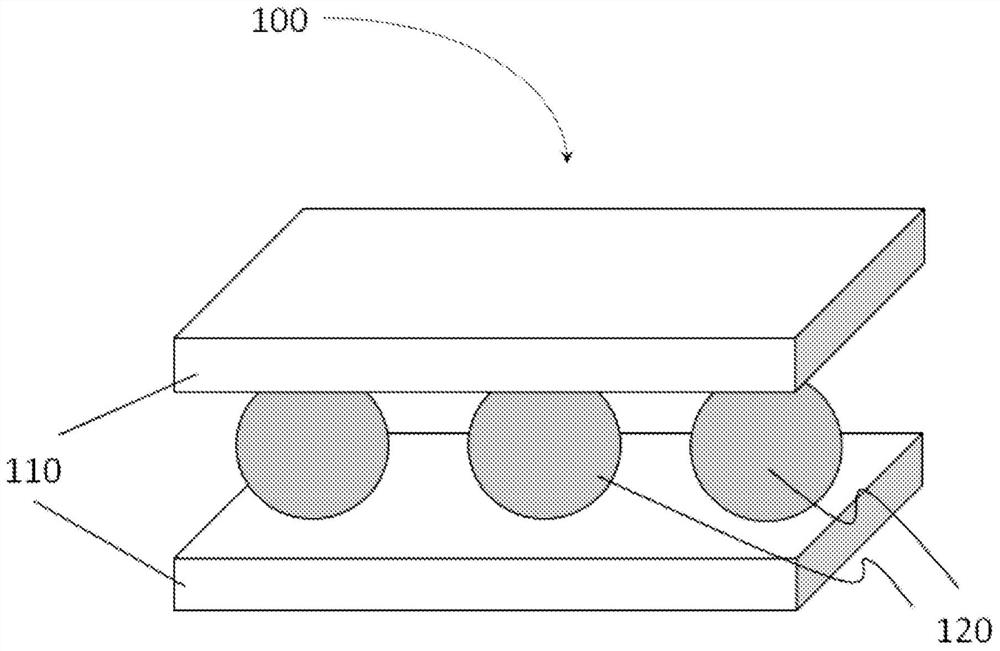
Nitrogen-based gas sustained release agent, nitrogen-based gas sustained release body comprising same, nitrogen-based gas sustained release method using sustained release body, breathing instrument, wrapping body, and sustained release device
A slow-release agent and slow-release nitrogen technology, applied in the direction of respirators, chemical instruments and methods, medical preparations containing active ingredients, etc., can solve the problems of sustained release of inorganic solid materials and potential safety hazards, and achieve safety Excellent performance, easy operation, and excellent stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
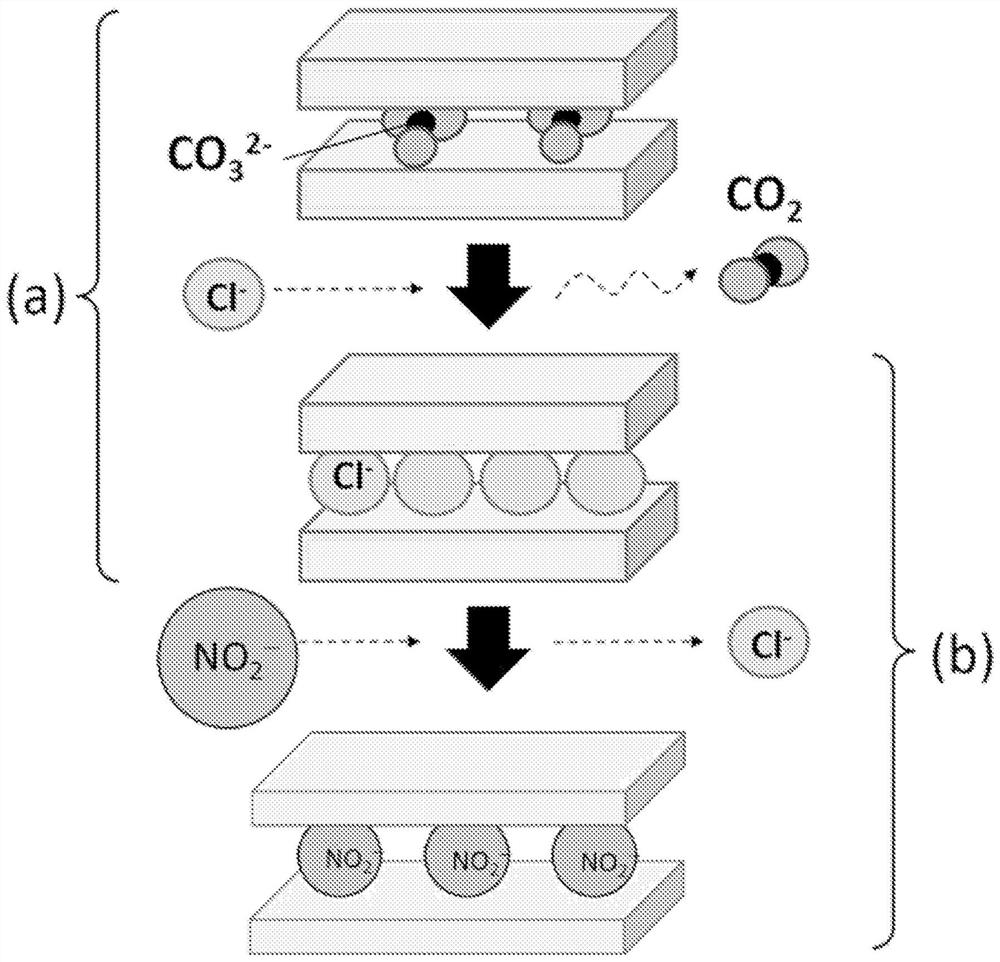
[0126] Here, among the nitrite ion / nitrate ion-containing LDH, the method for synthesizing the nitrite ion-containing LDH will be described in detail. The method for synthesizing LDH containing nitrite ions by an ion exchange method includes: preparing a layered double hydroxide and a solvent in which monovalent anions other than nitrite are included between layers; making the solvent contain nitrite ions The solution is prepared; the layered double hydroxide containing monovalent anions other than the above-mentioned nitrite ion between the layers is contacted with the above-mentioned solution; the solid substance of the LDH containing the nitrite ion synthesized by the contact The solution is separated and washed and dried.
[0127]As a layered double hydroxide (hereinafter referred to as "raw material LDH") used as a starting material in which monovalent anions other than nitrite ions are included between layers, as long as the anions can be converted by ion exchange or the...
Embodiment 1
[0300] As the carbonate-type LDH, the divalent metal ion is used as Mg ion, the trivalent metal ion is used as Al ion, and the general formula Mg 3 Al(OH) 8 (CO 3 2- ) 0.5 2H 2 Commercially available carbonic acid-type layered double hydroxide represented by O (DHT-6, manufactured by Kyowa Chemical Industry Co., Ltd., particle size distribution about 0.1 to 1 μm, Mg / Al molar ratio 2.99 (±0.06)). Hereinafter, this LDH is described as CO 3 2- MgAl-LDH3.
[0301] First, by the method described in Japanese Patent No. 5867831, carbonic acid-type LDH is converted into Cl-type LDH. Specifically, first, weigh 2.0 g of CO 3 2- MgAl-LDH3 was put into a three-necked flask, and 300 mL of ethanol was added. Next, 16.1 mL of hydrochloric acid alcohol solution (3 mass%) was added dropwise to the suspension while stirring it with a magnetic stirrer under a nitrogen flow (500 mL / min), and the mixture was stirred and reacted at 35° C. for 2 hours. Then, filtration was performed with ...
Embodiment 2
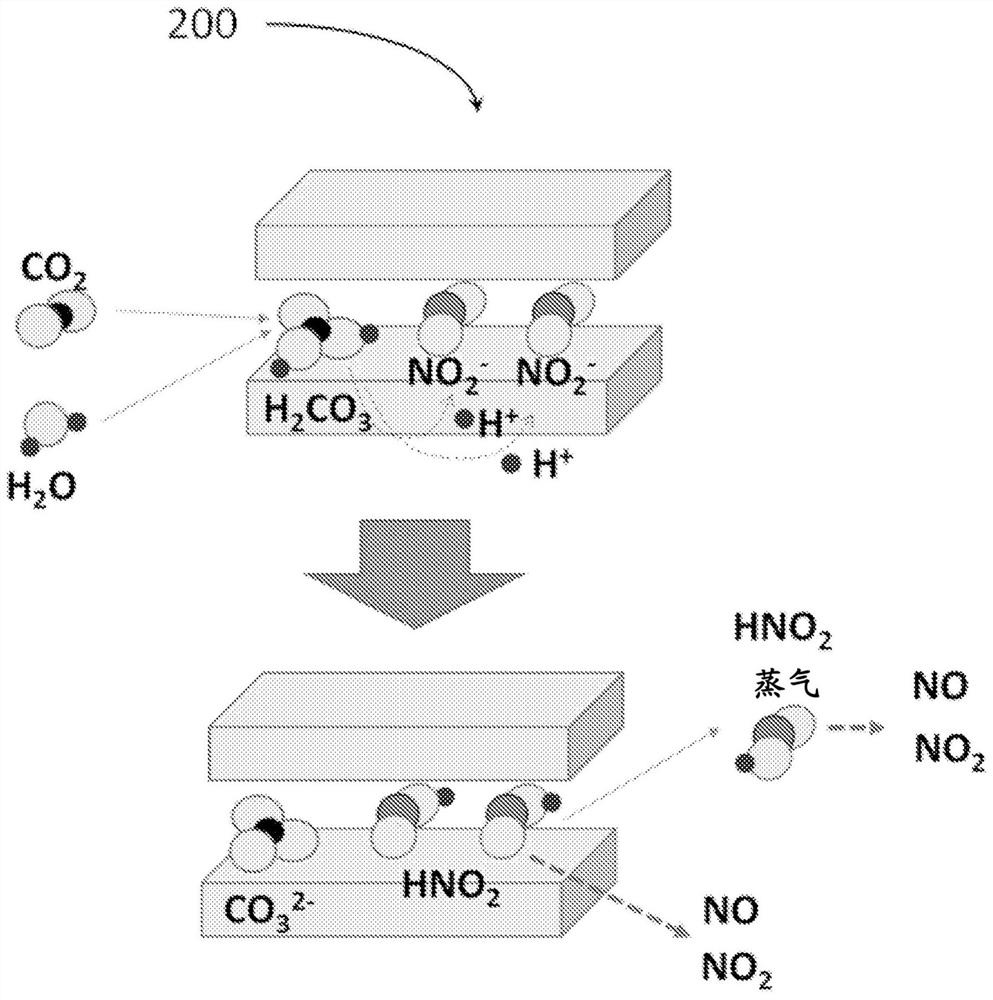
[0314] Next, use Figure 9 With the apparatus shown, a nitrogen-based gas release experiment was performed on the LDH containing nitrite ions obtained in Example 1, and the measurement was performed using a detection tube.
[0315] Figure 9 It is a schematic diagram showing the apparatus and experimental system used in Example 2.
[0316] Specifically, the powdery sample 720 (100 mg) of LDH containing nitrous acid in a glass container was sent to the atmosphere (20° C., 35% relative humidity) using a pump 710 at a flow rate of 100 mL / min for about 1 hour. After the stabilization of , various detector tubes 910 are used to measure the components in the gas after contact with the LDH containing nitrite ions. It should be noted that although the pump 710 is used to send the atmosphere (20°C, relative humidity 35%) to the LDH containing nitrite ions at a flow rate of 100mL / min, the detection tube 910 is used to suck an amount of 50mL / min as needed, The remaining 50mL / min part is...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com