Pharmaceutical composition containing ribose polymerase inhibitor
A technology of ribose polymerase and composition, applied in the field of pharmaceutical compositions containing olaparib nanoparticles and preparation thereof, can solve the problems of unfavorable industrialized production of preparations, narrow access routes, high safety production pressure, etc. Drug bioavailability, low content of impurities in preparations, and the effect of promoting absorption in vivo
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Preparation of Olaparib Nanoparticle Tablets
[0054]
[0055] 1) Add 400ml of water to sodium lauryl sulfate and stir to dissolve, then add the raw material of olaparib (manufacturer: Shanghai Yuang Chemical Co., Ltd.), and stir to form a coarse suspension;
[0056] 2) Transfer the coarse suspension to a nano grinder and grind until the average particle size is less than 300nm to obtain a nano suspension;
[0057] 3) Add lactose to 1000ml water and stir to dissolve, then mix with the nanosuspension evenly;
[0058] 4) Spray-dry the suspension obtained in step 3) to obtain a drug dry powder containing nanocrystals;
[0059] 5) After the dry powder is granulated by dry method, it is uniformly mixed with microcrystalline cellulose, crospovidone, micropowder silica gel, and magnesium stearate, and pressed into tablets to obtain the product.
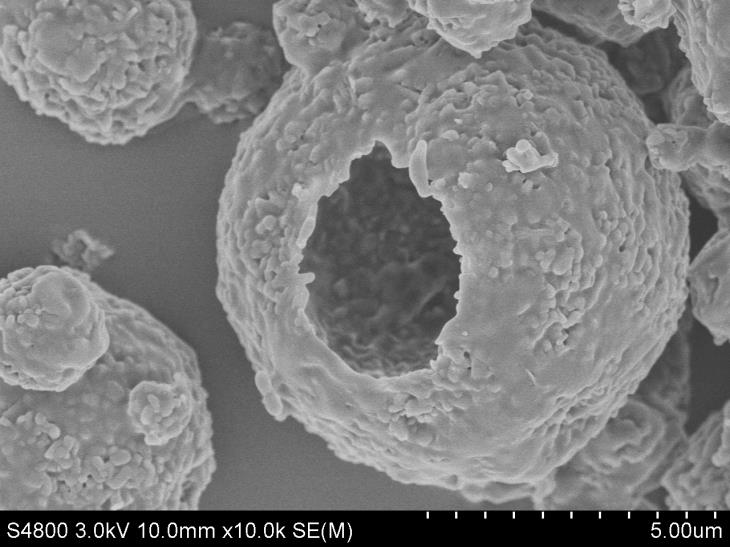
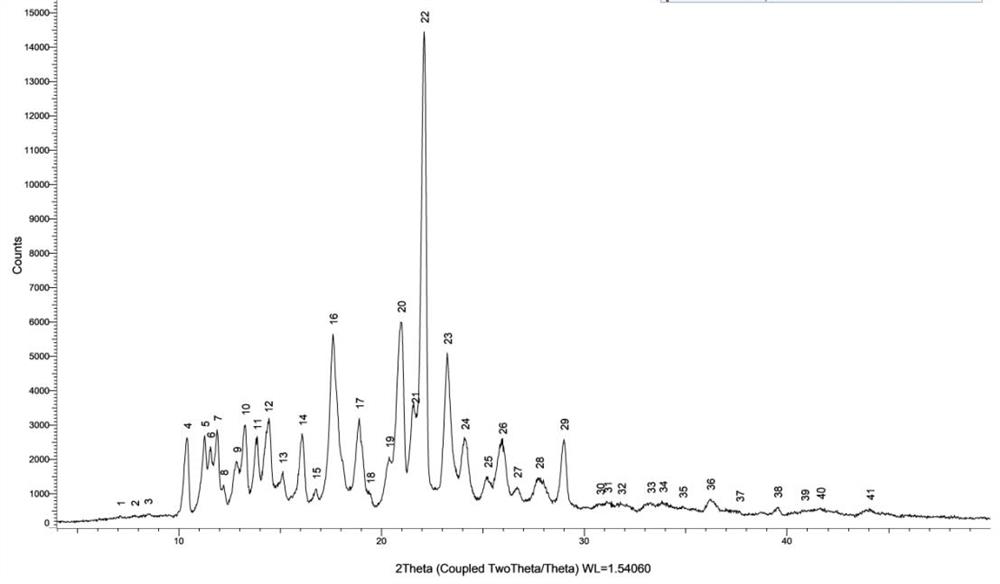
[0060] Nanoparticle Size Analysis:
[0061] In order to further clarify the size of olaparib nanoparticles, take an appropriat...
Embodiment 2
[0067] Preparation of Olaparib Nanoparticle Tablets
[0068]
[0069] 1) Add 400ml of water to polysorbate 80 and stir to dissolve, then add the raw material of olaparib and stir to form a coarse suspension;
[0070] 2) Transfer the coarse suspension to a nano grinder and grind until the average particle size is less than 300nm to obtain a nano suspension;
[0071] 3) Add 1000ml of water to mannitol and stir to dissolve, then mix with the nanosuspension evenly;
[0072] 4) Spray-dry the suspension obtained in step 3) to obtain a drug dry powder containing nanocrystals;
[0073] 5) The dry powder is granulated by dry method, then mixed with calcium hydrogen phosphate, pregelatinized starch, micropowder silica gel, and sodium fumar stearate, and then pressed into tablets.
[0074] The nanosuspension in Example 2 was analyzed by a laser particle size analyzer, and its average particle size was 240.0nm. The dry drug powder containing nanocrystals could be quickly dispersed an...
Embodiment 3
[0076] Preparation of Olaparib Nanoparticle Tablets
[0077]
[0078] 1) Add 400ml of water to polysorbate 80 and stir to dissolve, then add the raw material of olaparib and stir to form a coarse suspension;
[0079] 2) Transfer the coarse suspension to a nano grinder and grind until the average particle size is less than 300nm to obtain a nano suspension;
[0080] 3) Add 1000ml of water to mannitol and stir to dissolve, then mix with the nanosuspension evenly;
[0081] 4) Spray-dry the suspension obtained in step 3) to obtain a drug dry powder containing nanocrystals;
[0082] 5) The dry powder is granulated by dry method, then mixed with microcrystalline cellulose, croscarmellose sodium, micropowder silica gel, and sodium fumarstearate, and then pressed into tablets to obtain the product.
[0083] The nanosuspension in Example 3 was analyzed by a laser particle size analyzer, and its average particle size was 198.3nm. The dry drug powder containing nanocrystals could be...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com