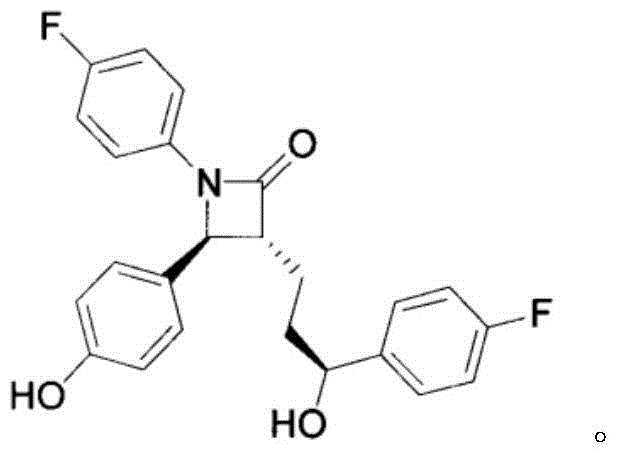
Ezetimibe solid orally-taken preparation for degrading cholesterol and preparing method of ezetimibe solid orally-taken preparation for degrading cholesterol
A technology for ezetimibe and oral preparations, applied in the field of pharmaceutical preparations, can solve problems such as poor water solubility of ezetimibe and general solubilizer effect, and achieve the effects of easy industrialized large-scale production, rapid drug dissolution, and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023]
[0024]
[0025] Preparation Process:
[0026] Dissolve trometamol in prescription quantity in ethanol, add prescription quantity of ezetimibe, stir to dissolve, dry at 50°C to remove ethanol to obtain mixture, pass this mixture through 60 mesh sieve, and then mix with prescription quantity of microcrystalline Cellulose, sodium carboxymethyl starch, and magnesium stearate are mixed evenly, and compressed into tablets.
Embodiment 2
[0028]
[0029] Preparation Process:
[0030] Dissolve trometamol in prescription quantity in ethanol, add prescription quantity of ezetimibe, stir to dissolve, dry at 55°C to remove ethanol to obtain mixture, pass this mixture through 80 mesh sieve, and then mix with prescription quantity of microcrystalline Cellulose, lactose, crospovidone, and magnesium stearate are mixed evenly and compressed into tablets.
Embodiment 3
[0032]
[0033] Preparation Process:
[0034] Dissolve tromethamine in prescription quantity in ethanol, add prescription quantity of ezetimibe, stir to dissolve, dry at 50°C to remove ethanol to obtain mixture, pass this mixture through 65 mesh sieve, and then mix with prescription quantity of microcrystalline Cellulose, sodium carboxymethyl starch, and magnesium stearate are mixed evenly, and compressed into tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com