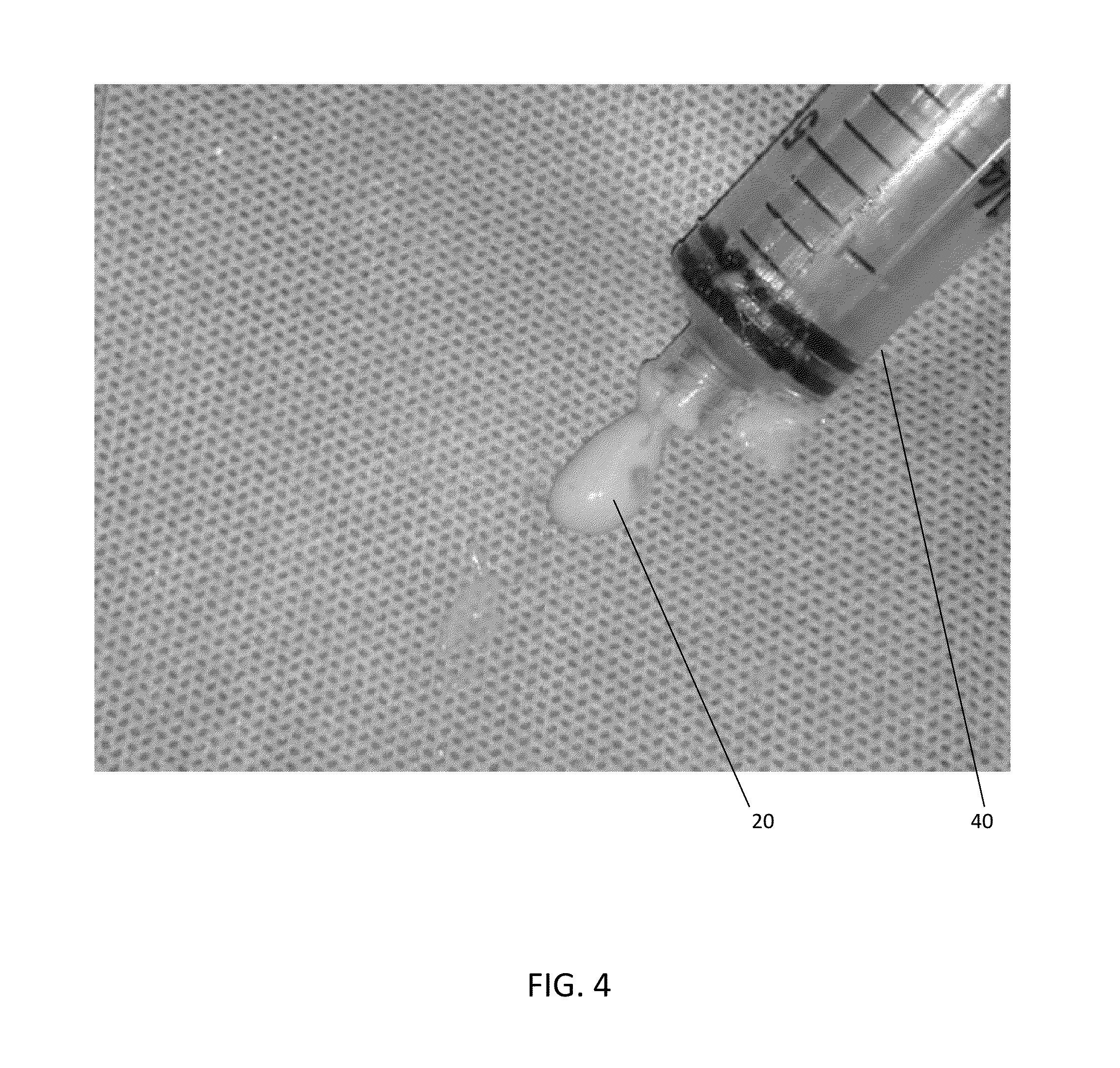
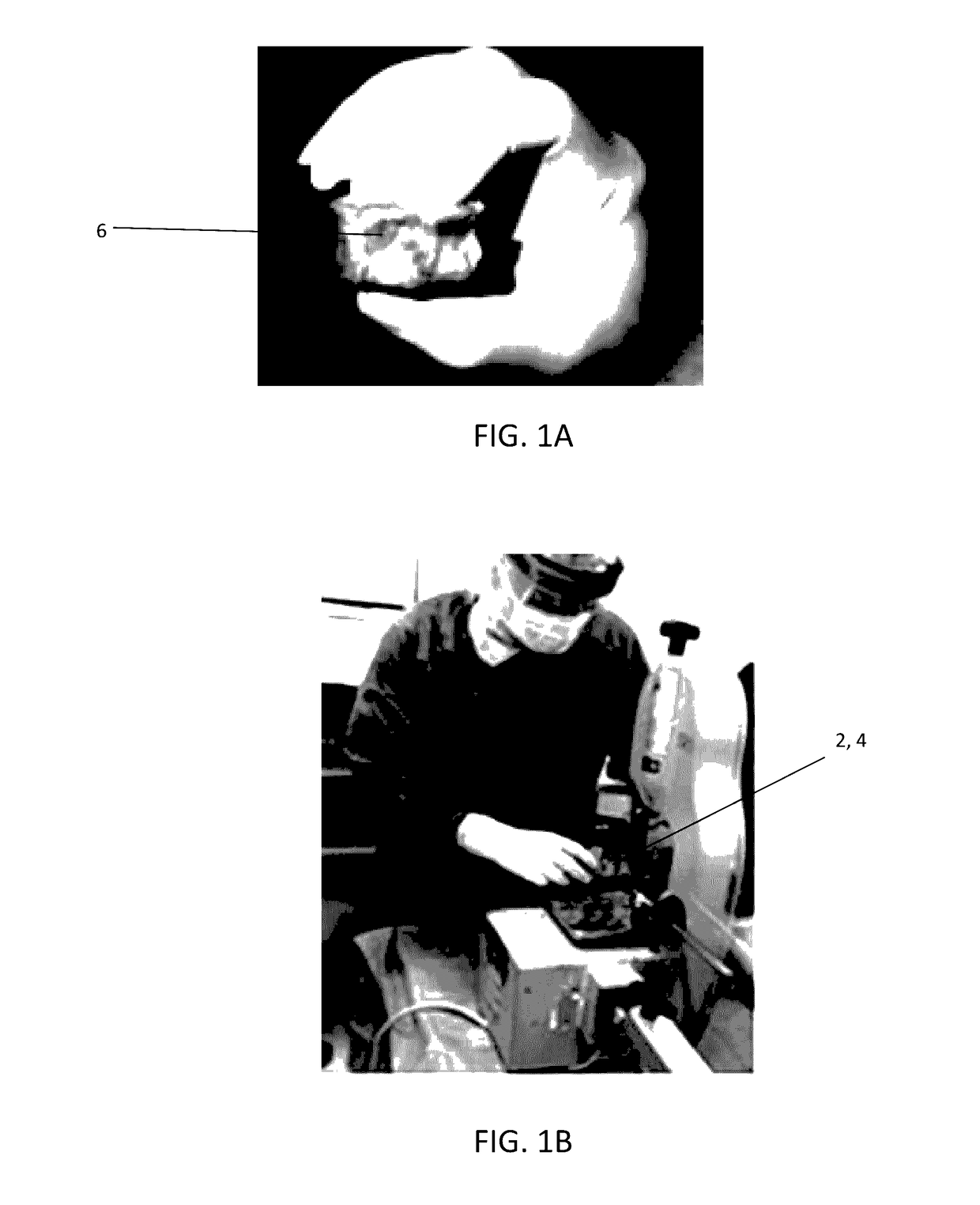
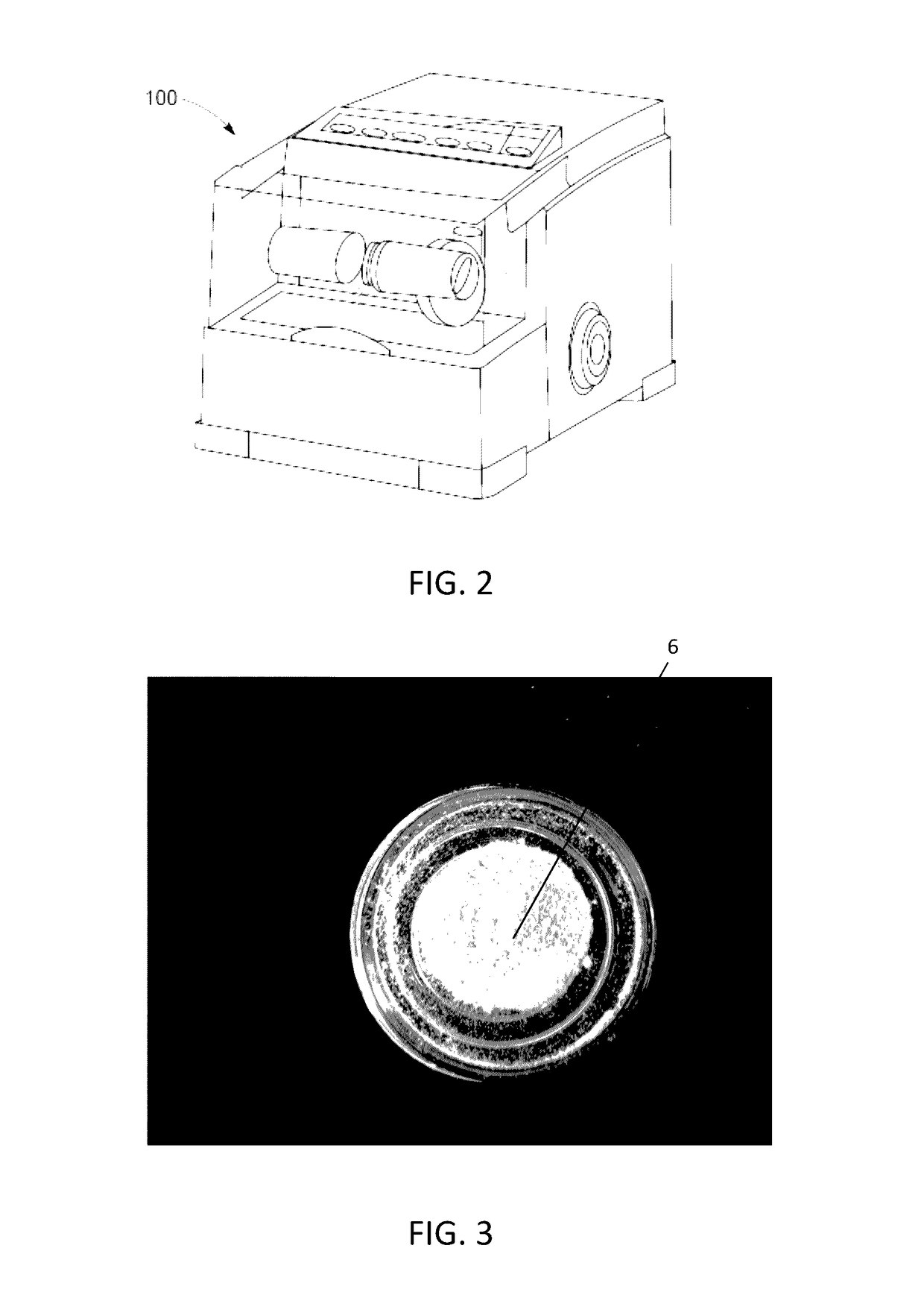
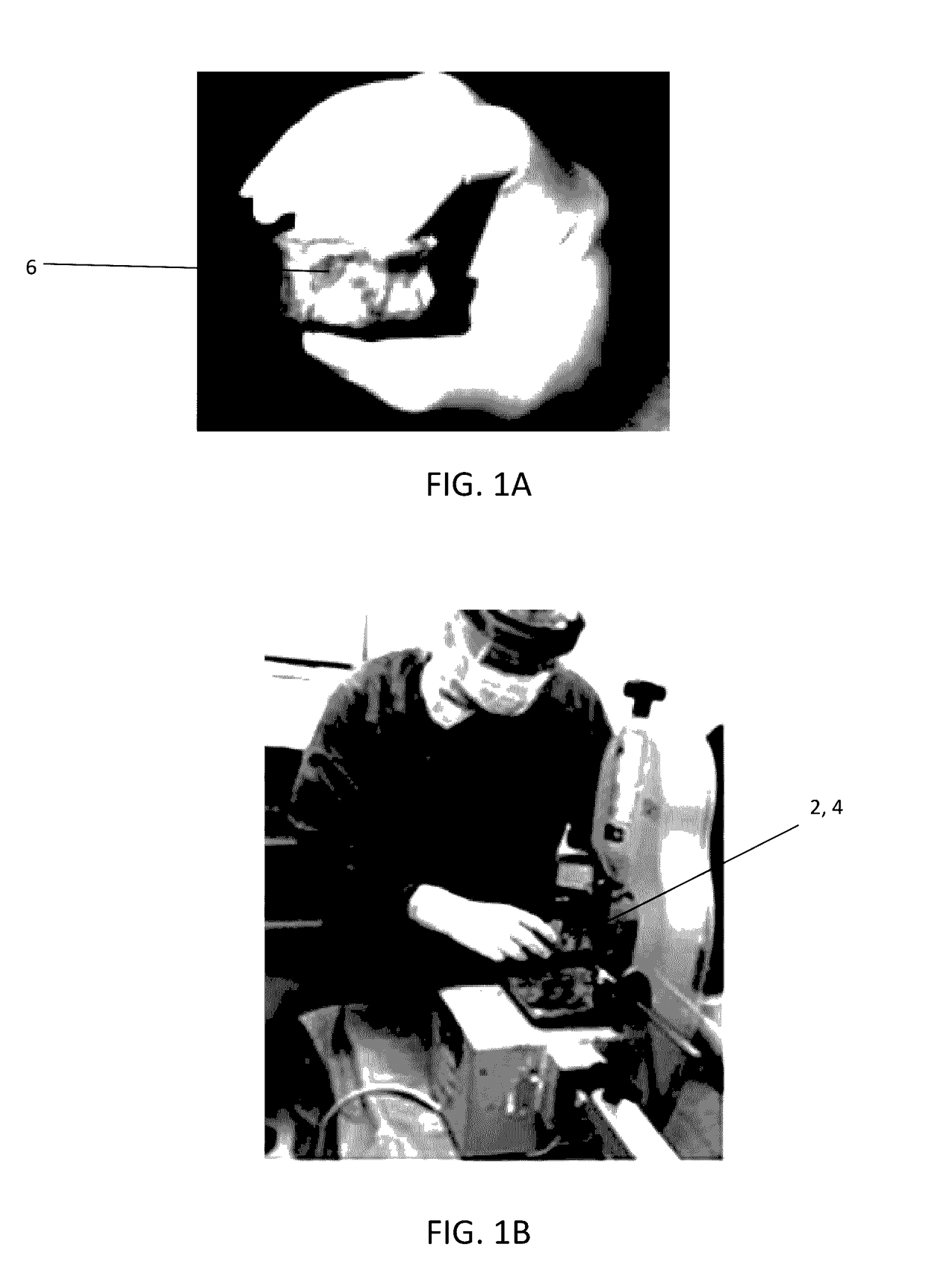
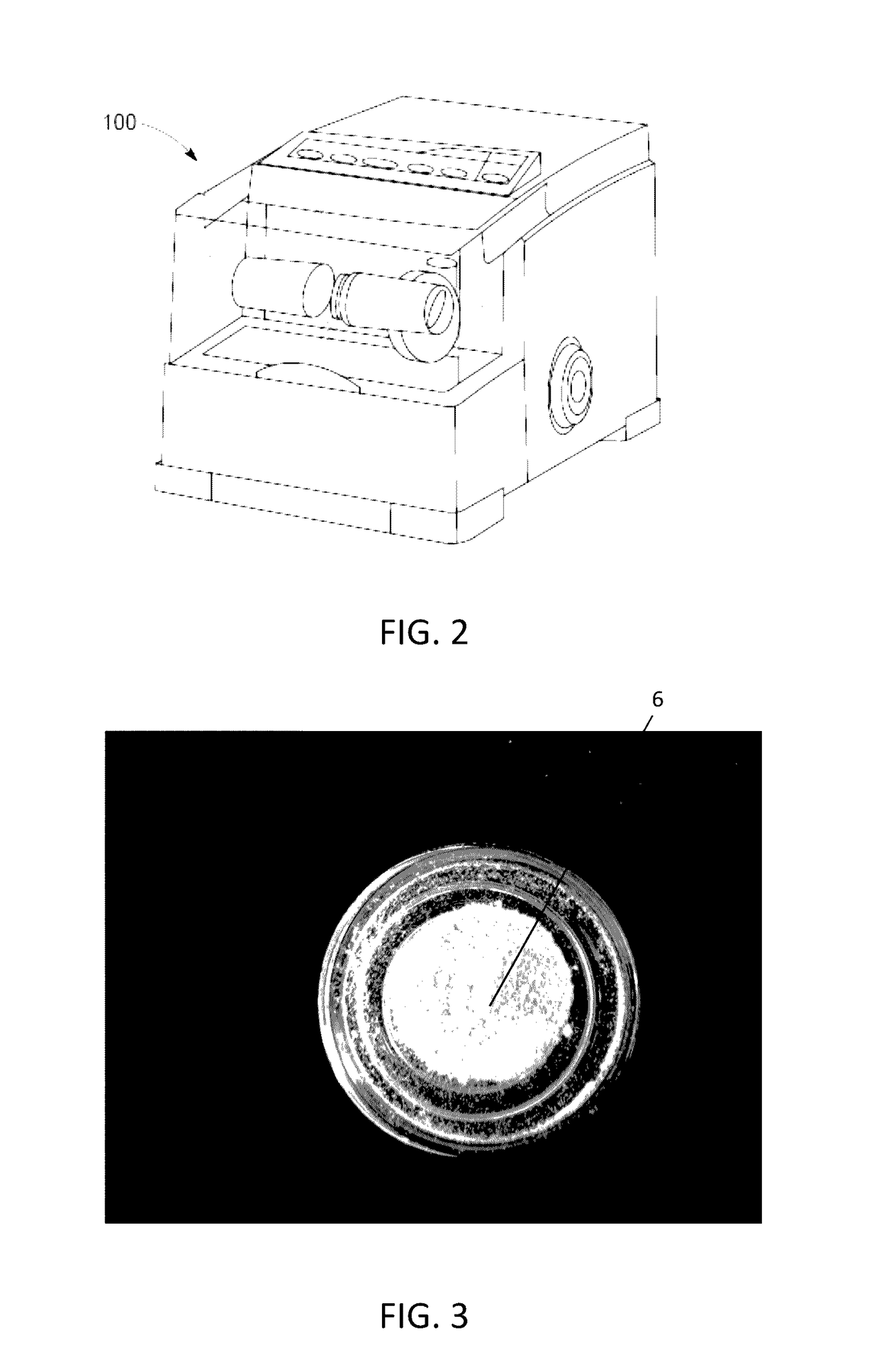
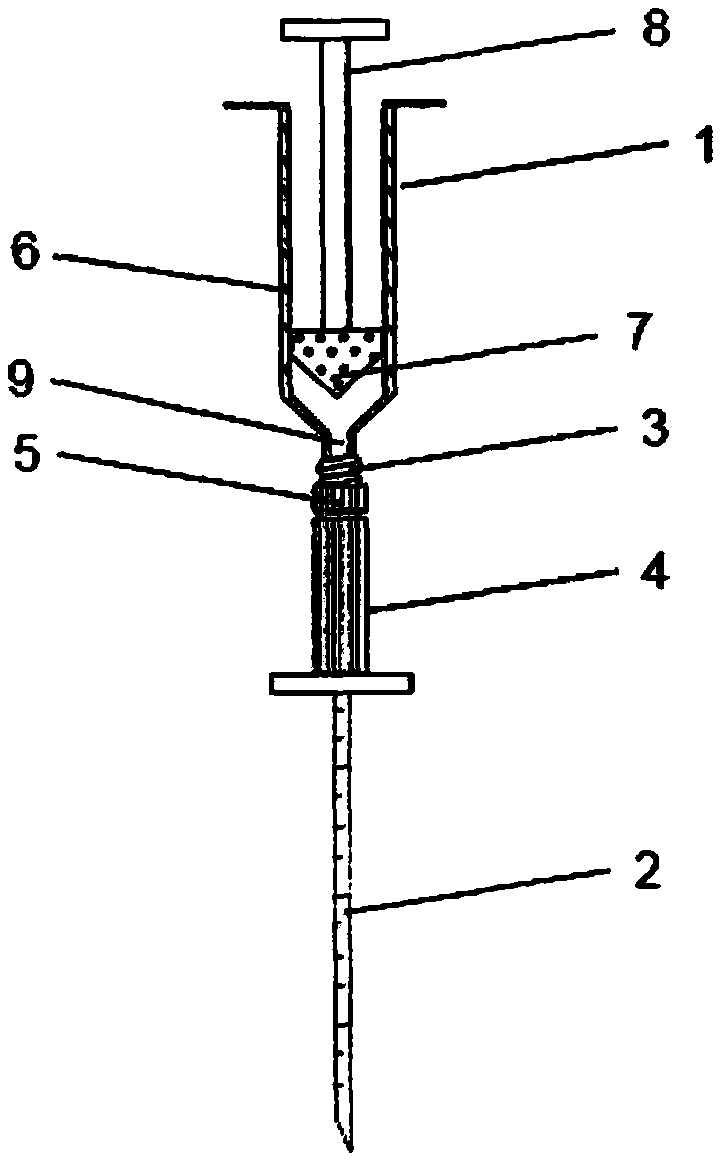
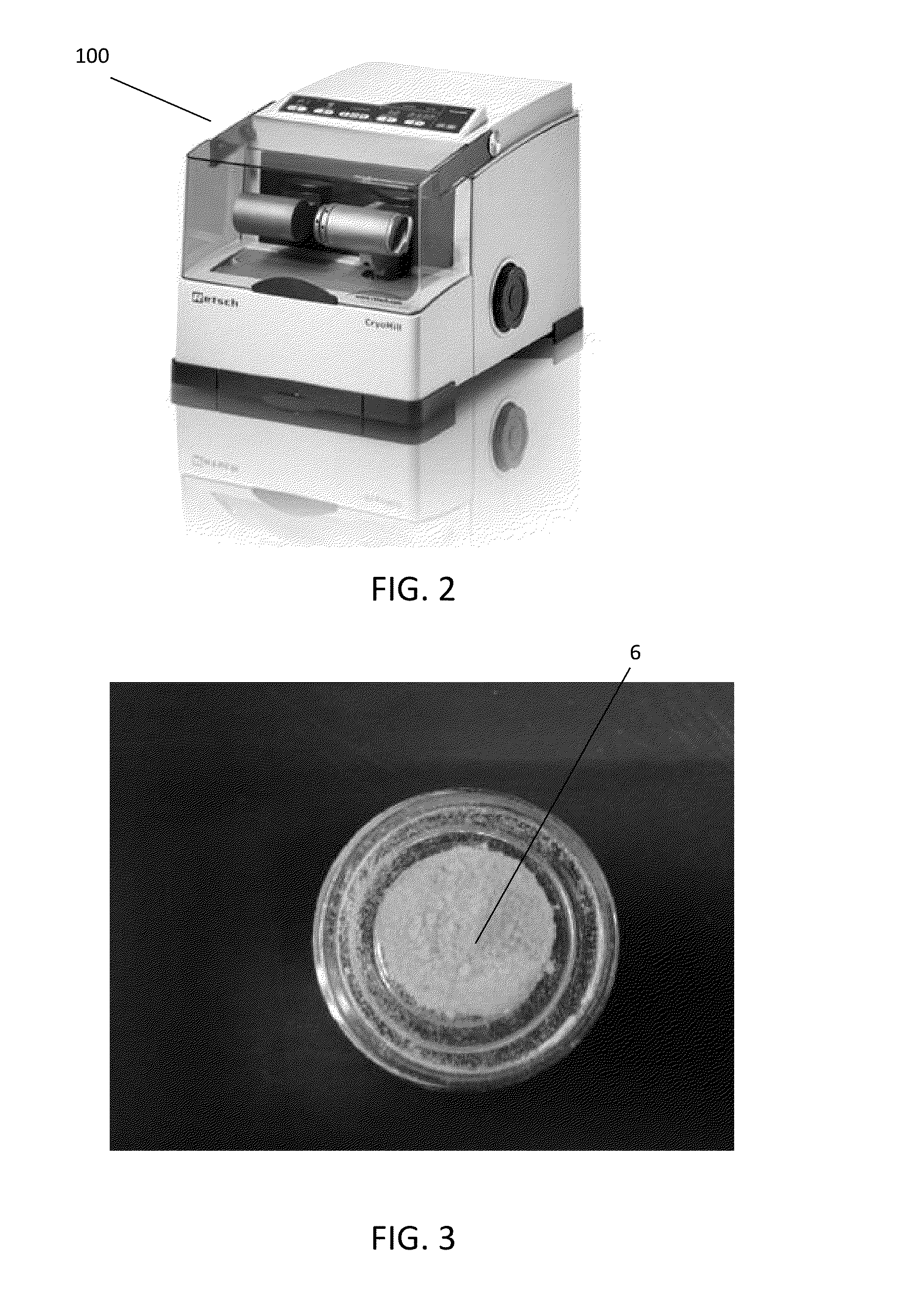
The present invention provides a novel way to replenish the disc using retooled disc compositions to repair degenerative discs. There is no better source of proteoglycans than the actual disc material (6) itself. To this end, there has been developed a technique to remove the nucleus pulposus and retool the morphology of the nucleus pulposus to create a powder material (10) that is dry and can be stored at room temperature for long periods of time. This powder (10) can then be reconstituted with a variety of fluids, the most suitable being normal saline or lactated ringers to form a flowable mixture (20).
Owner:VIVEX BIOLOGICS GRP INC
Compositions and methods comprising growth factors, chondroitin and glucosamine for degenerative disc regeneration
Owner:UNIV HEALTH NETWORK +1
Compositions and methods for degenerative disc regeneration
Owner:UNIV HEALTH NETWORK +1
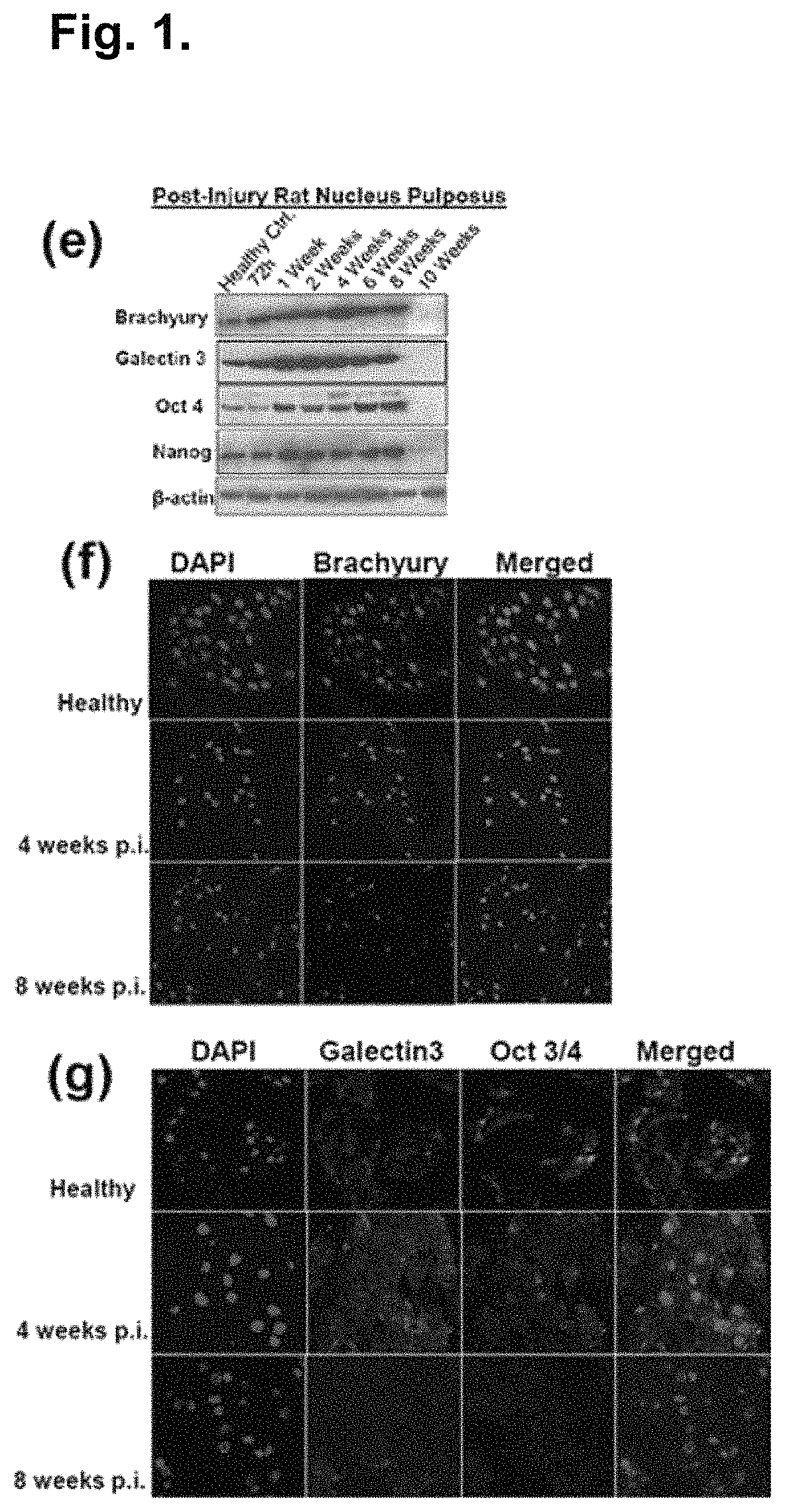
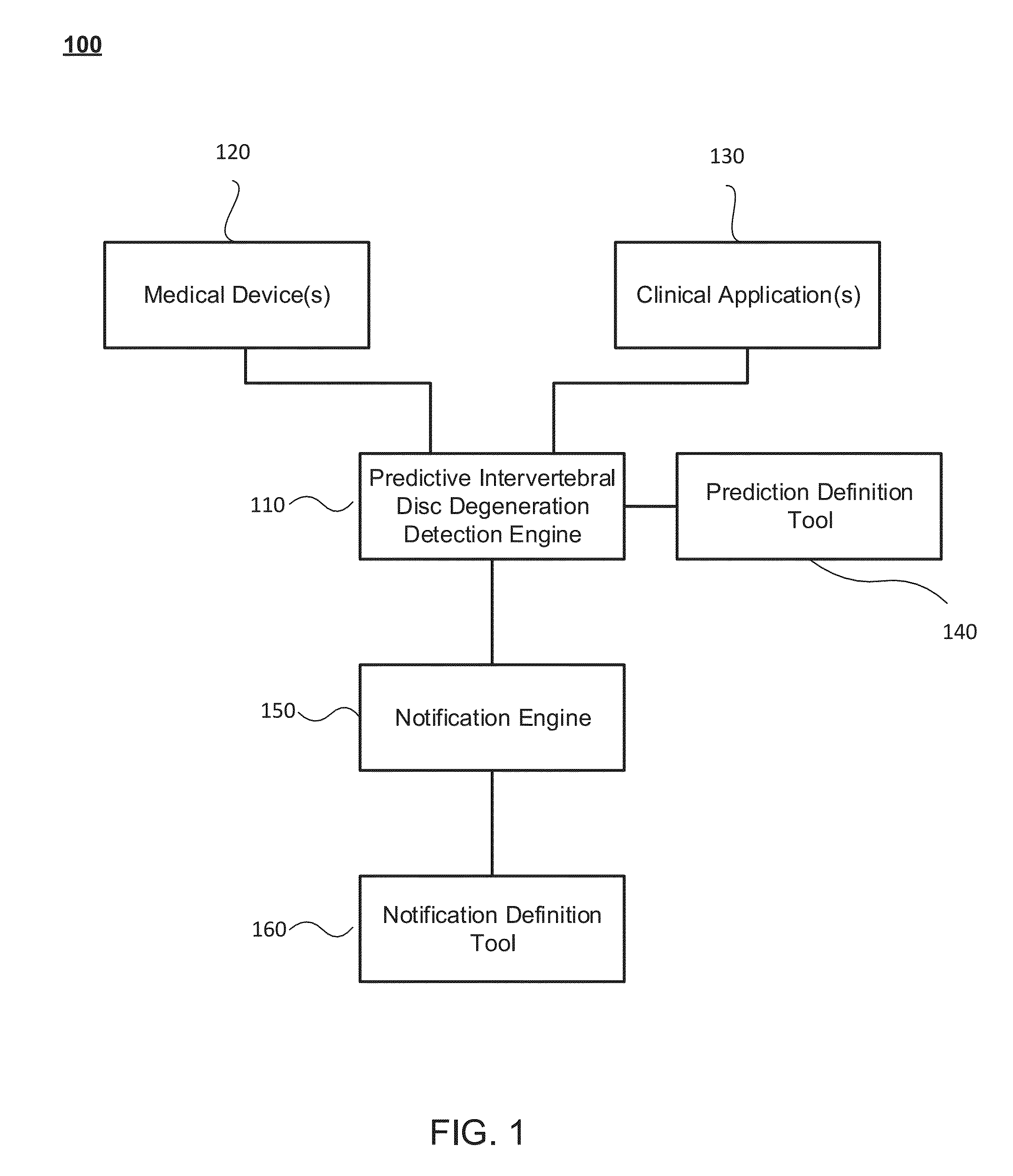
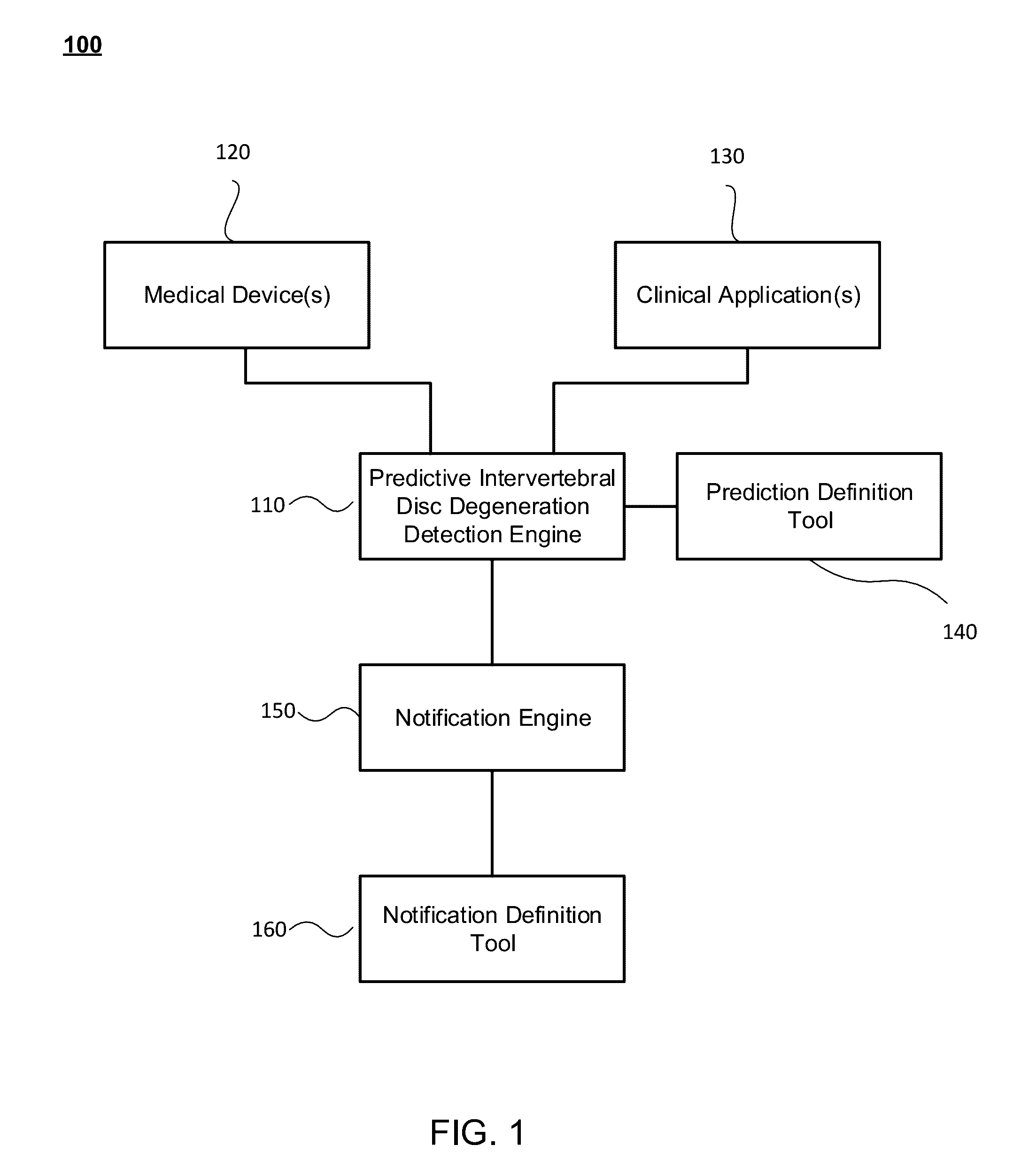
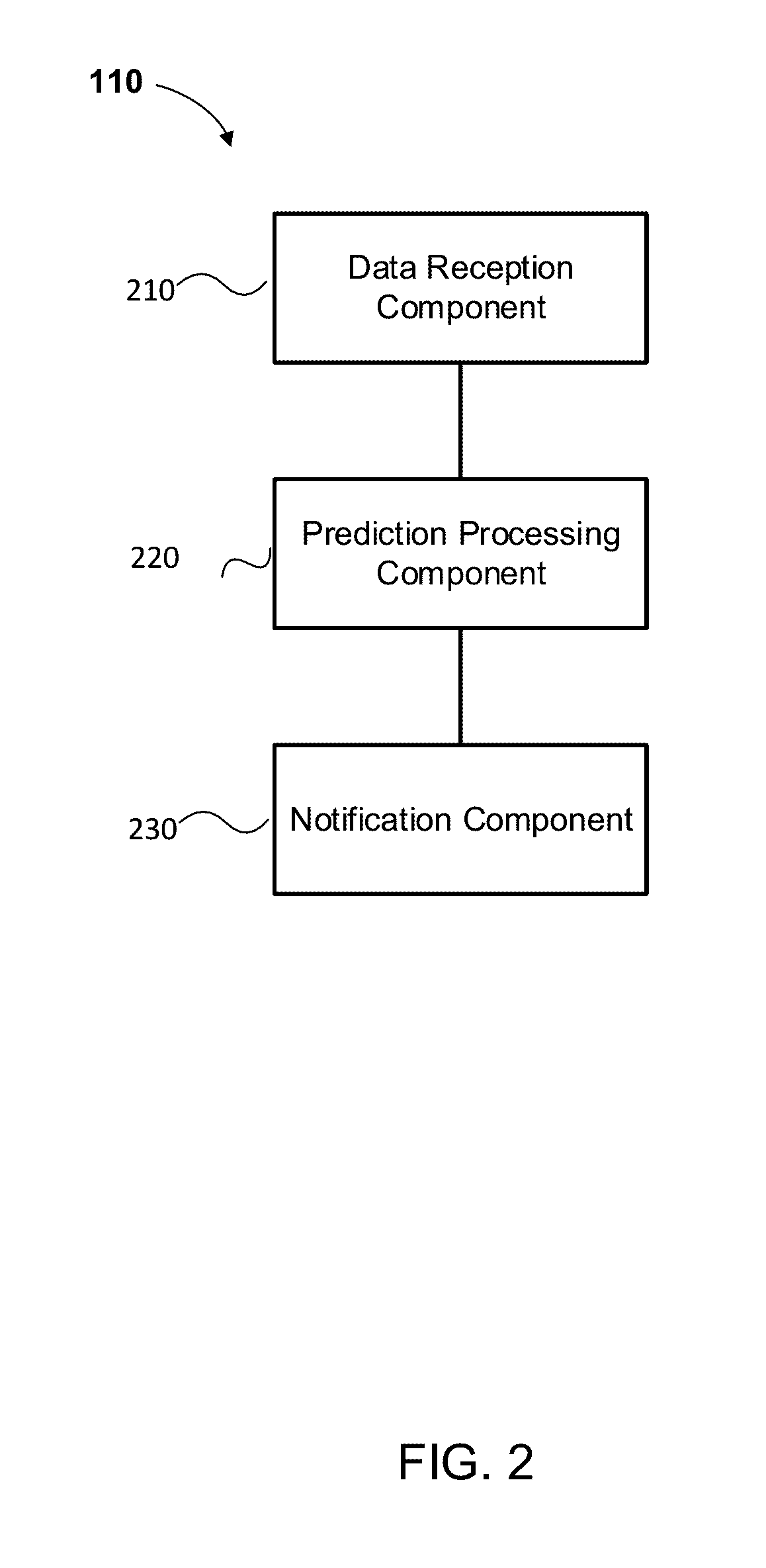
Predictive intervertebral disc degeneration detection engine
ActiveUS20150201887A1Improved prognosisMedical imagingProgram initiation/switchingDiseaseSystems approaches
Systems, methods and computer program products for facilitating the prognosis of degenerative disc disorder (DDD) are provided. In one aspect, intervertebral disc loss is predicted based on receiving image data comprising one or more images of the human spine; segmenting, using a processor, disc regions of said one or more images; generating, using the processor, individual biomarkers based on texture features of said segmented disc regions; generating, using the processor, a predicting intervertebral disc loss based in part on the prognostic marker generated from the individual biomarkers.
Owner:GENERAL ELECTRIC CO
Enhancement of fibroblast plasticity for treatment of disc degeneration
PendingUS20210230551A1Enhances more more activityEnhances one and more and one and more activityPeptide/protein ingredientsGenetically modified cellsMedicineFibroblast
Embodiments of the disclosure include methods and compositions related to preparation of fibroblasts for use of treatment and prevention of a degenerative disc in an individual. In particular cases, fibroblasts are subject to de-differentiation that results in enhancement of their therapeutic activity and such methods include exposure of the fibroblasts to one or more agents and / or conditions.
Owner:SPINALCYTE
Spinal disc regenerative composition and method of manufacture and use
ActiveUS9655928B2High viscosityImprove grinding efficiencyPowder deliveryInternal osteosythesisSaline waterRoom temperature
The present invention provides a novel way to replenish the disc using retooled disc compositions to repair degenerative discs. There is no better source of proteoglycans than the actual disc material (6) itself. To this end, there has been developed a technique to remove the nucleus pulposus and retool the morphology of the nucleus pulposus to create a powder material (10) that is dry and can be stored at room temperature for long periods of time. This powder (10) can then be reconstituted with a variety of fluids, the most suitable being normal saline or lactated ringers to form a flowable mixture (20).
Owner:VIVEX BIOLOGICS GRP INC
Predictive intervertebral disc degeneration detection engine
Systems, methods and computer program products for facilitating the prognosis of degenerative disc disorder (DDD) are provided. In one aspect, intervertebral disc loss is predicted based on receiving image data comprising one or more images of the human spine; segmenting, using a processor, disc regions of said one or more images; generating, using the processor, individual biomarkers based on texture features of said segmented disc regions; generating, using the processor, a predicting intervertebral disc loss based in part on the prognostic marker generated from the individual biomarkers.
Owner:GENERAL ELECTRIC CO
Compositions and methods for degenerative disc regeneration
There is disclosed herein compositions, methods, uses and systems for reducing pain in a patient that emanates from a body area, preferably spine or joint. Methods of treatment or prevention are described for a disease or condition selected from degenerative disc disease, disc injury, pain, arthritis, or suspected arthritis.
Owner:NOTOGEN INC +1
Spinal disk regenerative composition and method of manufacture and use
ActiveUS20160015754A1High viscosityImprove grinding efficiencyBiocidePowder deliveryMedicineRoom temperature
The present invention provides a novel way to replenish the disc using retooled disc compositions to repair degenerative discs. There is no better source of proteoglycans than the actual disc material (6) itself. To this end, there has been developed a technique to remove the nucleus pulposus and retool the morphology of the nucleus pulposus to create a powder material (10) that is dry and can be stored at room temperature for long periods of time. This powder (10) can then be reconstituted with a variety of fluids, the most suitable being normal saline or lactated ringers to form a flowable mixture (20).
Owner:VIVEX BIOLOGICS GRP INC
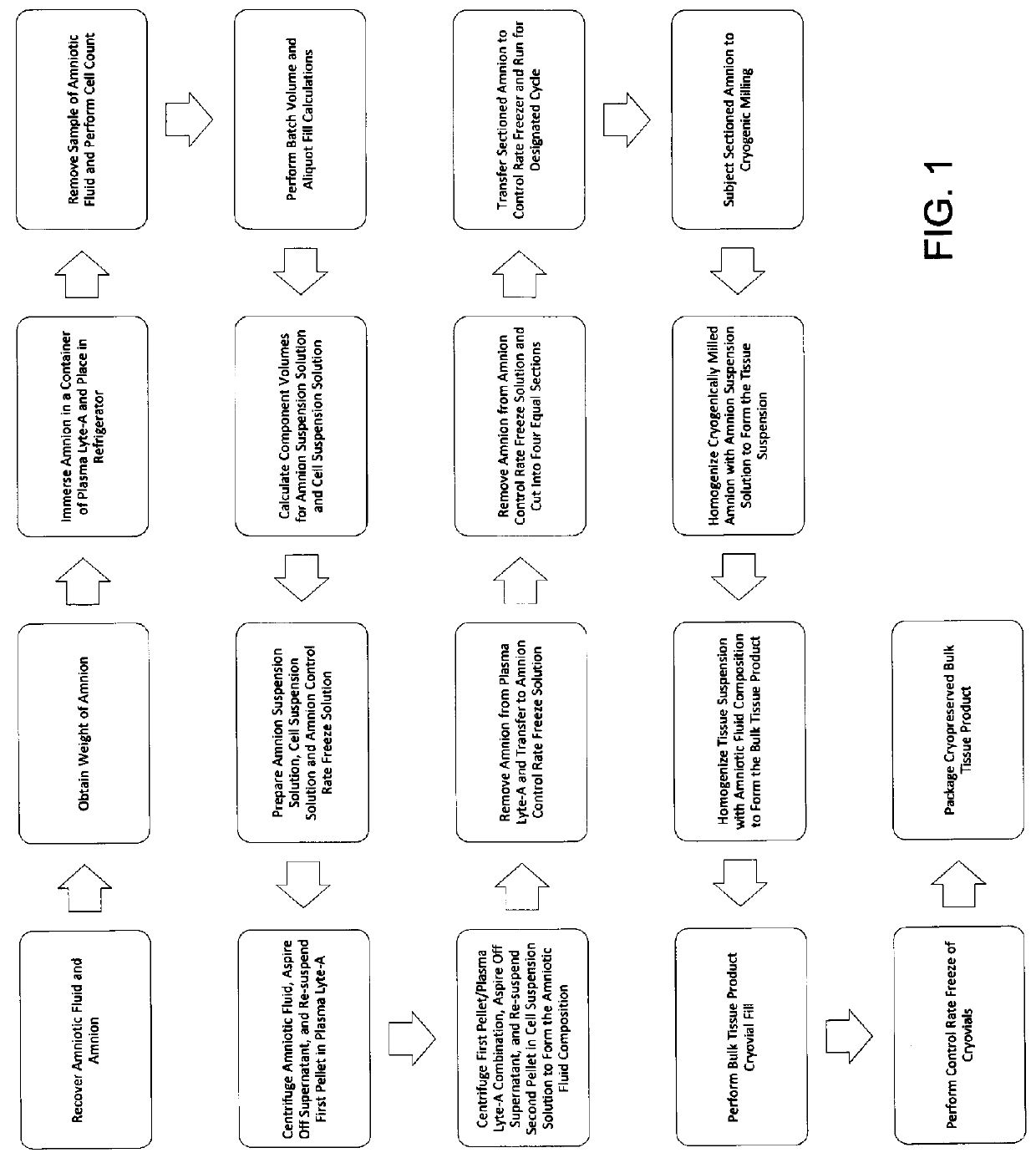
Preparation for treating degenerative disc diseases and preparation method thereof
PendingCN111067920AImprove survival rateAvoid discomfortInfusion syringesAerosol deliveryDiseaseLumbar intervertebral disc
The invention relates to the technical field of lumbar intervertebral disc medical treatment, in particular to a preparation for treating degenerative disc diseases and a preparation method. The preparation for treating the degenerative disc diseases comprises mesenchymal stem cells, and the mesenchymal stem cells are induced and cultured by a conditional culture medium of chondrocytes, so that the differentiation of the mesenchymal stem cells is facilitated; and the mesenchymal stem cells are wrapped with hydrogel, and the survival rate and activity of the mesenchymal stem cells in the body are enhanced. The invention also provides a heat preservation injector used for the preparation for treating degenerative disc diseases, and the tube wall of the heat preservation injector is filled with a heat preservation material, so that the temperature of contents is ensured, the activity of cells is favorably maintained, and the stress reaction of an organism is reduced.
Owner:中国人民解放军联勤保障部队第九二〇医院
Methods for the treatment of degenerative disc diseases by human birth tissue material composition
ActiveUS11224617B1Pharmaceutical delivery mechanismMammal material medical ingredientsDiseaseTissue material
Methods for treating degenerative disc disease by administering a human birth tissue material composition are provided. The method includes the step of administering a human birth tissue material composition onto or into at least one intervertebral disc or intervertebral space in need of treatment.
Owner:BIODLOGICS
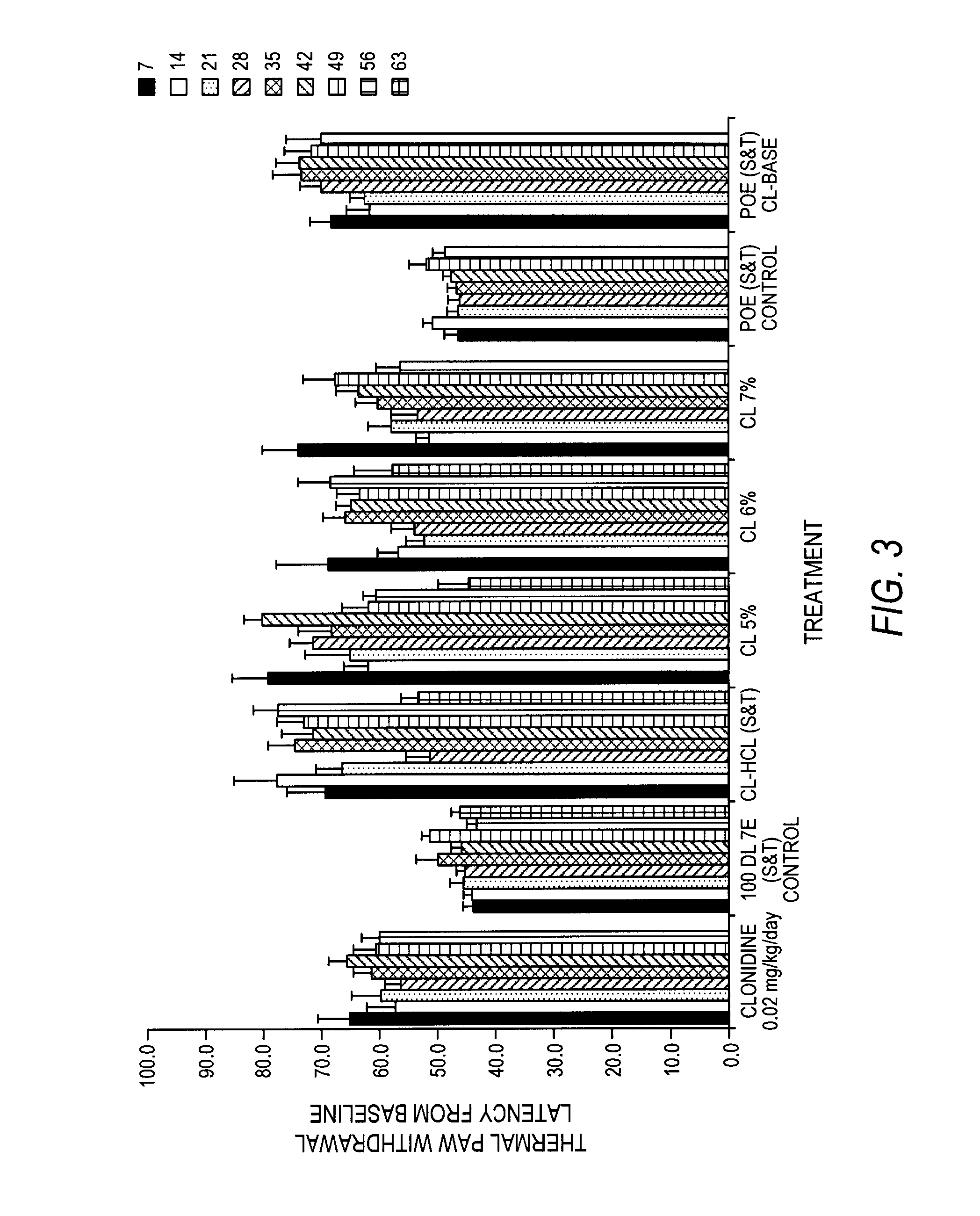
Alpha adreneric receptor agonists for treatment of degenerative disc disease
ActiveUS20150265578A1Reduce inflammationReduce painBiocideOrganic active ingredientsDiseaseDegenerated intervertebral disc
Effective treatments of pain and / or inflammation from degenerative disc disease and / or facet joint are provided. Through the administration of an effective amount of at least one alpha adrenergic agonist at or near a degenerative disc and / or facet joint, one can reduce, prevent or treat pain and / or inflammation caused by the degenerative disc disease and / or facet joint.
Owner:WARSAW ORTHOPEDIC INC
Alpha adrenergic receptor agonists for treatment of degenerative disc disease
InactiveCN101842086AOrganic active ingredientsNervous disorderAdrenergic receptor agonistsDegenerated intervertebral disc
Effective treatments of pain and / or inflammation from degenerative disc disease and / or facet joint are provided. Through the administration of an effective amount of at least one alpha adrenergic agonist at or near a degenerative disc and / or facet joint, one can reduce, prevent or treat pain and / or inflammation caused by the degenerative disc disease and / or facet joint.
Owner:WARSAW ORTHOPEDIC INC
Spinal disk regenerative composition and method of manufacture and use
ActiveUS20160015755A1High viscosityImprove grinding efficiencyInternal osteosythesisSurgical adhesivesRoom temperatureLactate ringer
Owner:VIVEX BIOLOGICS GRP INC
Intervertebral disc repair, methods and devices therefor
The present application discloses compositions, methods and devices for treatment of a degenerative intervertebral disc. A composition can comprise chondrocytes expressing type II collagen. These chondrocytes can be obtained from human cadavers up to about two weeks following death, and can be grown in vitro. The compositions can further comprise one or more biocompatible molecules. Treatment of a degenerative disc can comprise injecting or implanting a composition comprising the chondrocytes into a degenerative disc through an aperture or incision. If the aperture or incision is closed with a suture or a glue after introduction of the chondrocytes, the closure can withstand over 400 N of compression force.
Owner:ISTO TECH
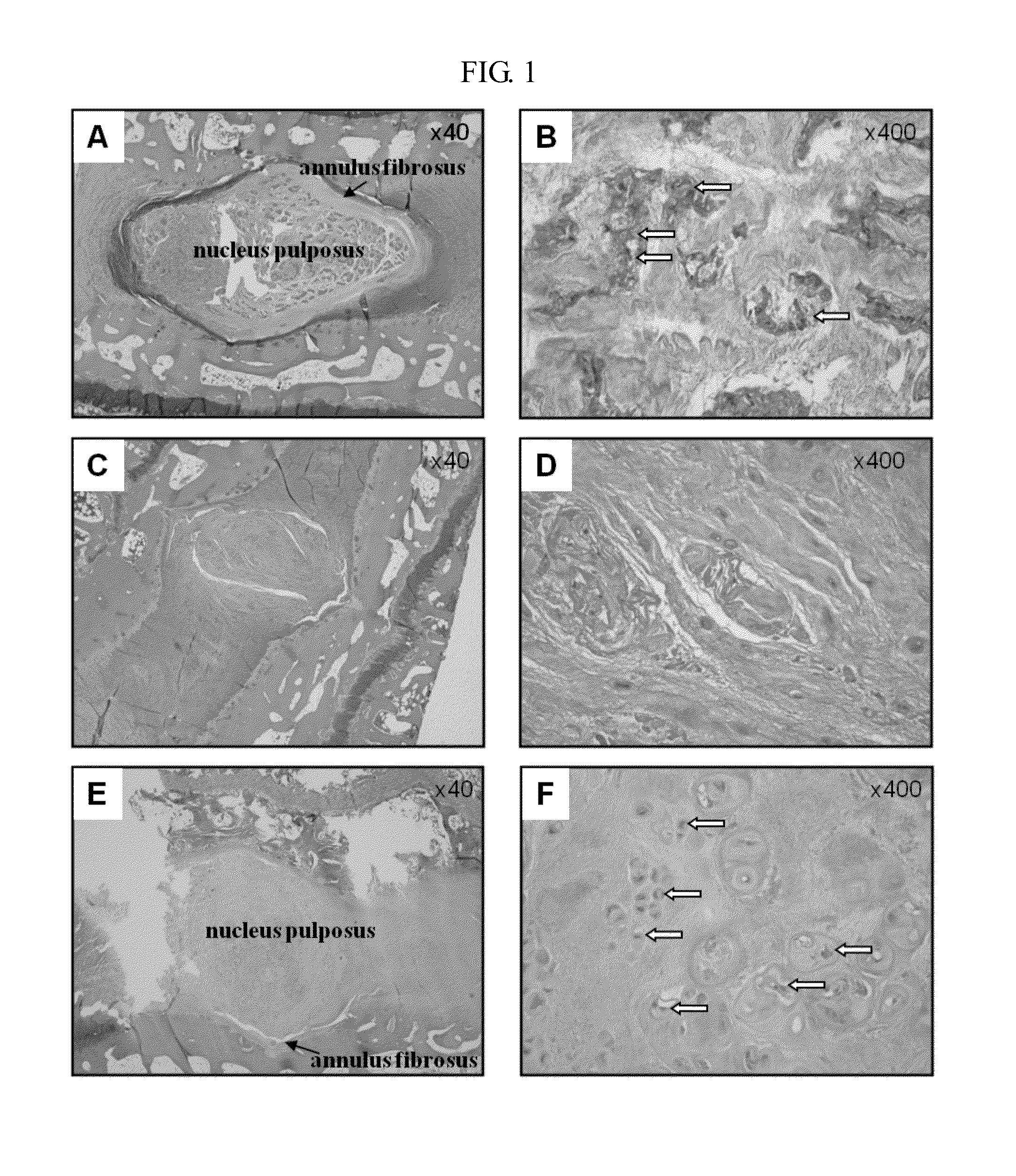
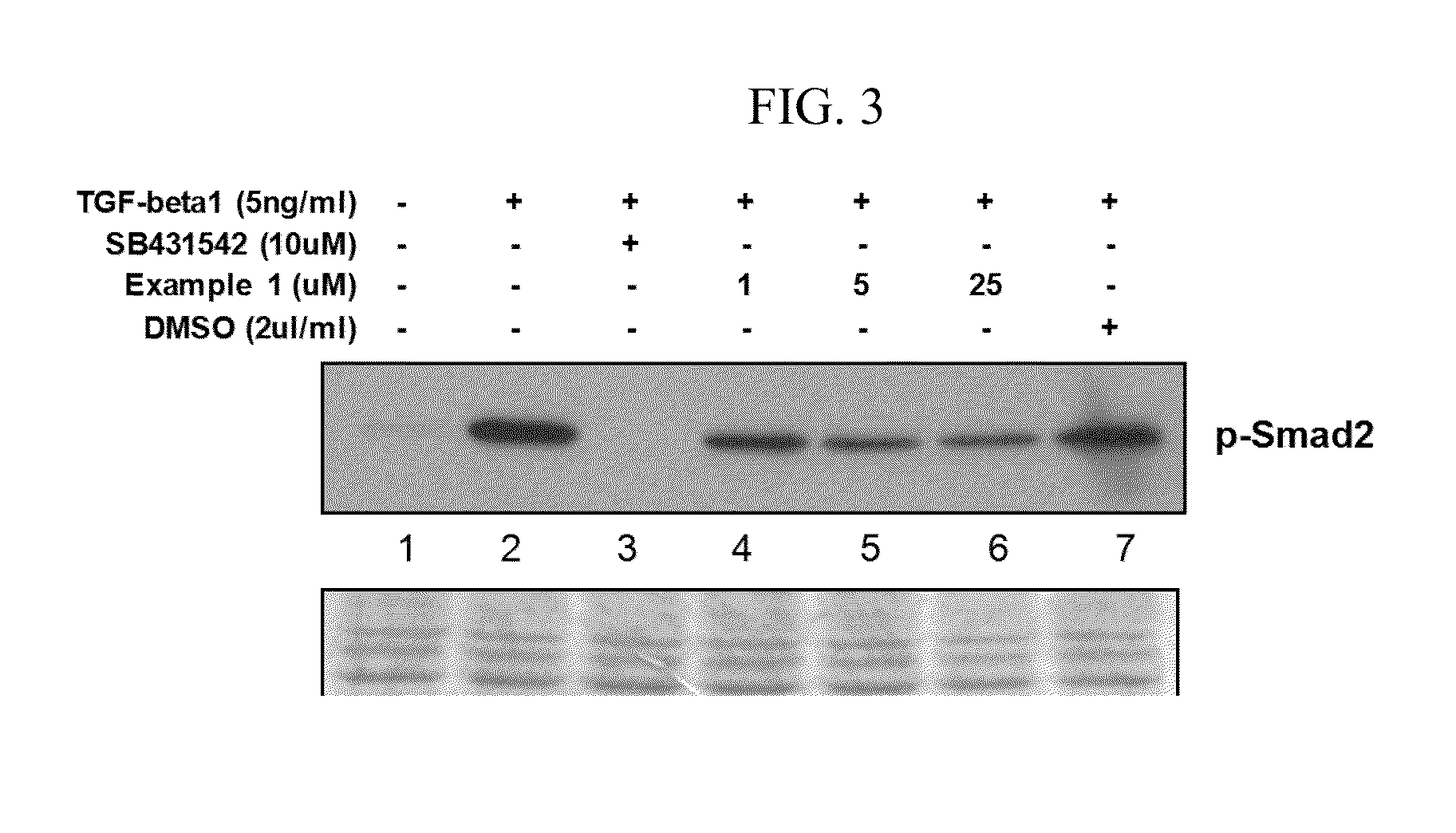
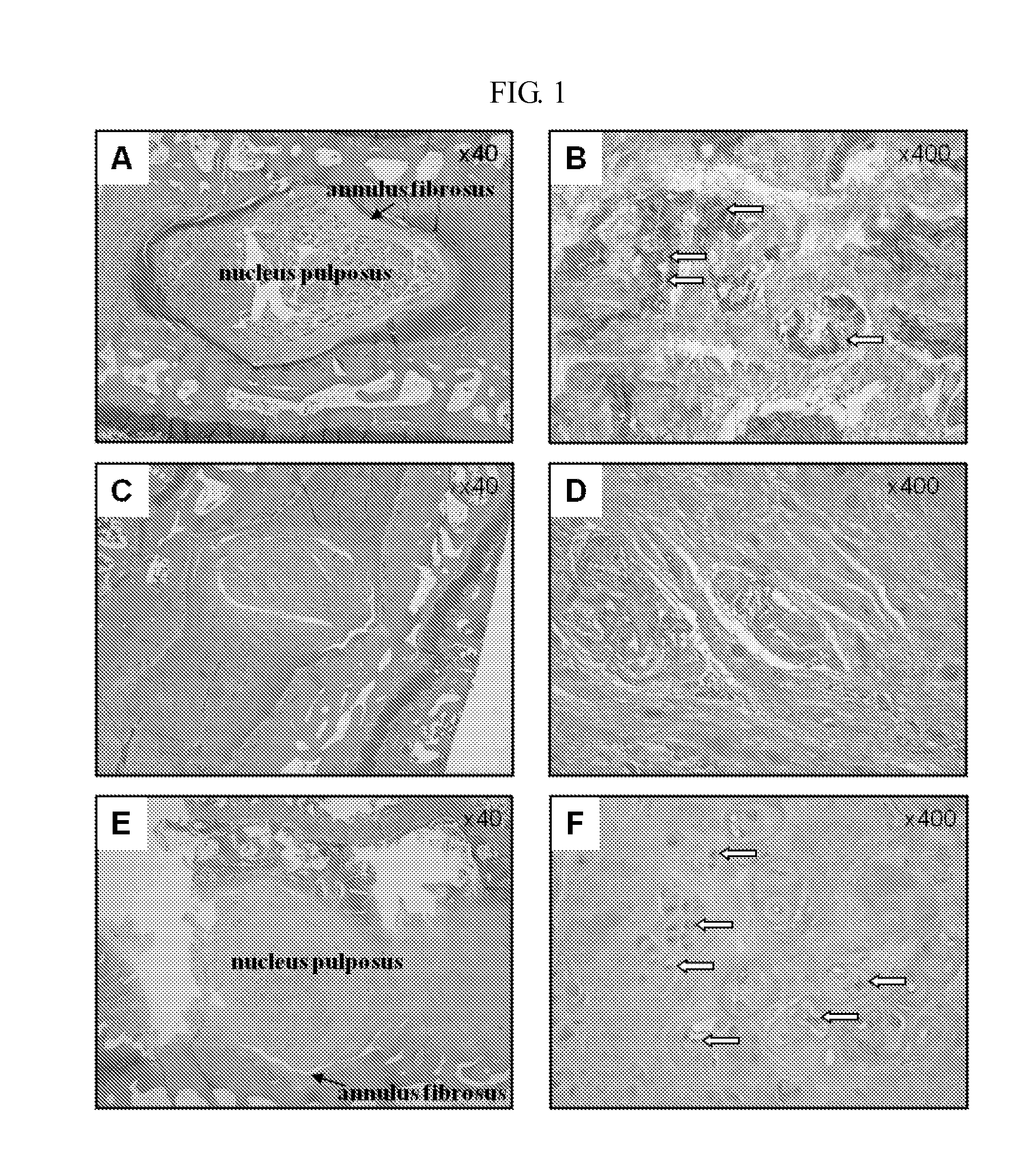
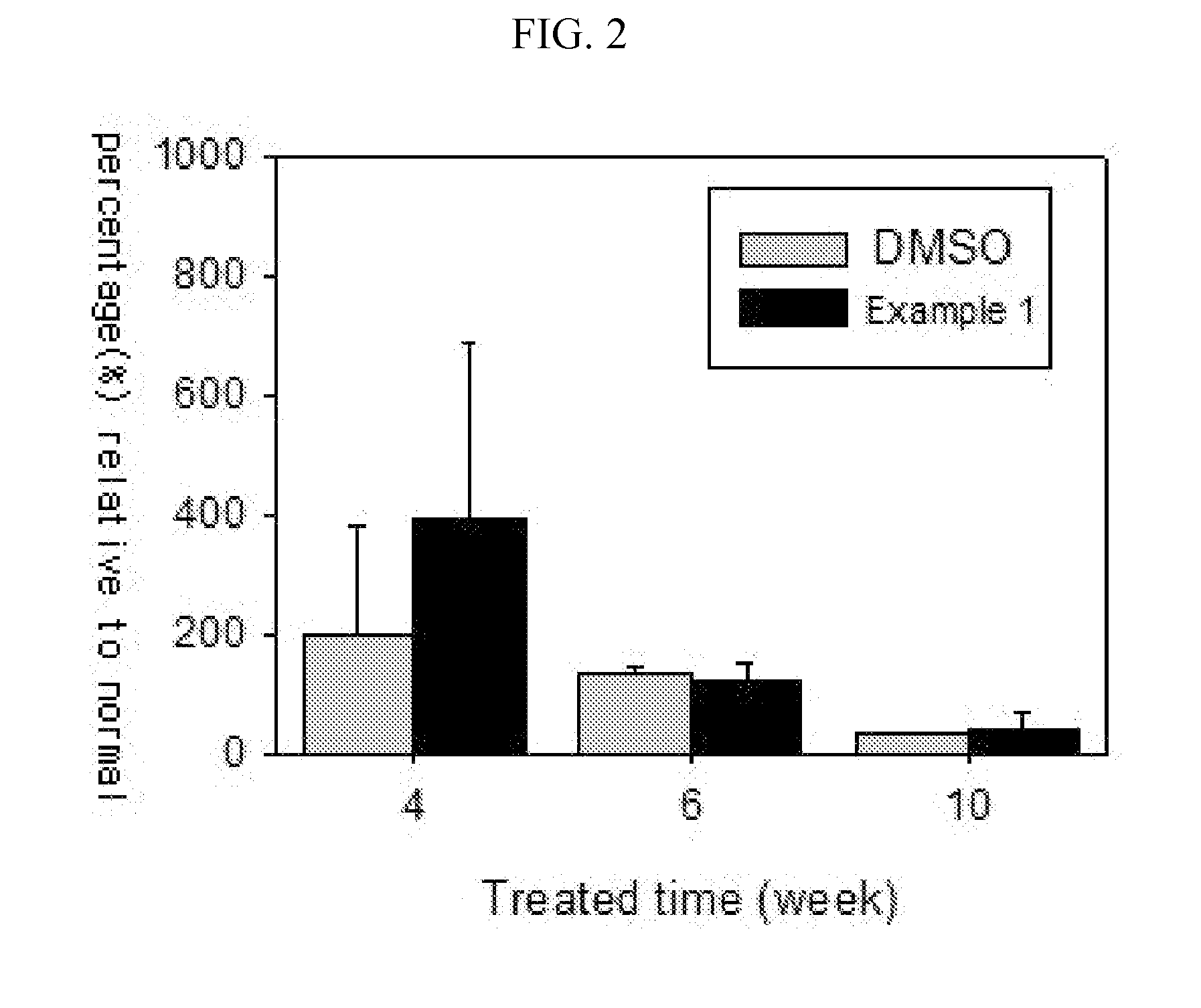
Peptide and use thereof
ActiveUS8691749B2Inhibition is effectivePeptide/protein ingredientsSkeletal disorderBody organsMedicine
The present invention provides a peptide comprising an amino acid sequence of SEQ ID NO: 1 and a pharmaceutically acceptable salt thereof effective for treating degenerative disc diseases, treating body organ fibrosis, treating cancer and / or treating glomerulosclerosis, and effective for the inhibition of TGF-beta 1 signaling.
Owner:ENSOL BIOSCI
Novel peptide and use thereof
ActiveUS20120190625A1Inhibition is effectiveTreating and/or preventingPeptide/protein ingredientsSkeletal disorderDiseaseFibrosis
The present invention provides a peptide comprising an amino acid sequence of SEQ ID NO: 1, a variant thereof and a pharmaceutically acceptable salt thereof.A novel peptide of the present invention, a variant thereof and a pharmaceutically acceptable salt thereof are effective for treating and / or preventing degenerative disc diseases, treating body organ fibrosis, treating cancer and / or treating glomerulosclerosis, and are effective for the inhibition of TGF-beta1 signaling.
Owner:ENSOL BIOSCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com