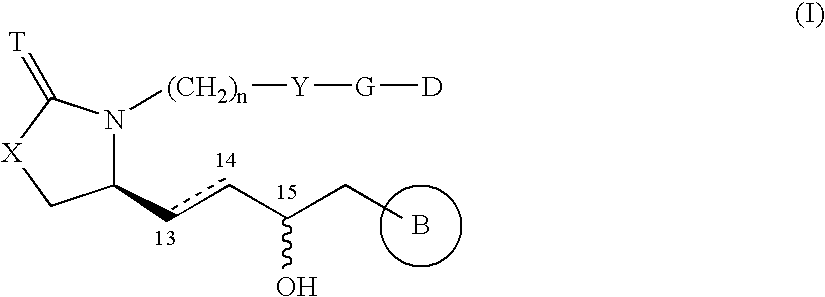
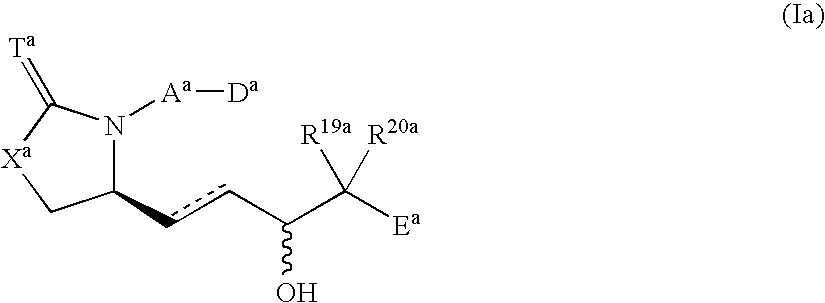
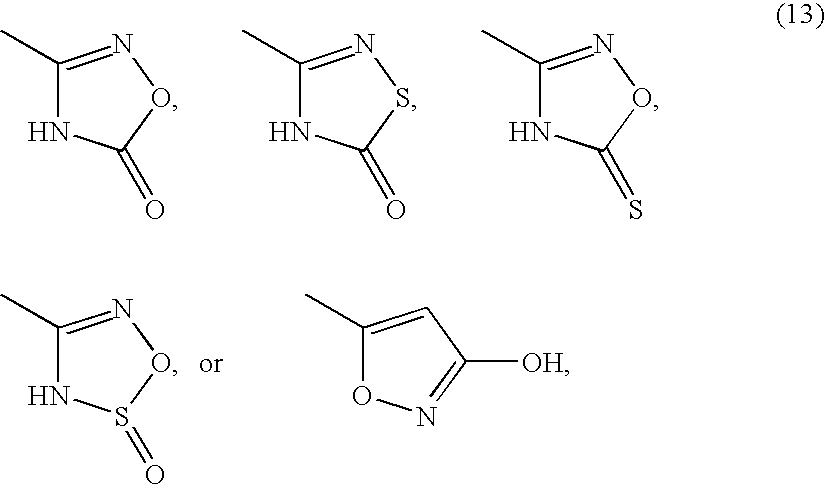
8-azaprostaglandin derivatives and medical use thereof
a technology of azaprostaglandin and derivatives, applied in the field of 8azaprostaglandin derivatives, can solve the problems of limited safety of dose administration, and achieve the effects of enhancing the preventive and/or treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
(4R,5E)-4-tert-butoxycarbonylamino-7-oxo-8-(3,5-dimethylphenyl)oct-5-enoic acid ethyl ester
[0396] Under atmosphere of argon, a suspension of 60% sodium hydride (50 mg) was added by a solution of dimethyl (2-oxo-3-(3,5-dimethylphenyl)propyl)phosphonate (373 mg) in tetrahydrofuran (5 mL) at the temperature of 0° C. The mixture was stirred for an hour and then a solution of ethyl (4R)-4-(tert-butoxycarbonylamino)-4-formylbutanoate (298 mg) in tetrahydrofuran (5 mL) was added to the mixture. The mixture was stirred for an hour. To the mixture, methyl tert-butyl ether and water were added, and then IN a solution of sodium hydroxide was added. The organic layer was washed with saturated brine, dried over an anhydrous magnesium sulfate, concentrated and was purified by column chromatography on silica gel (hexane:ethyl acetate=4:1) to give the title compound (300 mg) having the following physical data.
[0397] TLC: Rf0.76 (hexane:ethyl acetate=1:1)
example 2
(4R,5E,7S)-4-tert-butoxycarbonylamino-7-hydroxy-8-(3,5-dimethylphenyl)oct-5-enoic acid ethyl ester
[0398] Under atmosphere of argon, a solution of the compound prepared in Example 1 (295 mg) in tetrahydrofuran (7.3 mL) was added by 1.0 mol / l (R)-2-methyl-CBS-oxazaborolidine / toluene solution (0.22 mL) at the temperature of 0° C. Then 1.0 mol / l borane tetrahydrofuran complex / tetrahydrofuran solution was dropped to the mixture, and then the mixture was stirred for 45 minutes. Additionally, 1.0 mol / l (R)-2-methyl-CBS-oxazaborolidine / toluene solution (0.22 mL) and 1.0 mol / l borane tetrahydrofuran complex / tetrahydrofuran solution were dropped to the mixture and then the mixture was stirred for 20 minutes at a temperature of 0° C. To the mixture, small quantity of ethanol and water was added and raised till room temperature. The mixture was extracted with ethyl acetate. The organic layer was washed with diluted hydrochloric acid, saturated sodium bicarbonate water and saturated brine succe...
example 3
(4R,5E,7S)-4-amino-7-hydroxy-8-(3,5-dimethylphenyl)oct-5-enoic acid ethyl ester hydrochloride
[0401]
[0402] A solution of the compound prepared in Example 2 (243 mg) in ethanol (1 mL) was dropped by 4N hydrochloride / dioxane (0.5 mL) at a temperature of 0° C. and the mixture was stirred for 3 hours at room temperature. The mixture was concentrated to give the title compound (205 mg) having the following physical data. The compound was not purified any more and as is to be used in the next reaction.
[0403] TLC: Rf0.29 (chloroform:methanol:acetic acid=9:1:0.1);
[0404] NMR: δ 6.83, 5.90, 5.54, 4.40-4.34, 4.14, 3.76-3.68, 2.82-2.67, 2.27, 2.26, 2.10-1.94, 1.85-1.72, 1.26
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com