Classical swine fever virus multimer vaccine and preparation method thereof
A technology of vaccines and recombinant vectors, applied in antiviral agents, chemical instruments and methods, medical preparations containing active ingredients, etc., can solve problems such as unsatisfactory immune effects, and achieve the effect of increasing stability and stabilizing the state
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Embodiment 1, fusion protein design and construction of recombinant expression vector
[0064] 1.1. Design of fusion protein
[0065] In order to improve the immune effect of CSFV-E2 protein, the gene encoding CSFV-E2 was fused with the gene encoding the constant region of the porcine IgG heavy chain, and the gene encoding CRM19L was fused with the gene encoding the constant region of the porcine IgG light chain. SpyTag is a polypeptide segment, and SpyCatcher is a corresponding protein. The two can recombine and spontaneously form iso-peptide bond coupling.
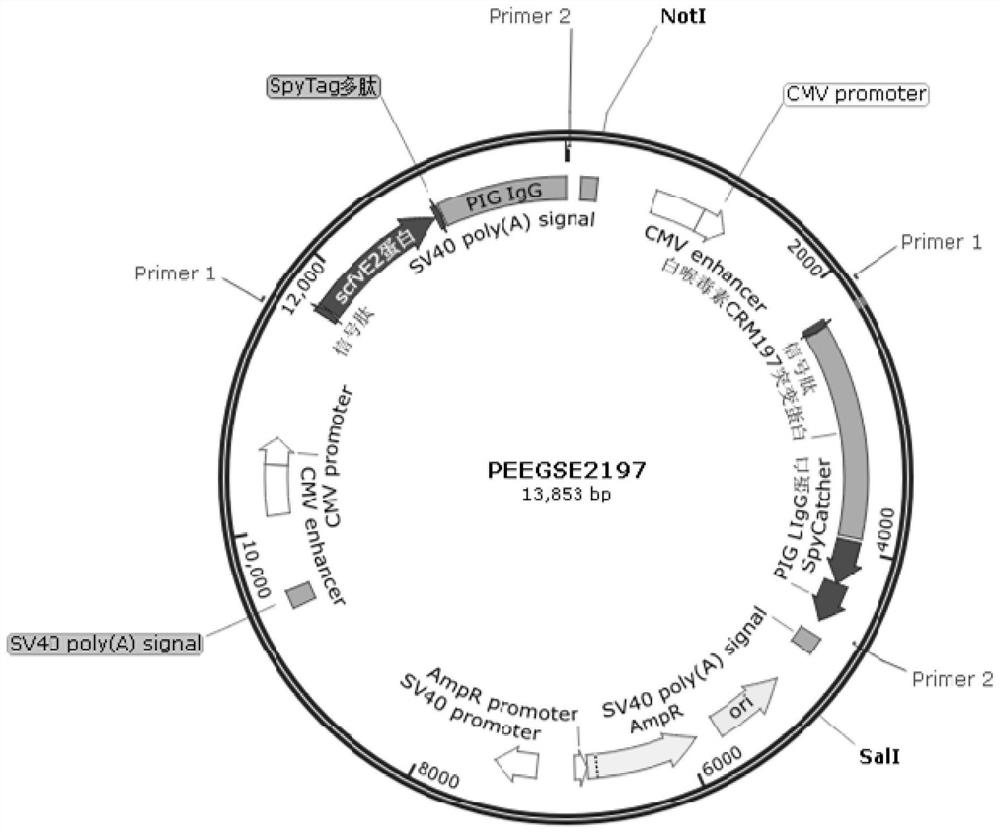
[0066] Entrusted Beijing Qingke Bio Co., Ltd. to synthesize fusion protein (CSFV-E2)-SpyTag-HIgG encoding gene (SEQ ID No. 1) and fusion protein CRM197-SpyCatcher-LIgG encoding gene (SEQ ID No. 2), wherein (CSFV -E2)-SpyTag-HIgG gene replaces the fragment between the HindIII and ECORI restriction sites of the PEE12.4 vector (a small fragment between the HindIII and ECORI restriction sites) to obtain a recombinant...
Embodiment 2
[0074] Example 2. Construction of stably transfected cell line and protein expression
[0075] 2.1. Construction of stably transfected cell line
[0076] The recombinant expression vector PEEGSE2197 of Example 1 was digested with Pvu I and recovered to obtain a linearized expression vector PEEGSE2197. The linearized expression vector PEEGSE2197 was transfected into CHO cells, and the transfection steps included:
[0077] Step 1: 24h before transfection, adjust the cell density to 2.5-3×10 6 cells / mL, cultured overnight.
[0078] Step 2: On the day of transfection, dilute the cell density to 3 x 10 with fresh medium 6 cells / mL, cell viability ≥95%.
[0079] Step 3: Prepare the DNA-PEI complex, dilute DNA and PEI with Opti-MEM respectively, the final DNA concentration during transfection is 1-2 μg / mL, the mass ratio of PEI to DNA is 5:1, and the diluted DNA is PEIs were allowed to stand for 5 minutes and then mixed 1:1, then gently inverted and mixed several times.
[0080...
Embodiment 3
[0100] Embodiment 3, E2 vaccine safety and effect verification
[0101] The E2 vaccine prepared in 2.4 in Example 2 was used for this step of the experiment, and the commercial vaccine was Kewenjing (a swine fever virus E2 protein recombinant baculovirus inactivated vaccine (WH-09 strain)).
[0102] 3.1. Safety experiment of recombinant fusion protein
[0103] Take 6-8 weeks old Balb / C mice (purchased from Shanghai Slack Laboratory Animal Co., Ltd.) with a body weight of 18-22 g, 20 (half male and half male), and randomly divided into groups, 5 mice in each group, a total of 4 groups . The first group was E2 vaccine 50μg group, the second group was E2 vaccine 100μg group, the third group was commercial vaccine group (positive control), and the fourth group was PBS group (negative control).
[0104] Group 1: Each mouse was first subcutaneously inoculated with 0.1 mL of the E2 vaccine solution (500 μg / mL vaccine A) in 2.4.1 of Example 2, so that the dose of fusion protein E2 w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com