SARS-CoV-2 receptor bind region glycosylation modification antigen and application thereof
A coronavirus-receptor binding technology, applied in the field of peptides, can solve the problems of low immunogenicity and insufficient cross-protection, and achieve the effects of simple preparation method, good cross-protection effect, and good application prospect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1 Glycosylation design and expression purification of the RBD monomer in the receptor binding region of the novel coronavirus
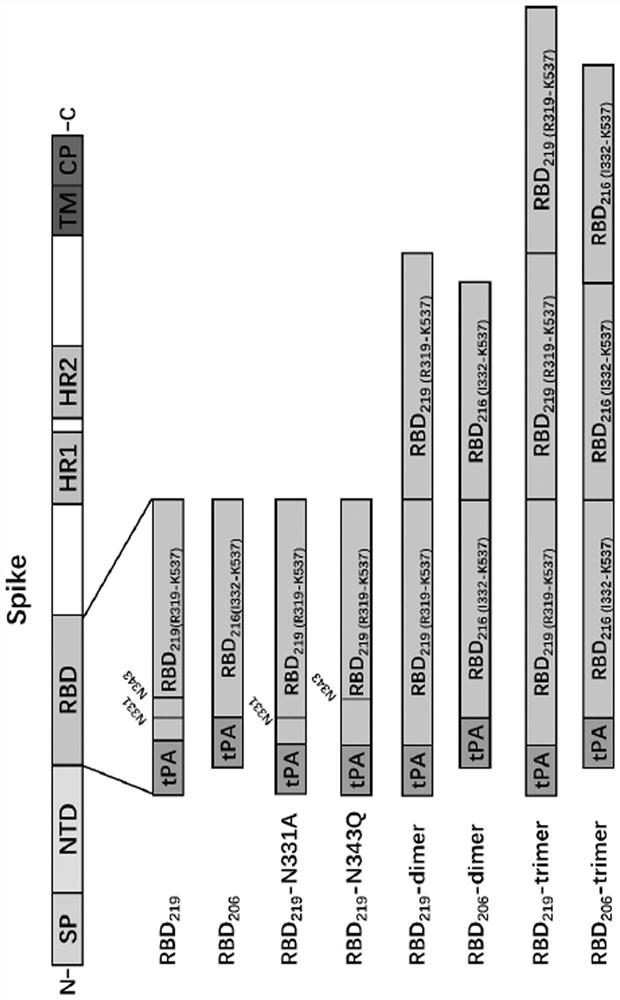
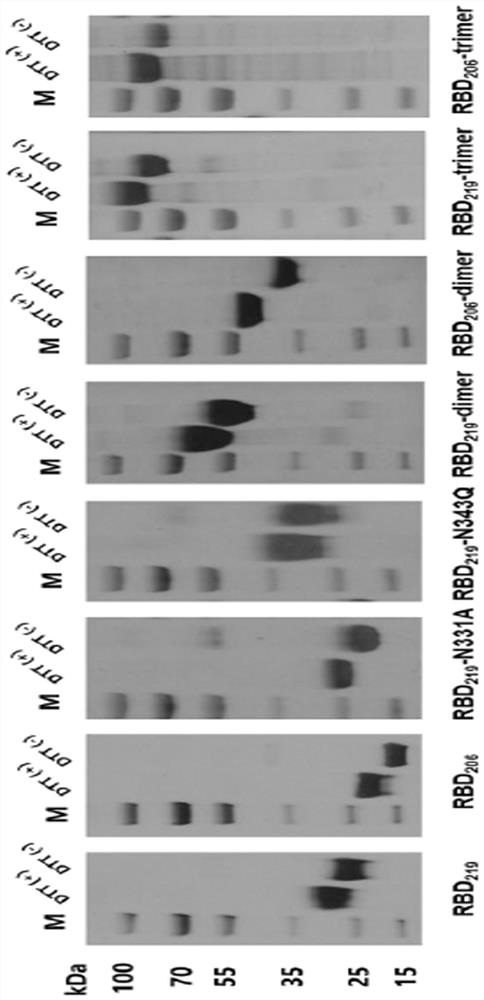
[0036] First, according to the two important N-glycosylation sites (located at the 331st and 343rd amino acids respectively) of the reported new coronavirus RBD protein (sequence such as Genebank, YP_009724390.1, R319-K537) (Science 369:330-333 (2020)), carried out N-glycosylation modification on RBD, removed glycosyl masking and exposed antigenic epitopes to enhance its immunogenicity. The transformation method is to first obtain the full-length RBD 219 Antigen (R319-K537, due to its C-terminal C538 cysteine may form a multimer due to the formation of an intermolecular disulfide bond, so it ends at K537), followed by truncating the full-length RBD 219(R319-K537) The N-glycosyl site of the region or its point mutation, design three kinds of glycosylation engineered antigen RBD 219 -N331A (mutate amino acid 331 from asparagine N to a...
Embodiment 2
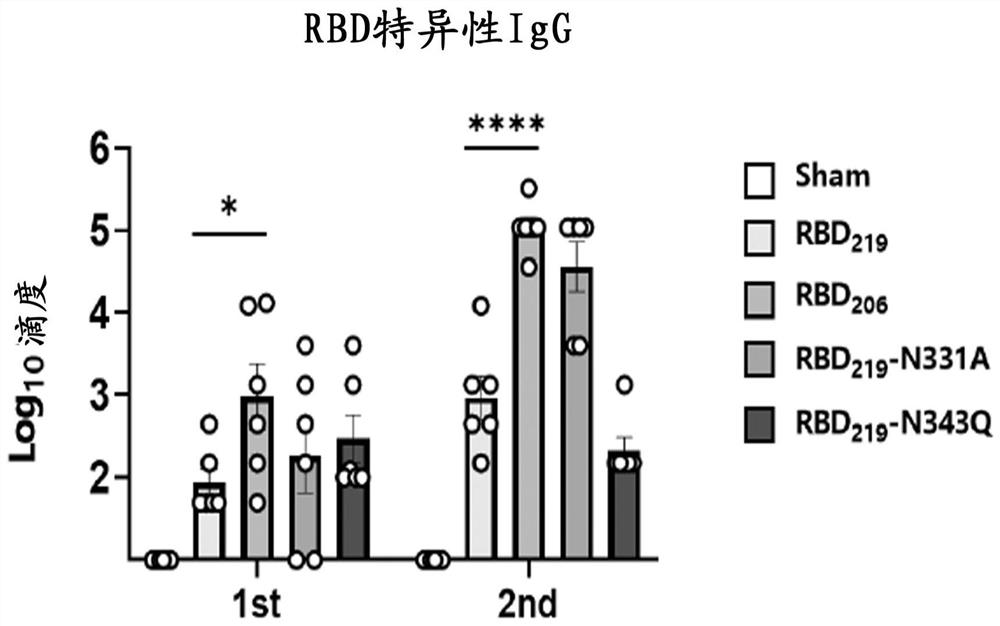
[0038] Example 2 Evaluation of Immunogenicity of Glycosylated RBD Monomer Antigen
[0039] The wild-type RBD prepared above 219 Protein and RBD monomer glycosylation engineered antigen RBD 219 -N331A, RBD 219 -N343Q (mutate amino acid 343 from asparagine N to glutamine Q to destroy N2 glycosylation site), RBD 206(I332-K537) ) protein for mouse immunization experiment to compare its immunogenicity. Select 6-8 weeks of female BALB / c mice, 6 in each group, dilute the protein obtained in Example 1 to 100 μg / mL with phosphate buffer, and mix with an equal volume of AL(OH) 3 The adjuvant (Alhydrogel, 1000 μg / mL) was mixed and adsorbed at room temperature for 1 h. Then carry out group immunization, and the grouping situation is shown in Table 1. By intramuscular injection, each mouse received two vaccine immunizations on day 0 and day 14 respectively, each inoculation volume was 100 μL (5 μg antigen, 50 μg aluminum adjuvant). On the 14th and 21d days, blood was collected from t...
Embodiment 3
[0043] Example 3 Novel coronavirus glycosylation engineered antigen RBD 206 -dimer and RBD 206 -Design and expression purification of trimer
[0044] On the basis of Example 2, through structural optimization design, the monomer RBD 206 Glycoengineered antigens undergo tandem repeats to form single-chain multimeric forms, such as dimeric RBD 206 -dimer (two sequences shown in SEQ ID NO: 1 in forward tandem) and trimeric RBD 206 -trimer (three sequences shown in SEQ ID NO: 1 for forward tandem), see sequence design figure 1 , to further enhance its immunogenicity (Cell182:722–733(2020)). See Example 1 for the method of expressing RBD using the Expi293F mammalian cell expression system 206 - dimer, RBD 206 -trimer antigen protein, using GE's His-trap affinity chromatography column for protein purification. After obtaining the recombinant protein, use BCA method and UV spectrophotometer to measure the protein concentration, use SDS-PAGE experiment to identify the purified ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| antibody titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com