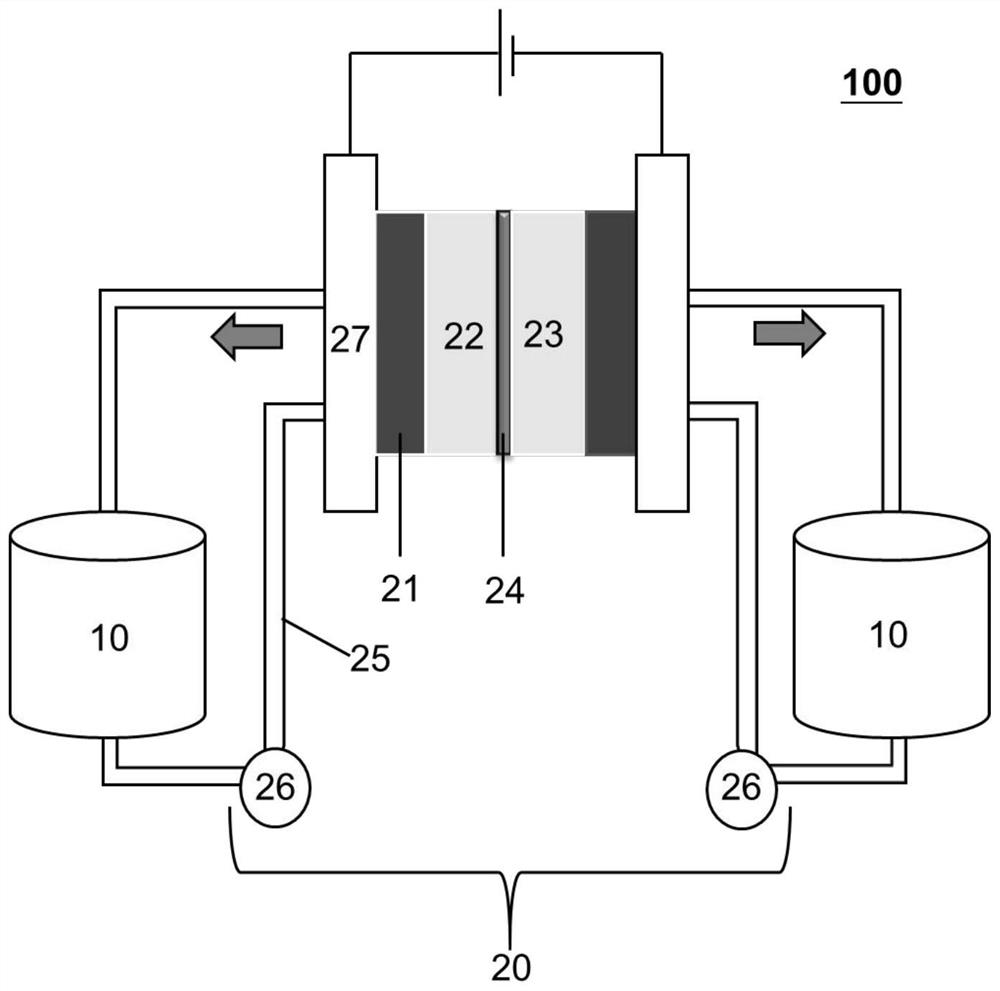
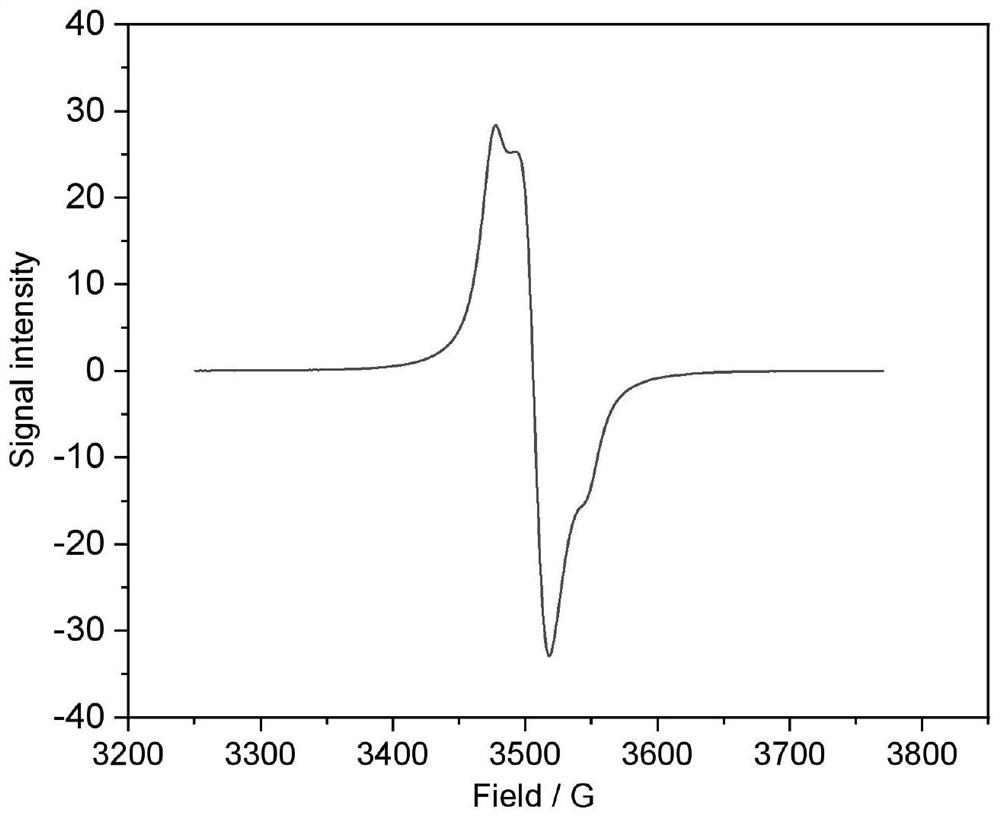
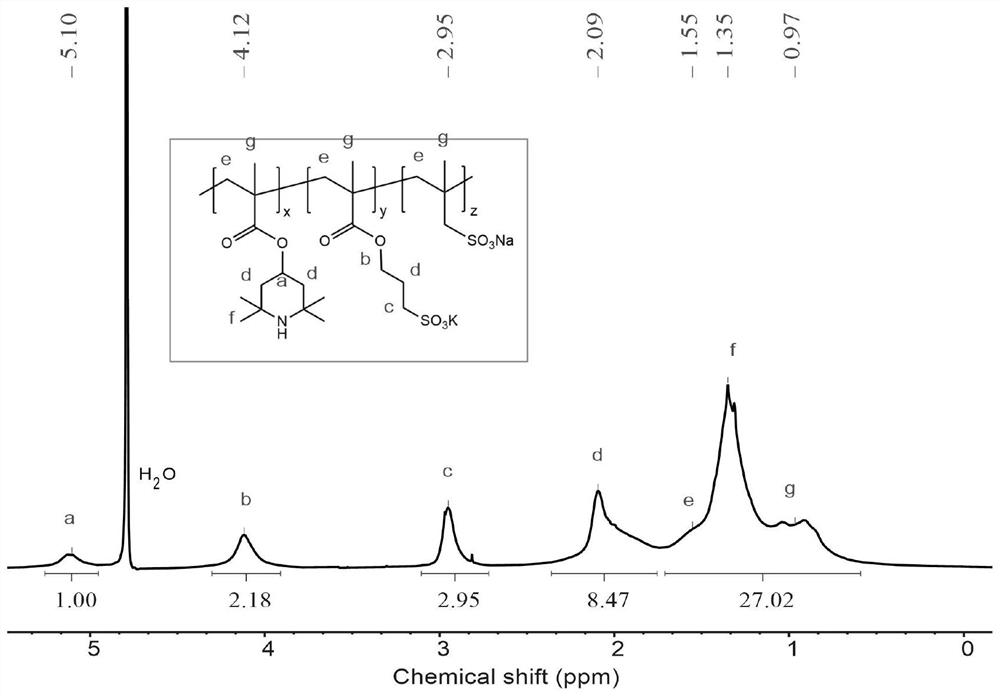
Aqueous positive electrode polymer, preparation method thereof and small molecule flow battery system
A technology for flow batteries and polymers, applied in fuel cells, regenerative fuel cells, circuits, etc., can solve the problems of restricting the large-scale application of flow batteries, cross-contamination of porous membranes, and large pore size of porous membranes, and achieve suppression of concentration differences. Polarization phenomenon, meet the needs of large-scale energy storage, the effect of fast charge and discharge rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0049] The present invention also provides a method for preparing the above-mentioned water-based positive electrode polymer, comprising the following steps: Ester (TEMPMA), monomer 3-sulfonic acid propyl methacrylate potassium salt (SMPS) and monomer 2-methyl-2-propene-1-sulfonic acid sodium (SMAS) as raw materials, adding initiator, through free The method for preparing the water-based positive electrode polymer by base polymerization, the chemical reaction formula is as shown in formula (1):
[0050]
[0051] Specifically, the method for preparing the water-based positive electrode polymer includes the following steps: S11. In a container, sequentially add TEMPMA, SMPS, SMAS and dilute hydrochloric acid. After all the solids are dissolved, the initiator is added, and the 2 Stir and heat to remove oxygen in the environment, react for 18-24h; after the reaction, cool to room temperature; add NaOH dropwise to adjust to alkalinity, and then use a dialysis bag with a molecul...
Embodiment 1
[0081] Preparation of an Aqueous Positive Electrode Polymer by Free Radical Polymerization
[0082] In a 250 mL flask with a branch tube, TEMPMA (5.633 g 25 mmol), SMPS (7.512 g 30.5 mmol), SMAS (1.58 g 10 mmol) and 100 mL 0.75 M dilute hydrochloric acid were added sequentially. After all the solids were dissolved, the initiator 4,4'-azobis(4-cyanovaleric acid) (ABCVA, 0.84 g) was added to obtain a suspension. Pass N 2 After 30min, heat to 80°C. The solution turned from cloudy to clear. Reaction 24h. After the reaction, cool to room temperature. NaOH (20wt%) was added dropwise until the pH was around 8. Carry out dialysis with a dialysis bag with a molecular weight cut-off of 1000, and freeze-dry the solution after the dialysis to obtain a white flocculent polymer;
[0083] Oxidation: Dissolve the polymer in 30 mL deionized water, add Na 2 WO 4 (0.6g), heated to 50°C, added dropwise H within 20h 2 o 2 (30wt%) 22mL, NaOH was added dropwise throughout the process to m...
Embodiment 2
[0085] In a 250 mL flask with a branch tube, TEMPMA (4.50 g 20 mmol), SMPS (4.92 g 20 mmol), SMAS (1.58 g 10 mmol) and 10 mL of 0.75 M dilute hydrochloric acid were sequentially added. After all the solids were dissolved, the initiator 4,4'-azobis(4-cyanovaleric acid) (ABCVA, 0.84 g) was added. Pass N 2 After 30min, heat to 65°C. The solution turned from cloudy to clear. Reaction 24h. After the reaction, cool to room temperature. NaOH (20wt%) was added dropwise until the pH was around 8. Carry out dialysis with a dialysis bag with a molecular weight cut-off of 1000, and freeze-dry the solution after the dialysis to obtain a white flocculent polymer;
[0086] Oxidation: Dissolve the polymer in 50 mL deionized water, add Na 2 WO 4 (0.6g), stirring at room temperature, adding H dropwise within 20h 2 o 2 (30wt%) 22mL, NaOH was added dropwise throughout the process to maintain the pH at 8-9. Reaction at room temperature for 24h. A dialysis bag with a molecular weight cut...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com