Intravenous injection drug-loading targeting nano-carrier for RA (Rheumatoid Arthritis) and preparation method of drug-loading targeting nano-carrier
A nano-carrier, targeting technology, applied in non-active ingredients medical preparations, medical preparations containing active ingredients, pharmaceutical formulas, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
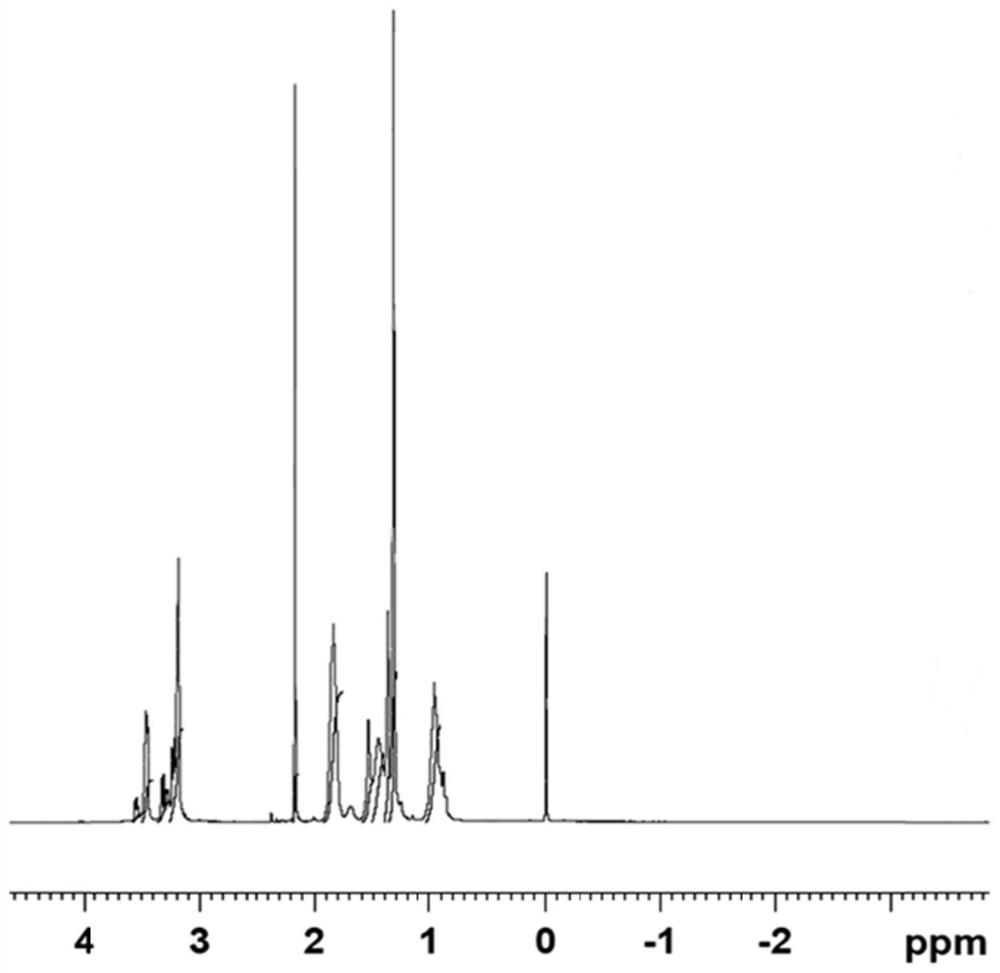
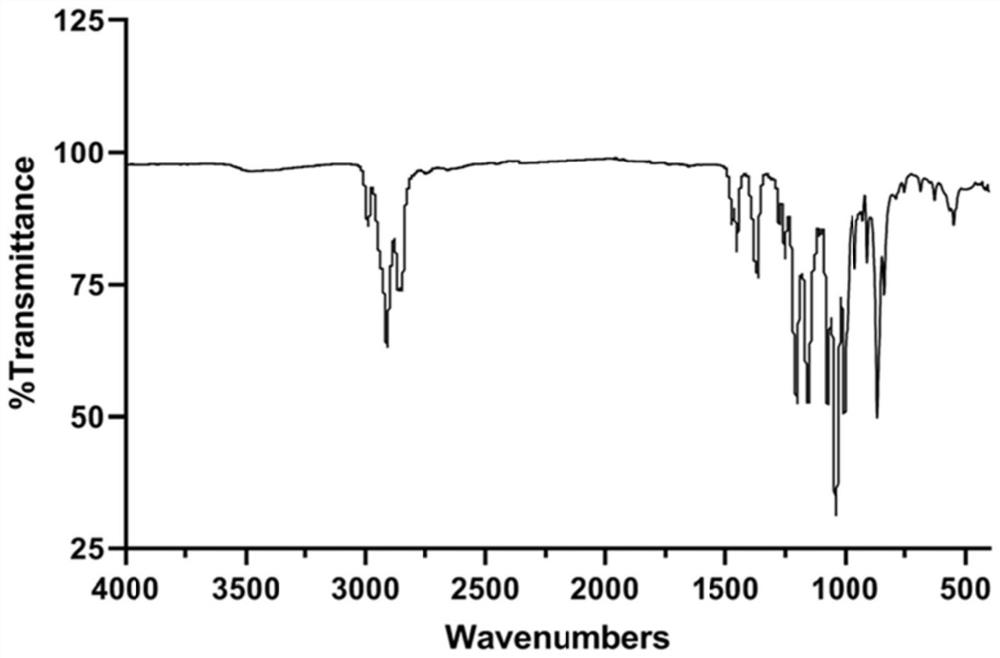
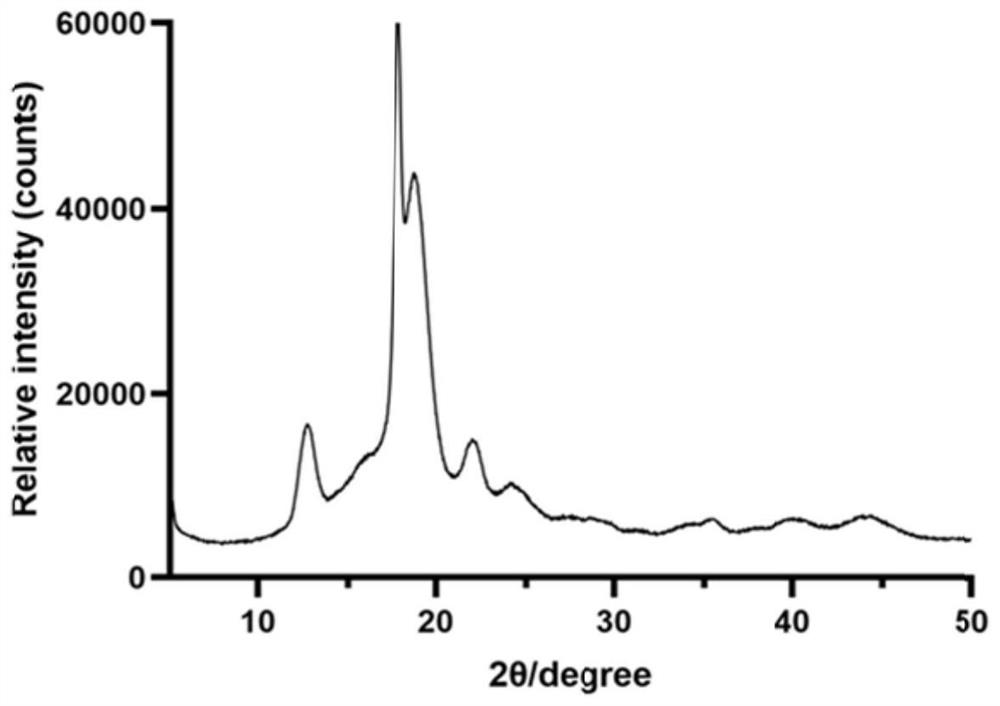
[0023] Embodiment 1 (synthesis and characterization of PCADK)
[0024] 1. Materials and methods
[0025] 1.1 Experimental reagents and instruments
[0026] 1,4-cyclohexanedimethanol (Aladdin C105684); redistilled benzene (Aladdin B116191); p-toluenesulfonic acid (McLean T823839); 2,2-dimethoxypropane (Aladdin D105750); Ethylamine (McLean T818772); n-Hexane (McLean H811141).
[0027] Constant temperature magnetic stirring (Dalong); X-ray diffractometer (Rigaku, Japan); Fourier transform infrared spectrometer (Shimadzu IR Prestige-21, Japan); Nuclear magnetic resonance (BRUKER DMX300, Switzerland).
[0028] 1.2 PCADK material preparation
[0029] Connect the 3-neck flask and the spherical condenser in the fume hood, and use a heatable magnetic stirring water bath to heat the experiment. Accurately weigh 12.5031g of 1,4-cyclohexanedimethanol in a three-necked flask, connect the water separator and the condensing reflux tube, add 30ml double-distilled benzene, heat to 87°C on ...
Embodiment 2
[0047] Embodiment 2 (preparation and characterization of PCADK composite nanomaterial)
[0048] 1. Materials and methods
[0049] 1.1 Experimental cells
[0050] MH7A cells, HTX1833, Shanghai Tong Biotech.
[0051] 1.2 Experimental reagents and instruments
[0052] High-glucose complete DMEM (KGM31800S-500, KGI Bio); trypsin-EDTA digestion solution (T1300, Solarbio); PBS (02-024-1ACS BI); Trizon Reagent (CW0580S, CWBIO); Ultrapure RNA ultrapure RNA extraction Kit (CW0581M, CWBIO); HiScript II Q RT SuperMix for qPCR (+gDNA wiper) (R223-01, Vazyme); 2×SYBR Green PCR Master Mix (A4004M, Lifeint); RIPA cell lysate (C1053, Beijing Pulilai); BCA protein quantification kit (CW0014S, Kangwei Century); egg yolk lecithin (L8260, Suo Laibao); vasoactive intestinal peptide ([D-p-Cl-Phe6,Leu17]-VIP) (HY-P1159, MCE); Sodium hyaluronate (S8190 Solebol); Polyvinyl alcohol (PVA), polyethyleneimine (PEI) Aladdin reagent; PCADK material (prepared in the previous experiment); CO 2 Incubator ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com