A kit for detecting antigen myosin 1-igg antibody
A technology of tropomyosin and antigenic protein, which is applied in the field of kits for detecting antigenic myosin 1-IgG antibodies in serum, can solve the problems of non-existence and other problems, and achieve simple operation, fast reaction speed, and less reagent consumption Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] 1. Expression of Tropomyosin 1 protein antigen: the corresponding antigen protein was expressed by bioengineering method.
[0039] 1.2 Antigen protein immobilization method: the antigen coating solid phase carrier method of the present invention uses the direct coating method: (1) the antigen is bound to the polystyrene microwell plate or the nitrocellulose membrane by physical adsorption or non-covalent bond mode; ( 2) The antigen is bound to the magnetic particles containing carboxyl functional groups through chemical coupling.
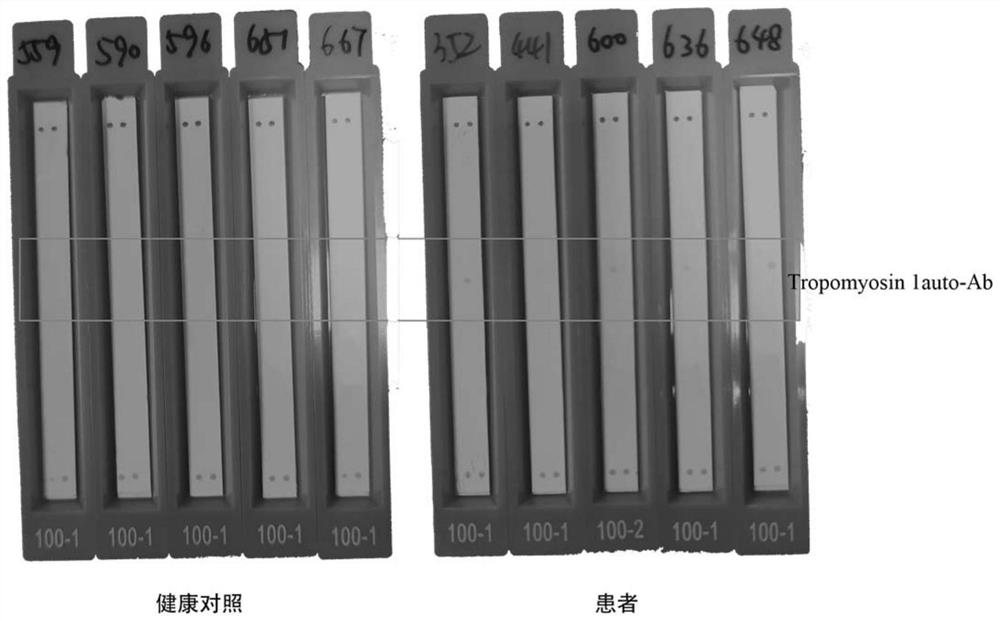
[0040] 1.3 Positive quality control substance and standard substance: The positive quality control substance and standard substance selected in the present invention are IgG or human anti-tag peptide IgG extracted and quantified from patient serum.
[0041] 1.4 Labeled antibody and substrate chromogenic reagent: the selected labeled antibody of the present invention can be acridinium ester-labeled anti-human IgG, horseradish peroxidase-labele...
Embodiment 2
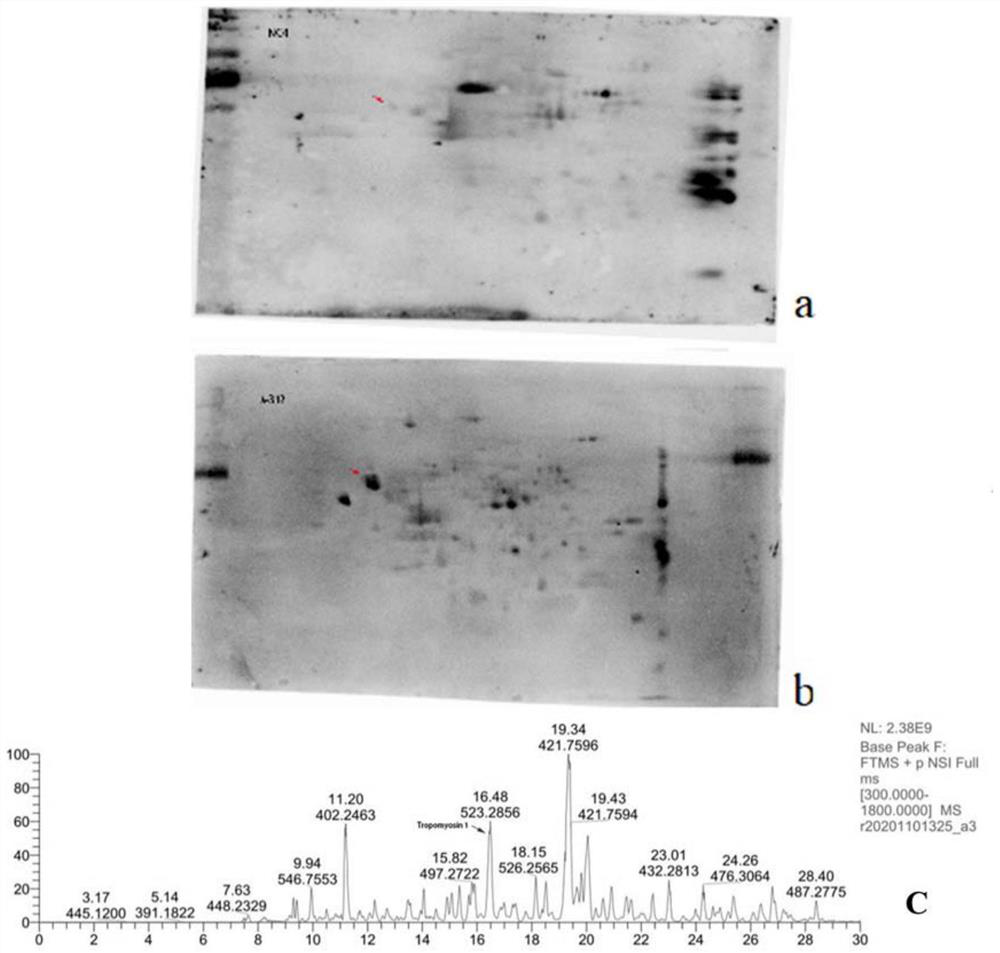
[0056] Example 2 Tropomyosin 1 on podocytes is one of the main target antigens targeted by autoantibodies in patients with autoimmune nephrotic syndrome
[0057] Culture podocyte strain (MPC5), wash 2-3 times with PBS, then use focused ultrasonic instrument (Covaris S220, Gene) to contain 30mm Tris-HCl, 8m urea, 4% CHAPS and protease inhibitor (#ab65621; Abcam, 1:200 dilution) in the lysis buffer for sufficient lysis on ice, and then the sample was placed in a centrifuge at 12000g, 4°C for 30min. The supernatant was collected, which was the collected glomerular podocyte total protein. The BCA protein concentration assay kit was used to measure the total protein concentration of the collected glomerular podocytes to obtain purified total protein of the glomerular podocytes. Then carry out two-dimensional electrophoresis, transfer to the membrane, and then incubate with the serum of the healthy control group and the patient respectively, and then add the secondary antibody for ...
Embodiment 3
[0058] Example 3 Expression of Tropomyosin 1 antigen protein
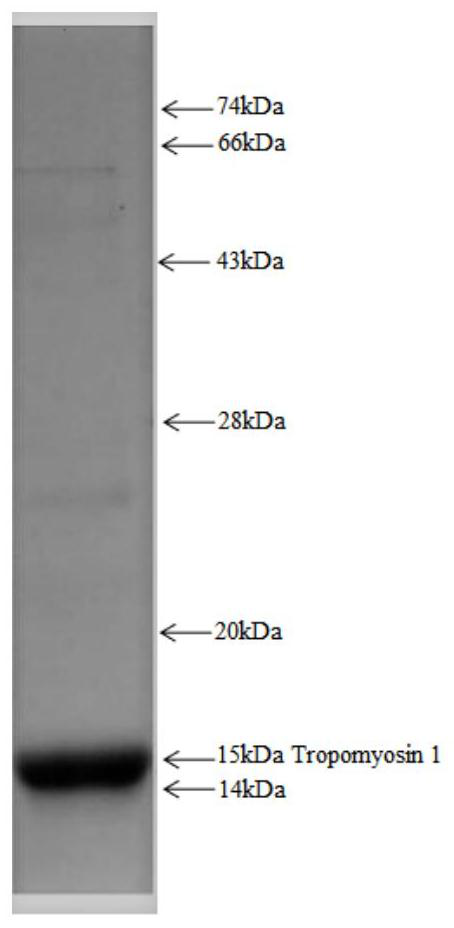
[0059] The expression and purification of the antigenic protein Tropomyosin 1 was carried out by PCR using the gene encoding the Tropomyosin 1 protein as a template by means of genetic engineering, and then an expression vector was constructed for protein expression. The antigenic protein expressed in the present invention contains the tag peptide of the His tag. The expressed recombinant protein was purified by nickel column affinity chromatography, ion affinity chromatography, hydrophobic column, molecular sieve, etc. Finally, the molecular weight of the synthetic protein Tropomyosin 1 was identified and quantified by SDS-PAGE. Such as figure 2 shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com