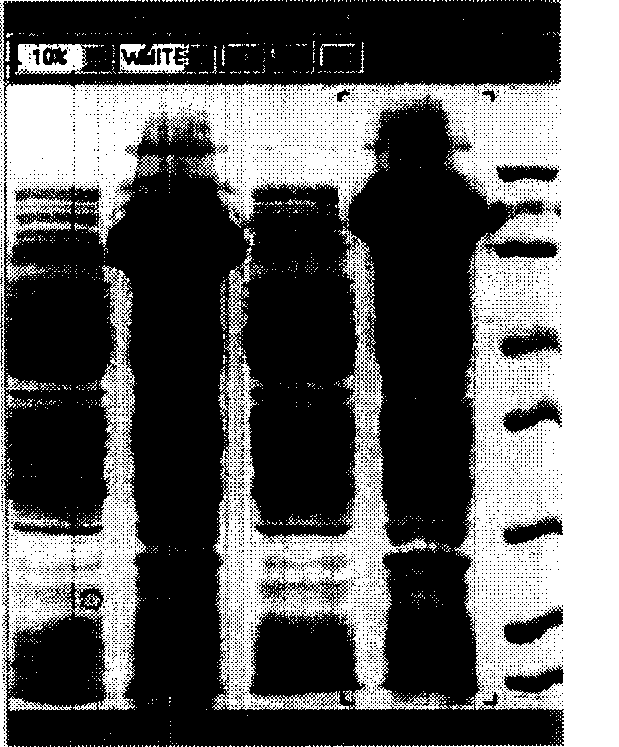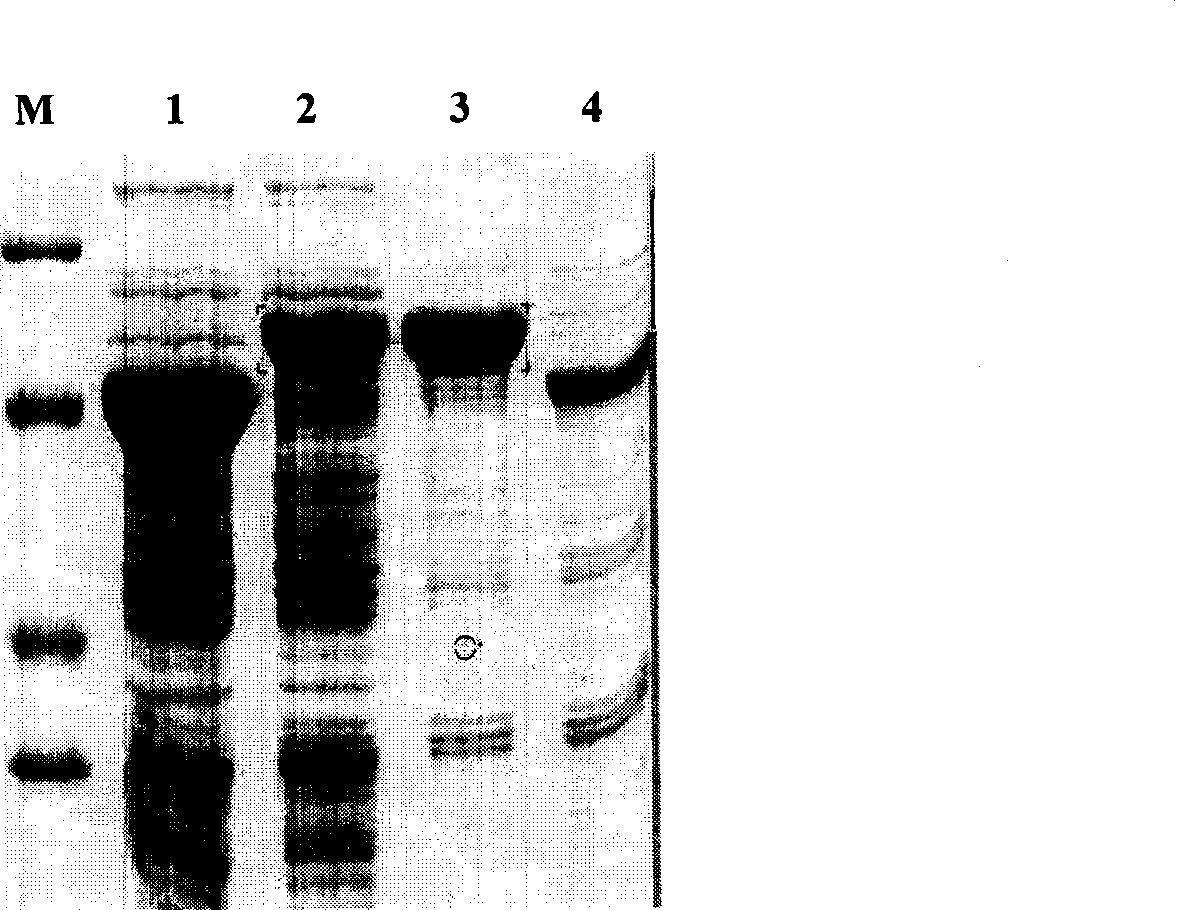Recombination human endothelium chalene expression strain and solubility expression method
An endostatin and expression method technology, applied in the field of genetic engineering, can solve the problems of low activity recovery rate, low renaturation rate, complicated process and the like, and achieve the effect of being beneficial to purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1 Construction of Recombinant Human Endostatin Expression Plasmid pET-Endo
[0036] According to the gene sequence of human endostatin, the N-terminal and C-terminal primer sequences were synthesized, and the primer sequences were as follows:
[0037] Primer 1: 5'-GCATAGCCATCGTGATTTC-3' (SEQ ID NO: 2) and
[0038] Primer 2: 5'-CGGGATCCCTACTTGGAGGCAGTCAT-3' (SEQ ID NO: 3)
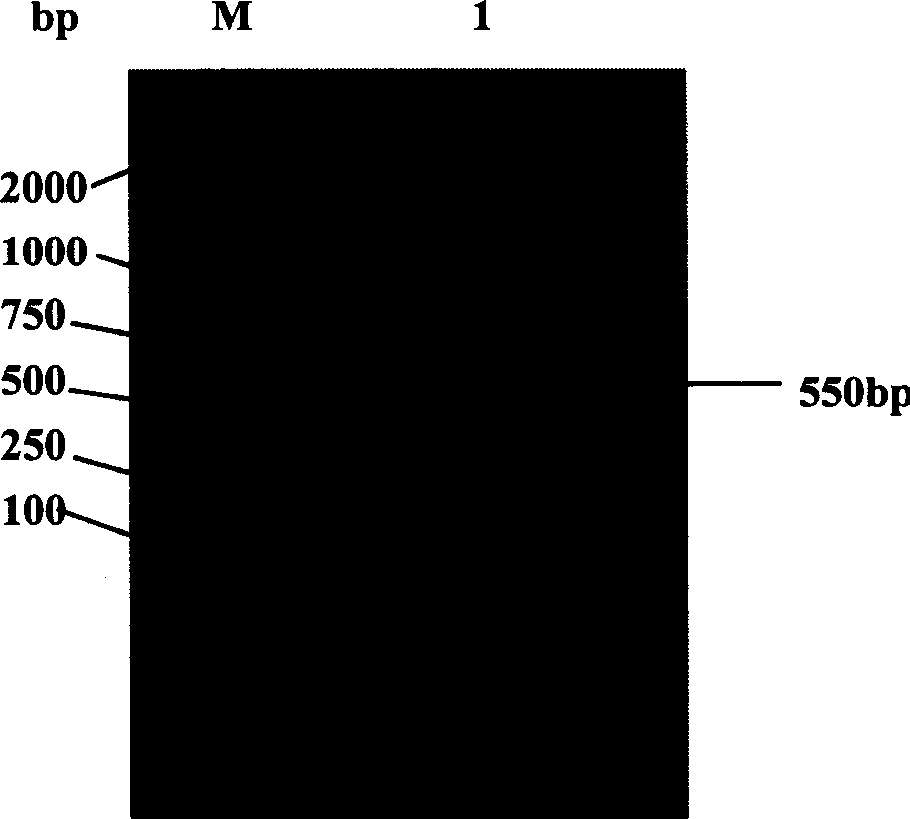
[0039] Using the plasmid pMD-Endo as a template, using the above two primers, the human endostatin gene was amplified by the PCR method, and analyzed by agarose electrophoresis, the amplified fragment was about 550bp ( figure 1 ), which is consistent with the expected. The PCR fragment and the vector pET43.1a were digested with PshAI and BamHI respectively, and ligated using TaKaRa DNA Ligation Kit (TaKaRa DNA Ligation Kit) to obtain the recombinant human endostatin expression vector pET-Endo.
[0040] The above-mentioned vectors were transformed into Escherichia coli Top10 (commercial str...
Embodiment 2
[0041] Example 2 Construction and expression of recombinant human endostatin expression strain
[0042] The recombinant plasmid pET-Endo was transformed into competent Escherichia coli Origami (DE3) by the heat shock method (the operation was the same as in Example 1). On the second day, single clones were selected, transferred to LB culture medium containing 100 mg / L ampicillin, cultured overnight at 37°C with shaking, and plasmids were extracted, identified by restriction enzyme digestion, and induced to express. Cultivate the monoclonal E.coli Origami-Endo containing the expression plasmid in LB medium (containing 100mg / L ampicillin) at 37°C until OD 600 was about 0.5, added IPTG with a final concentration of 1mmol / L to induce expression for 6h, collected the bacteria by centrifugation, and after breaking the bacteria by ultrasonic, carried out SDS-PAGE analysis on the whole bacteria, supernatant and precipitated (inclusion body) proteins respectively, SDS- The concentrati...
Embodiment 3
[0044] Embodiment 3 The influence of temperature on the soluble expression of recombinant human endostatin
[0045] The expression strain E. coli Origami-Endo was inoculated in 5 ml of LB culture medium containing 100 mg / L ampicillin (Amp). In the bacterial incubator, 37°C, 250r / min shaking culture for 15h, then transfer the bacterial solution to LB culture solution containing 100mg / LAmp at a ratio of 1:100, 37°C, 250r / min shaking culture for 3-4h, Make bacterial solution OD 600At about 0.5, add IPTG with a final concentration of 1.0mmol / L to the bacterial solution to induce expression, and the induction temperature is 25°C, 30°C and 37°C, respectively. After 6 hours of induction and expression, collect the bacterial cells, and break the bacteria by ultrasonic. The supernatant and precipitated (inclusion body) proteins were analyzed by SDS-PAGE to detect the soluble expression effect of recombinant human endostatin. After automatic gel scanning density quantitative analysis,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com