Synthesis and purification method of (R)-3-aminobutyric acid
A technology of aminobutyric acid and amino acids, applied in the field of bioengineering, can solve problems such as clogging of biotransformation reaction liquid, affecting efficiency, membrane fouling, etc., and achieve high-efficiency expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Embodiment 1 Aminotransferase gene and the acquisition of genetically engineered cells
[0068] Through gene prediction and database comparison, a gene encoding aminotransferase was obtained from the metagenome of marine samples, its nucleotide sequence is shown in SEQ ID NO: 1, and its protein amino acid sequence is shown in SEQ ID NO: 2 Show. The gene was optimized according to the codon preference of Escherichia coli, and the codon-optimized gene was obtained, the nucleotide sequence of which was shown in SEQ ID NO:3. Entrust the synthesis of the nucleotide sequence shown in SEQ ID NO: 3 and clone the aminotransferase gene into the pET30 expression vector NdeI and XhoI to obtain a plasmid expressing the aminotransferase, which is named pET30-AT plasmid. The plasmid is transformed into BL21(DE3) cells to obtain aminotransferase gene-positive genetically engineered bacteria BL21(DE3)[pET30-AT].
Embodiment 2
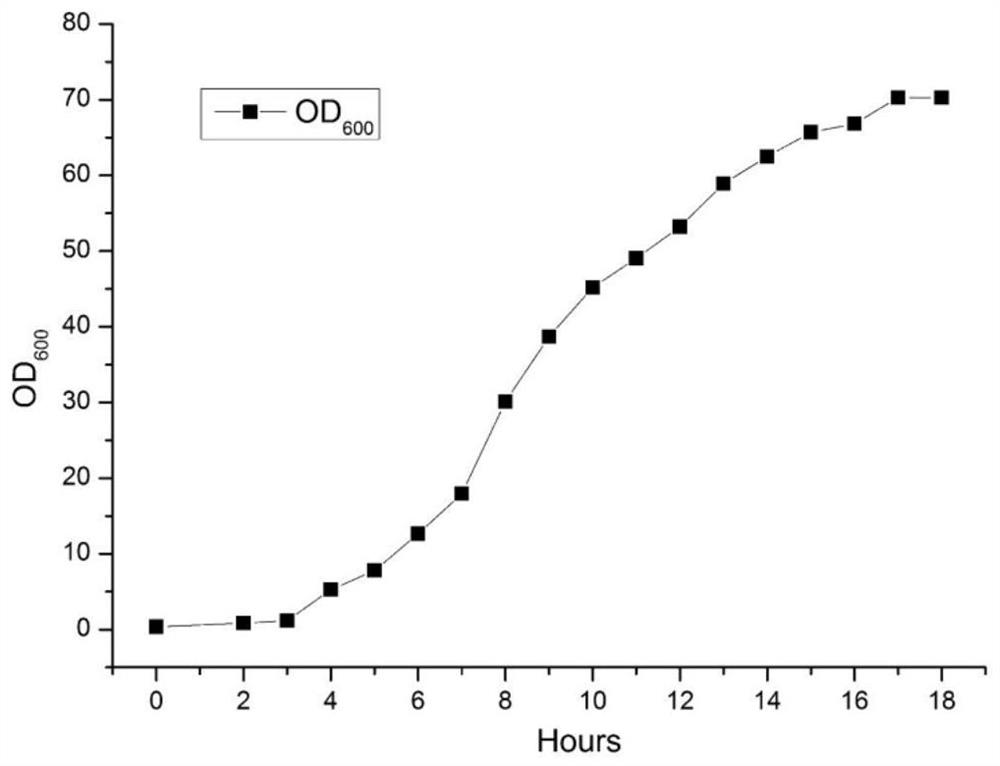
[0069] The high-density fermentation culture of embodiment 2 aminotransferase engineering bacteria
[0070] 1) The cultivation of the seed liquid of aminotransferase engineering bacteria
[0071] Get the genetically engineered bacterium BL21 (DE3) [pET30-AT] that embodiment 1 prepares, it is inoculated on the petri dish flat plate to activate bacterial classification, culture time 14h; Pick single bacterium colony from flat plate and inoculate to LB medium (peptone 10g / L, yeast powder 5g / L, NaCl 10g / L), cultivated in a constant temperature shaker at 37°C and 180rpm for 16h to obtain a fermented seed culture solution.
[0072] 2) High-density fermentation culture of aminotransferase engineering bacteria
[0073] A 5L fermenter was used for high-density cell fermentation of the aminotransferase, and the filling volume was 2.5L. The seed liquid that step 1) is cultivated is inserted in the fermentation medium (peptone 30g / L, yeast powder 30g / L, glycerol 50g / L, Na2HPO4 16.4g / L, ...
Embodiment 3
[0074] Whole-cell catalytic synthesis of embodiment 3 (R)-3-aminobutyric acid
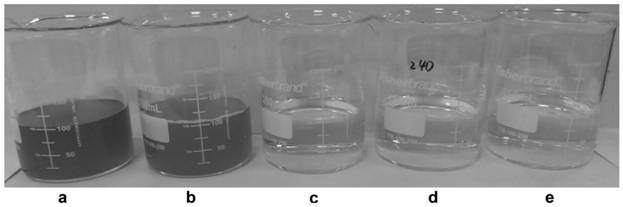
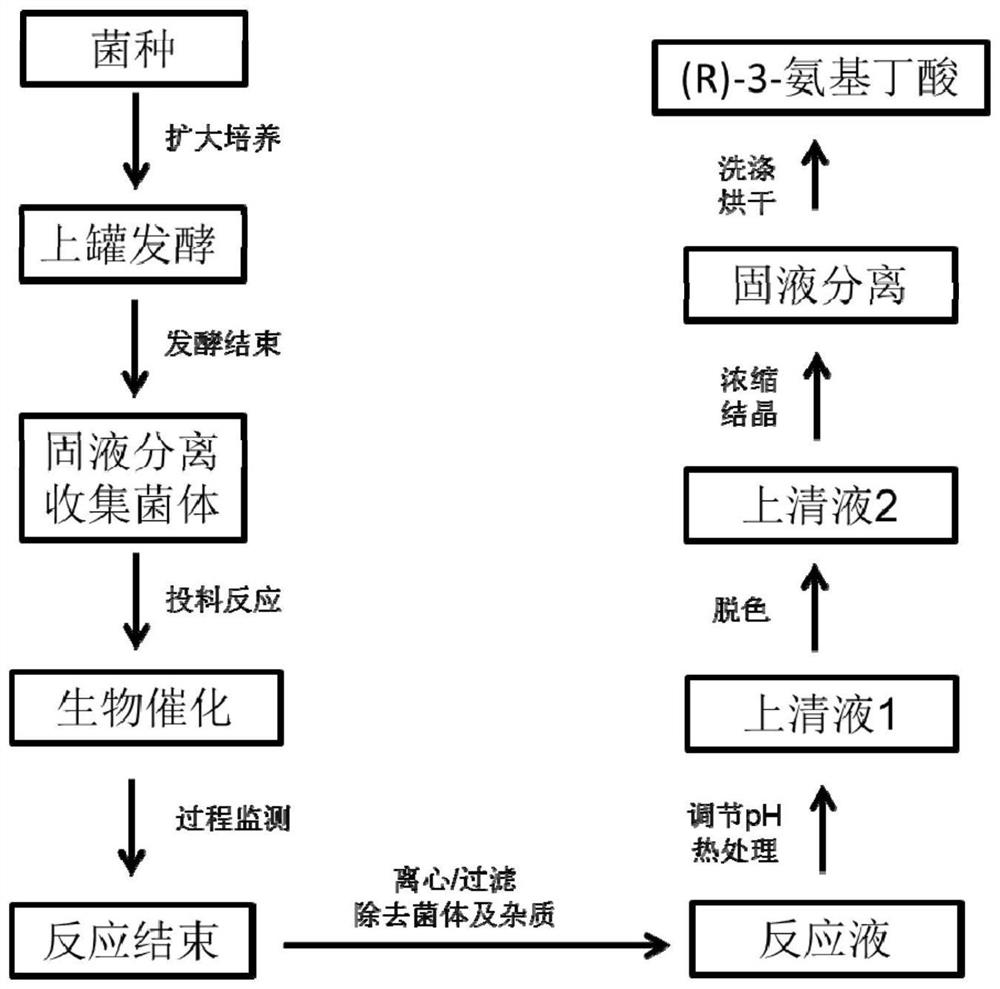
[0075] After the high-density fermentation in Example 2 was finished, the thalline was collected by centrifugation as a whole-cell catalyst. Preparation of biotransformation reaction liquid: add the thallus obtained after fermentation in Example 2 to water, the addition amount is 50g / L according to the wet weight of the thallus, add 200g / L crotonic acid, adjust the pH to 9.0 with ammonia water, and set the volume to 1000ml . The biotransformation reaction was carried out at 45°C. Sampling was taken at intervals in the middle process to detect the reaction between the substrate and the product. After 9 hours, the substrate was completely converted, and the concentration of (R)-3-aminobutyric acid in the supernatant was 227 g / L, and the conversion rate was 95%. The monitoring diagram of the conversion process is shown in the Figure 4 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com