Sesquilignan compound as well as separation method and application thereof
A technology of lignans and separation method, which is applied in the field of sesquilignans and their separation, and can solve the problems of undisclosed application of sesquilignans and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] The preparation of embodiment 1 sesquilignans
[0051] Include the following steps:
[0052] (1) Grind the dried noni seeds, and ultrasonically extract each kilogram of noni seeds with 4L petroleum ether and ethyl acetate solvent successively for 3 times, each extraction for 3 hours, combine the extracts, concentrate under reduced pressure to obtain the extract of ethyl acetate parts (about 110g);
[0053] (2) Take step (1) ethyl acetate part extract (about 60g), first through silica gel column chromatography, adopt sherwood oil-acetone mixed solvent to carry out gradient elution as eluent, the elution of sherwood oil-acetone mixed solvent The off-gradient is 100:1, 80:1, 20:1, 10:1, 5:1, 1:1, 1:10, 2 column volumes are collected for each gradient, and one fraction is obtained for each gradient, and a total of 7 components, namely Fr.1~Fr.7;
[0054] (3) Put Fr.5 through normal phase silica gel column chromatography first, and use the elution gradient of chloroform-m...
Embodiment 2
[0055] The preparation of embodiment 2 sesquilignans
[0056] Include the following steps:
[0057] (1) Grind the dried Noni seeds, and ultrasonically extract each kilogram of Noni seeds with 4L petroleum ether and ethyl acetate solvent sequentially for 4 times, each extraction for 2 hours, combine the extracts, concentrate under reduced pressure to obtain the extract of ethyl acetate parts (about 120g);
[0058] (2) Take step (1) ethyl acetate part extract (about 60g), first through silica gel column chromatography, adopt sherwood oil-acetone mixed solvent to carry out gradient elution as eluent, the elution of sherwood oil-acetone mixed solvent The off-gradient is 100:1, 80:1, 20:1, 10:1, 5:1, 1:1, 1:10, and each gradient collects 3 column volumes, and each gradient obtains one fraction, and a total of 7 components, namely Fr.1~Fr.7;
[0059] (3) Fr.5 was subjected to normal phase silica gel column chromatography first, and the elution gradient of chloroform-methanol mixe...
Embodiment 3
[0060] The structural identification of embodiment 3 sesquilignans
[0061] Using Spectrum ( 1 H NMR, 13 C NMR, HSQC, HMBC, NOESY) and modern structural identification techniques such as MS, determine the chemical structure of the compound III and IV obtained in embodiment 1 and embodiment 2.
[0062] The structural identification data are as follows:
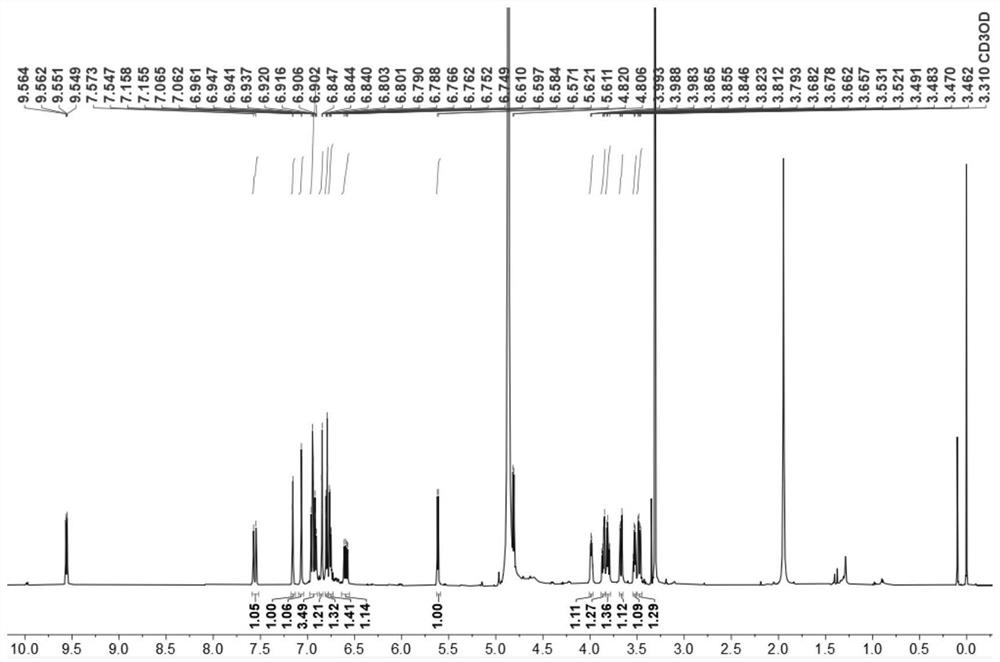
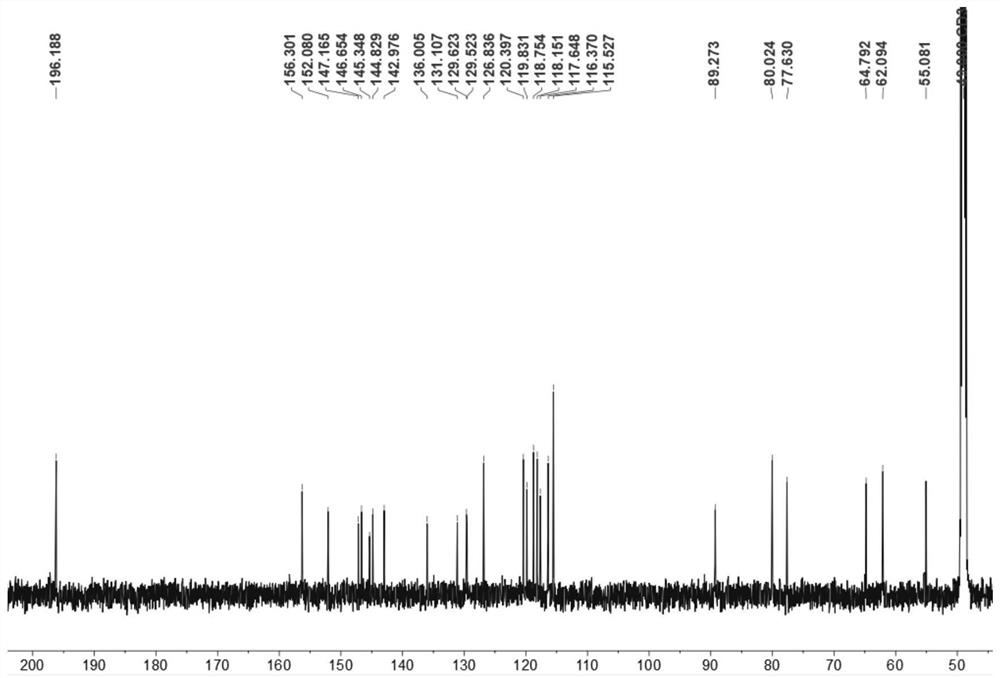
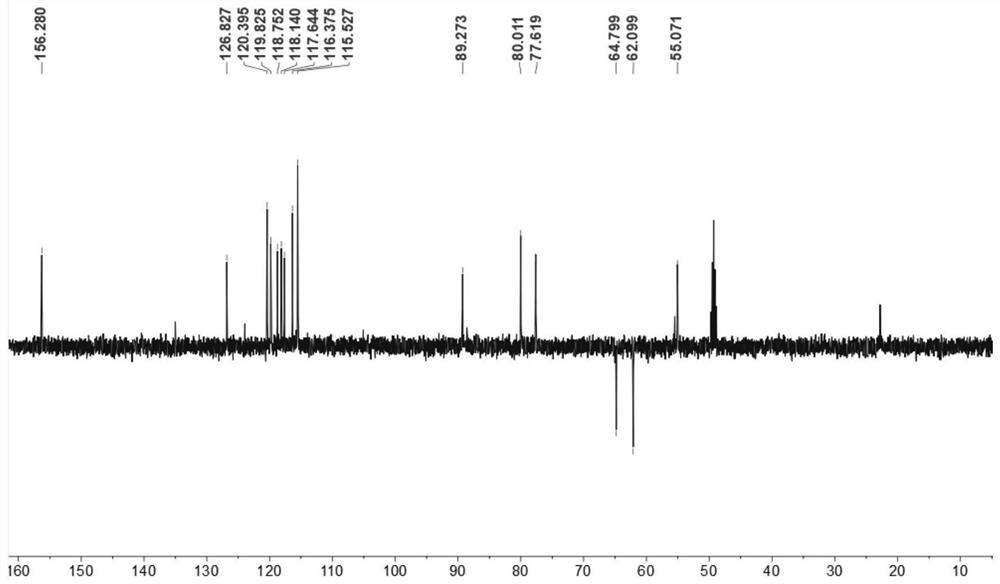
[0063] Compound Ⅲ: It is a yellow oily substance, easily soluble in methanol. High resolution mass spectrometry HRESI(-)MS(m / z491.1345[M-H] - , theoretical value 491.1342) determine its molecular formula as C 27 h 24 o 9 ;according to 1 H, 13 C and two-dimensional nuclear magnetic resonance data to determine its structure, the skeleton type is sesquilignan, that is, rel-(7'β,8'α,7"β,8"α)-3,3",4",9 ',9"-Pentahydroxy-1-propenyl-7',8'-chroman-3,7":7,9'-dioxa-sequilignan, named For moricitan A, its 1 H and 13 See Table 1 for C NMR data attribution. [600MHz( 1 H), 150 MHz ( 13 C), solvent: MeOD-d 4 ].
[0064] Compo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com