Pharmaceutical composition and its application, sterile container and kit
A technology for aseptic containers and compositions, applied in the directions of drug combinations, medical containers, infusion sets, etc., to achieve the effect of improving anti-tumor treatment effect and reducing side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
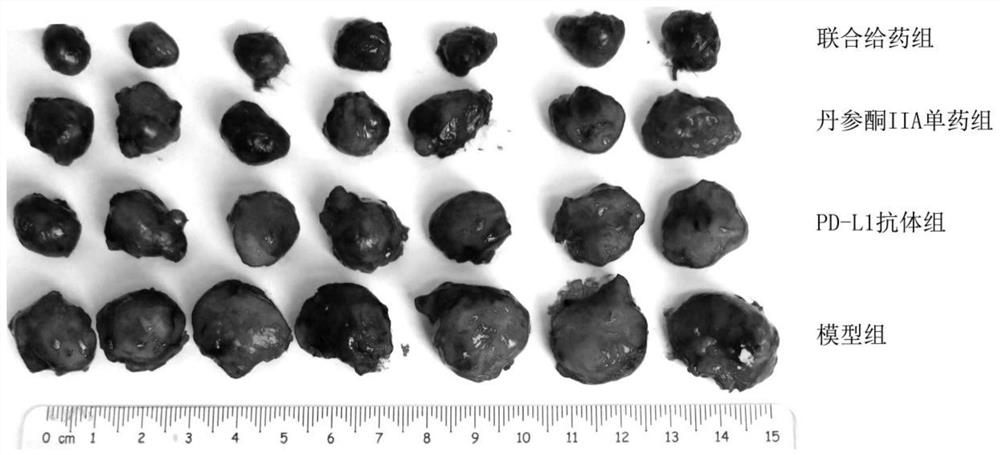
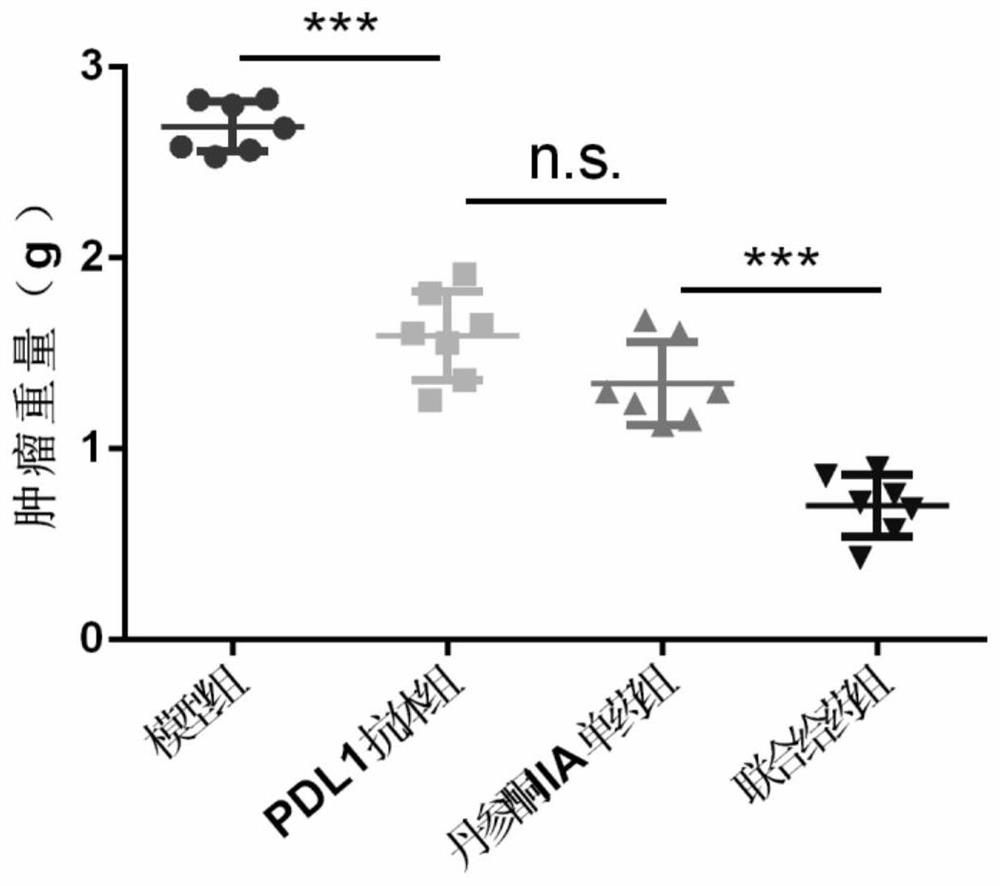
[0033] In order to prove that the present invention has a good curative effect, animal experiments verify the therapeutic effect of the combination of tanshinone IIA and PD-L1 on the mouse non-small cell lung cancer model as follows:
[0034] 1 material
[0035] 1.1 Cell culture
[0036]Lewis murine lung adenocarcinoma cells (LLC) were purchased from the Cell Culture Center of Chinese Academy of Medical Sciences (Cell Culture Center of Chinese Academy of Medical Sciences; CAMS; People's Republic of China, Beijing). 90% DMEM medium (Dulbecco's modified Eagle's medium, Gibco BRL, USA), 10% FBS fetal bovine serum (fetal bovine serum, Gibco BRL, USA), in 5% CO 2 , 95%O 2 cultured in a constant temperature and humidity incubator at 37°C. Replace the culture medium every 2 to 3 days or determine whether to pass passage according to the cell density.
[0037] 1.2 Experimental animals
[0038] Healthy SPF grade female C57BL / 6 mice were used, purchased from the Institute of Labora...
Embodiment 2
[0068] 1 material
[0069] 1.1 Experimental animals
[0070] Twelve female NOD / SCID mice (6-8 weeks, 18-22g) were purchased from the Institute of Laboratory Animal Science (Institute of Laboratory Animal Science) of CAMS, Beijing, People's Republic of China, and were subjected to 12 hours of light under pathogen-free conditions. / dark cycle feeding acclimation for one week. Food and water were given throughout the experiment. Lewis cells were trypsinized and resuspended in 10% calf serum (5×10 5 cells / mL) in DMEM medium, each mouse was subcutaneously injected with 0.1 mL of Lewis cell suspension in the armpit of the left forelimb for inoculation.
[0071] 1.2 Drugs
[0072] Monomer tanshinone IIA with a purity of >98% was purchased from Shanghai Yuanye Biotechnology Co., Ltd., and was first dissolved in 1% DMSO, and then dissolved in 0.9% saline.
[0073] 2 methods
[0074] Twelve NOD / SCID mice were randomly divided into 2 groups, 6 in each group. After grouping, each g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com