Polymer micelle material, preparation and application thereof, and targeted drug of polymer micelle material, preparation and application thereof
A technology of polymer micelles and drugs, which is applied in the direction of drug combinations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problem of low drug accumulation in tumor sites, non-selectivity of tumor cells, Insufficient tumor targeting effect and other problems, to achieve the effect of improving anti-tumor effect, improving treatment effect, improving efficacy and safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0112] Preparation of glucose ligand-type polymer micelles (Glu-PEG-b-P(Asp-g-NIDH)):
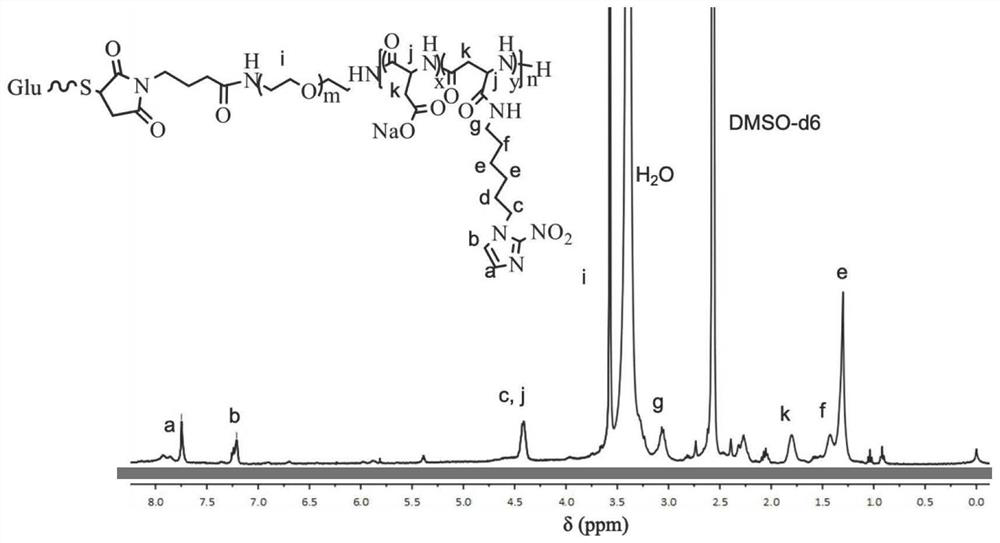
[0113] Thiolated glucose (Glu-SH) and maleimide-polyethylene glycol-poly(β-benzyl-L-aspartic acid) (Mal-PEG-b-PBLA) were dissolved in anhydrous DMSO The reaction was stirred overnight at room temperature, and after 24 hours of dialysis, the precipitate was lyophilized to obtain glucose-polyethylene glycol-poly(β-benzyl-L-aspartic acid) (Glu-PEG-b-PBLA).
[0114] Subsequently, glucose-polyethylene glycol-poly(β-benzyl-L-aspartic acid) (Glu-PEG-b-PBLA) was dissolved in acetonitrile at a concentration of 2 mg / mL, and 5-15 equivalents of 0.5M NaOH solution until the solution became clear, stirred vigorously at room temperature for 2 h, dialyzed the reaction solution in deionized water for 24 h, and freeze-dried to obtain polyethylene glycol-polyaspartic acid block copolymer (Glu-PEG-b-PAsp) ;
[0115] Glu-PEG-b-PAsp was dissolved in 2-(N-morpholine)ethanesulfonic acid buffer at pH 6.0, the ca...
Embodiment 2
[0117] Preparation of non-active targeting polymer (PEG-b-P(Asp-g-NIDH)):
[0118] Dissolve polyethylene glycol-poly(β-benzyl-L-aspartic acid) (PEG-b-PBLA) in acetonitrile at a concentration of 2 mg / mL, and then add 5-10 equivalents of 0.5M NaOH solution to The solution became clear, stirred vigorously at room temperature for 2 h, the reaction solution was dialyzed in deionized water for 24 h, and then freeze-dried to obtain PEG-b-PAsp;
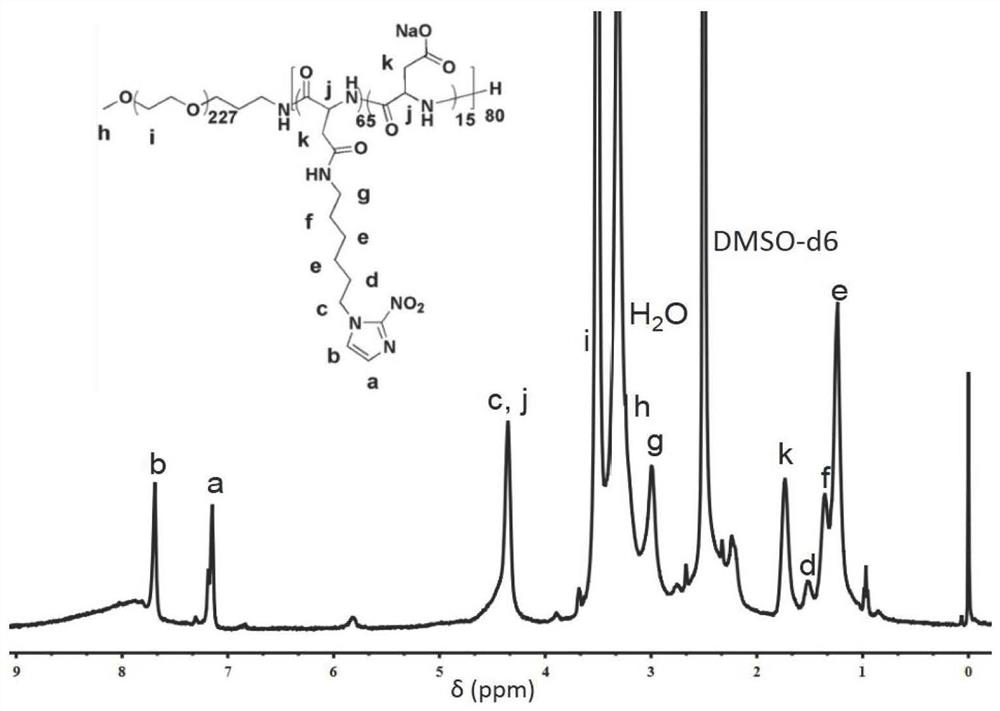
[0119] Dissolve PEG-b-PAsp in 2-(N-morpholine)ethanesulfonic acid buffer at pH 6.0, activate the carboxyl group by EDC / NHS reaction, add NI to PEG-b-PAsp, the reaction is at room temperature After 24 hours, the reaction solution was collected, dialyzed with deionized water for 2 days and freeze-dried to obtain the target product PEG-b-P(Asp-g-NIDH), which was characterized by H NMR spectrum 1 H NMR (DMSO) results are attached figure 2 Shown: δ7.69, 7.19(s, CH of midazole), 4.35(m, midazole-CH 2 , -CO-CH-), 3.51 (m, -O-CH 2 CH 2 -O-), 3.2...
Embodiment 3
[0121] Preparation of polymer micelles loaded with CDDP (cis-diammine dichloride) and MnTPPS and related reference substances:
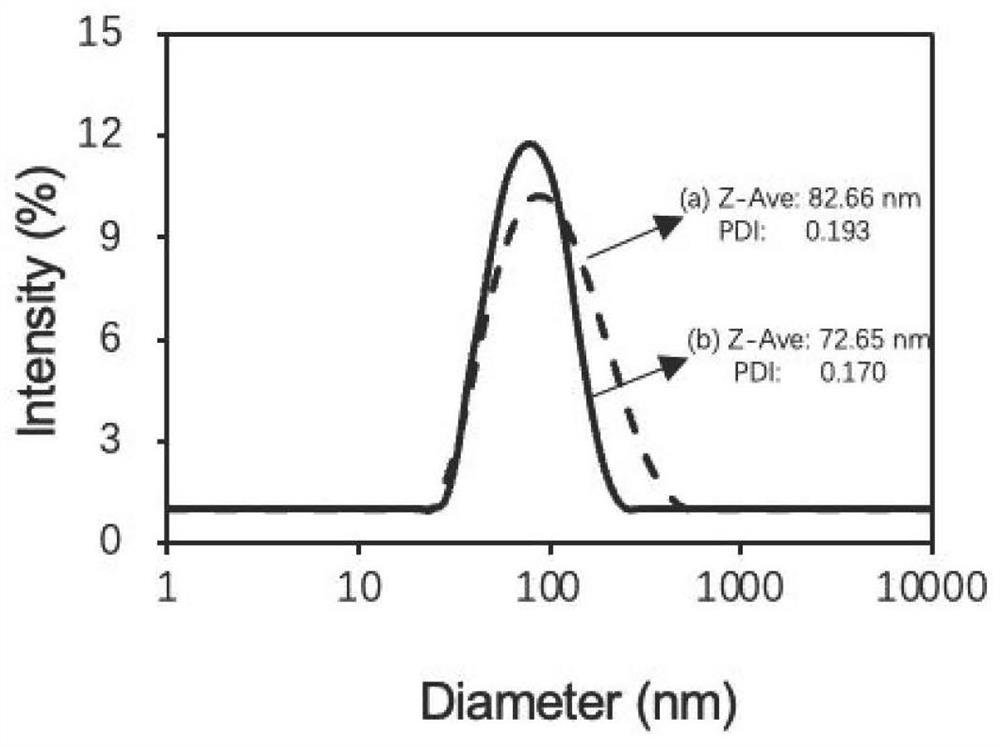
[0122] Weigh each 1.5 mg of PEG-b-P (Asp-g-NIDH) prepared in Example 2 and 0.5 mg of Glu-PEG-b-P (Asp-g-NIDH) prepared in Example 1 as raw materials and dissolve in two In methyl sulfoxide (DMSO), 0.8 mg CDDP and 1.6 mg MnTPPS were simultaneously dissolved in 1 mL Milli-Q ultrapure water. Under ultrasonic conditions, the drug mixture solution was quickly pushed into the DMSO solution of the high molecular polymer, and the ultrasonic was continued for 5 minutes. Subsequently, the obtained micellar solution was transferred to a constant temperature mixer, placed at 37° C., and reacted at 1000 rpm for 72 hours. Ultrafiltration centrifuge tubes (molecular weight cut-off: 35k Da) were added to Milli-Q water for multiple washes to remove free drugs and DMSO, and finally concentrated to obtain polymer micellar GluNPs loaded with CDDP and MnTPPS.
[0123] ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com