Catalyzed hydrogen desorption in Mg-based hydrogen storage material and methods for production thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
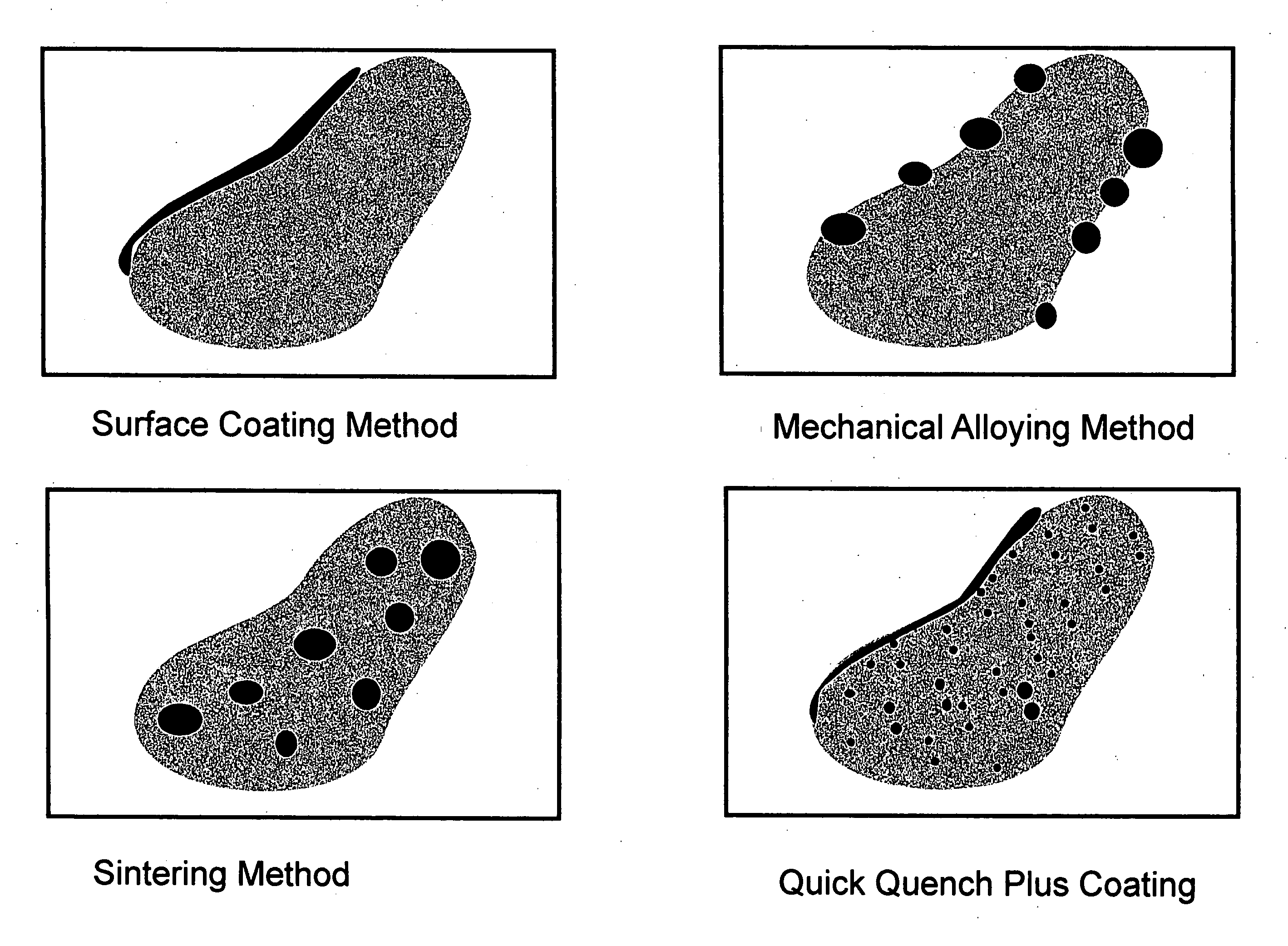
Method used
Image
Examples
example 1
[0076] Raw materials consisting of pure metal powders of magnesium (99.8%, −325 mesh), aluminum (99.5%, −325 mesh), iron (99.9+%, 10 micron) and other minor constituents were mixed in an agate mortar-pestle. Ten different compositions were produced, and their compositions in weight percent are listed in Table 1. A hardened steel die was used to press the mixed powders into a pellet of 1 cm diameter and 1 cm long. The pressed pellet was placed in a quartz tube and was sintered at a temperature above 500° C. for 22 hours under vacuum.
TABLE IChemical composition (all numbers are in weight percentage)Alloy NumberMgAlFeBCuPdVNiCScMM-188.82.78.5———————MM-287391——————MM-386392—————MM-48639—2—————MM-58736—4—————MM-68738——2————MM-78738———2———MM-887—9————4——MM-987—9—————4—MM-108737——————3
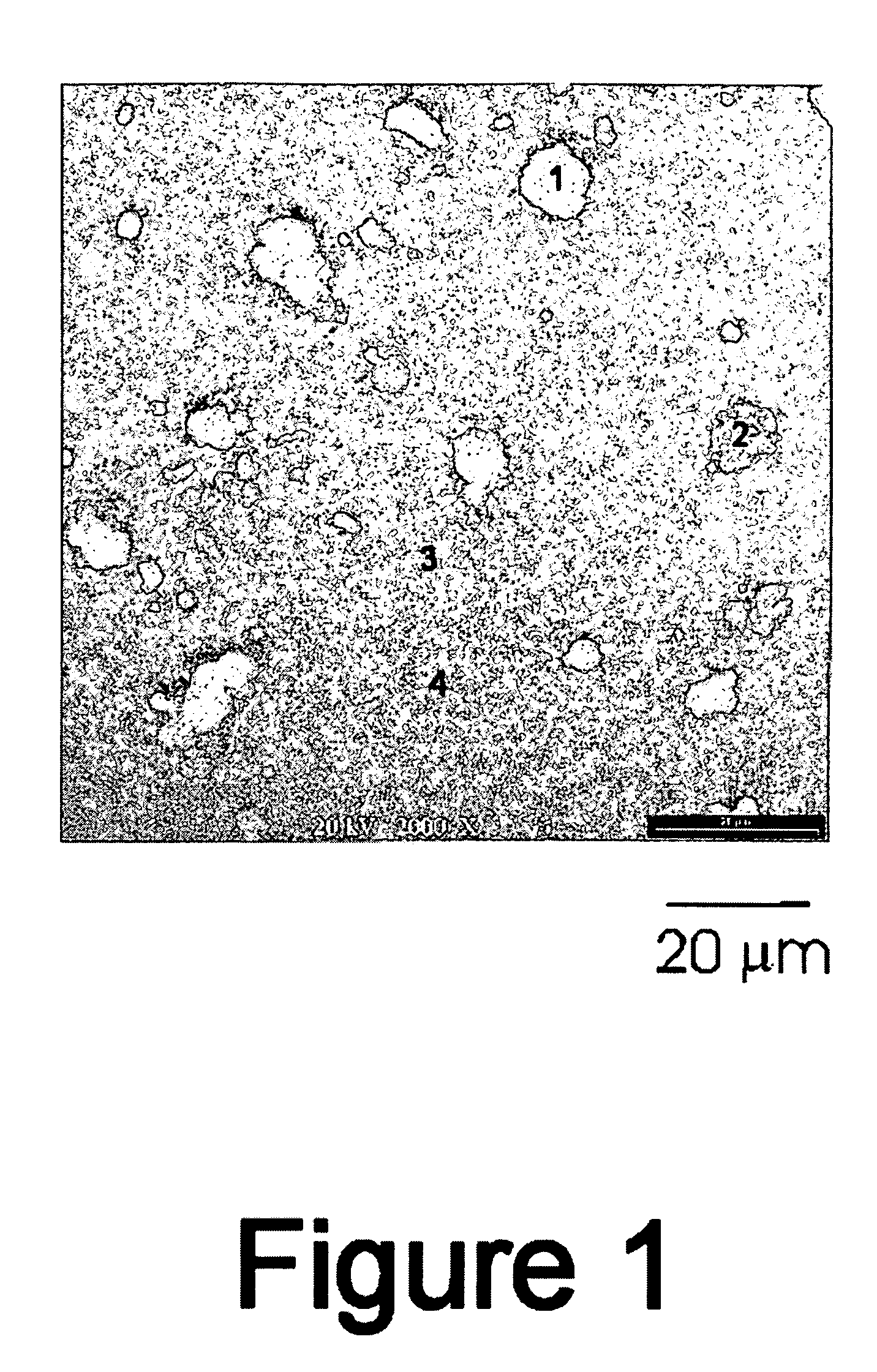
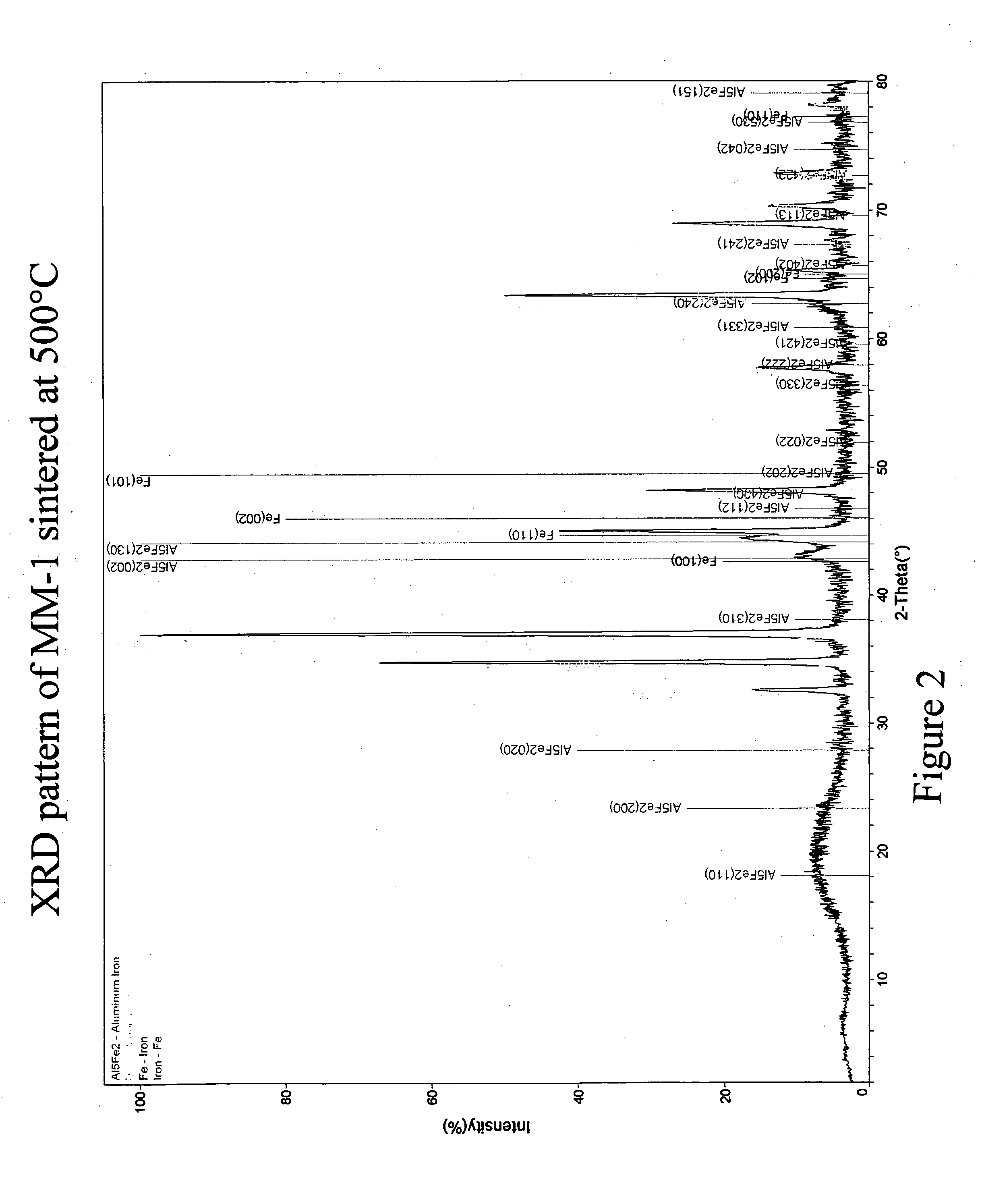
[0077]FIG. 1 is a scanning electron micrograph (SEM) taken in back-scattering mode of an MM-1 sample sintered / annealed at 500° C. The SEM indicates phase segregation of Fe and an Al5Fe2 intermetallic compou...
example 2
[0079] Another MM-1 material was produced by the process described in Example 1 with a change in sintering / annealing temperature. FIG. 6 plots the PCT curves of samples sintered / annealed at 570° C. and 600° C. respectively. While the PCT of the material sintered / annealed at 570° C. shows little deviation from that of the material of Example 1 (which was sintered / annealed at 500° C.), the material sintered / annealed at 600° C. provides an extended plateau at a slightly higher pressure.
example 3
[0080] The mechanically alloyed (MA) powders of MM-1 were prepared from mixtures of pure elemental magnesium (99.8%, −325 mesh), aluminum (99.5%, −325 mesh), and iron (99.9+%, 10 micron). The milling was carried out in an attritor loaded with Cr-steel grinding balls. The mechanical alloying process is performed under an argon atmosphere with the addition of 1% graphite and heptane to keep material from caking on the attritor walls. Typical milling time is two hours. FIG. 7 is an SEM back-scattering micrograph of this sample. The figure indicates severe phase segregation within the material. Region 1 (bright contrast on the picture) is filled with Fe and Al powder while Region 2 (central darker area) is all magnesium. FIG. 8, which is the XRD plot of the sample, shows no indication of any amorphous intermetallic product formed by this process. The MA-MM-1 powder was pressed onto an expanded nickel metal substrate and then coated on both sides with 100 angstroms of iron as surface cat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com