Analysis method for chiral purity of 1-naphthylethylamine
A technology of chiral purity and analysis method, applied in the field of analysis of chiral purity of 1-naphthylethylamine, which can solve problems such as low detection efficiency, increased reaction time, and difficulty in synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1: the liquid chromatography analysis of (D)-camphorsulfonyl chloride and 1-NEA derivative
[0040] 1-1: Liquid chromatography analysis of (D)-camphorsulfonyl chloride and (R)-1-NEA derivatives
[0041] Take (R)-1-NEA 5g (0.029mol), triethylamine 12mL (0.087mol) dissolved in 15g (11.32mL) of dichloromethane, stirred at 25 ° C, (D)-camphorsulfonyl chloride 6g (0.024 mol) was dissolved in 10g (7.55mL) of dichloromethane, stirred evenly, transferred to a constant pressure dropping funnel, slowly added dropwise to the system, TLC monitored the reaction, and evaporated to dryness after the reaction to obtain a yellow viscous liquid. The solid product can be better extracted, and further, a white solid (D)-camphorsulfonyl·(R)-1-naphthylethylamine derivative is precipitated by soaking in n-heptane, and the pure product is obtained by toluene recrystallization.
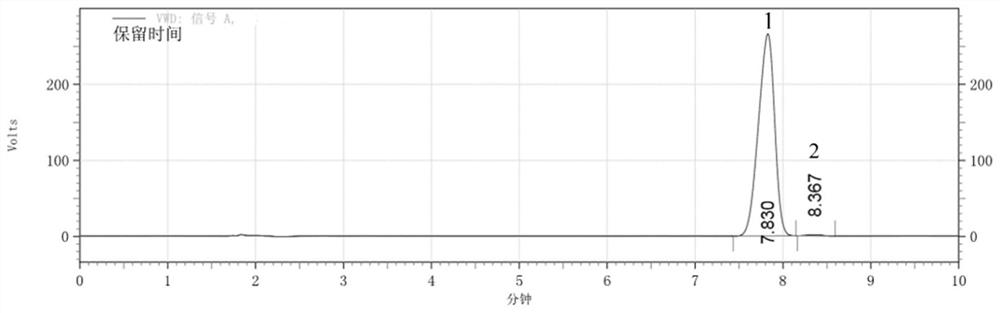
[0042] The (D)-camphorsulfonyl·(R)-1-naphthylethylamine derivative was dissolved in mobile phase at a co...
Embodiment 2
[0050] Example 2: 1-NEA and (D)-camphorsulfonyl chloride derivatization reaction conditions investigation test
[0051] 2-1: Dissolve (RS)-1-NEA 5g (0.029mol), triethylamine 4mL (0.029mol) in 25g (27.78mL) ethyl acetate, stir at 25°C, and (D)-camphorsulfonic acid Dissolve 7.3g (0.029mol) of acid chloride in 25g (27.78mL) of ethyl acetate, stir evenly, transfer to a constant pressure dropping funnel, slowly add it dropwise to the system, monitor the reaction by TLC, and evaporate to dryness to obtain a yellow Viscous liquid, soaked in n-heptane to precipitate a white solid (D)-camphorsulfonyl·(RS)-1-naphthylethylamine derivative.
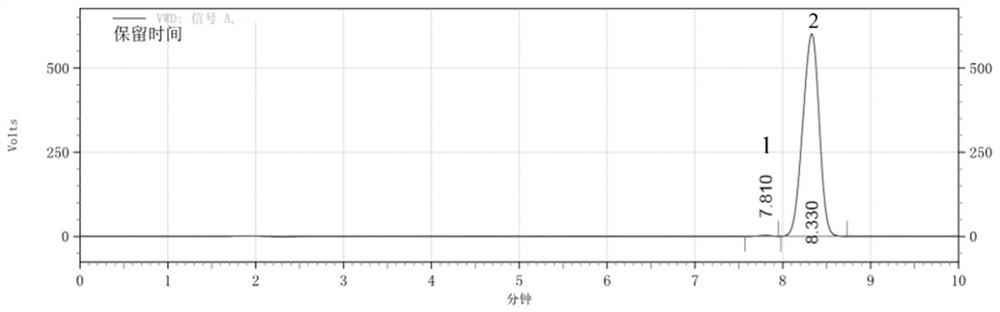
[0052] The (D)-camphorsulfonyl·(RS)-1-naphthylethylamine derivative was dissolved in mobile phase at a concentration of 30 μg / mL, and detected and analyzed by high performance liquid chromatography. Liquid chromatography conditions: Agilent C18 chromatographic column, mobile phase is acetonitrile-potassium dihydrogen phosphate solution (40mmol / L, K ...
Embodiment 3
[0061] Embodiment 3: chromatographic condition investigation test
[0062] (1) Derivatization
[0063] Take (RS)-1-NEA 5g (0.029mol), triethylamine 12mL (0.087mol) dissolved in 15g (11.32mL) of dichloromethane, stirred at 25 ° C, (D)-camphorsulfonyl chloride 6g (0.024 mol) was dissolved in 10g (7.55mL) of dichloromethane, stirred evenly, transferred to a constant pressure dropping funnel, slowly added dropwise to the system, TLC monitored the reaction, evaporated to dryness after the reaction to obtain a yellow viscous liquid, normal Soak in heptane to precipitate a white solid (D)-camphorsulfonyl·(RS)-1-naphthylethylamine derivative.
[0064] (2) Separation and detection of (D)-camphorsulfonyl chloride and (RS)-1-NEA derivatives using the following different chromatographic conditions
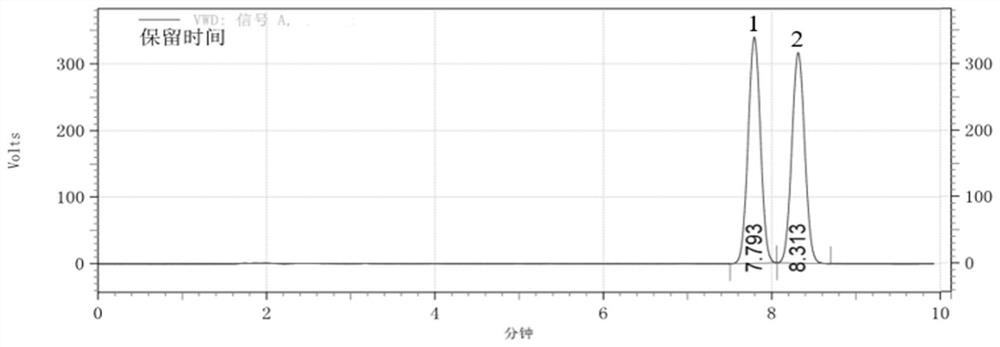
[0065] 3-1: The (D)-camphorsulfonyl·(RS)-1-naphthylethylamine derivative was dissolved in mobile phase at a concentration of 50 μg / mL, and detected and analyzed by high performance liquid ch...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com