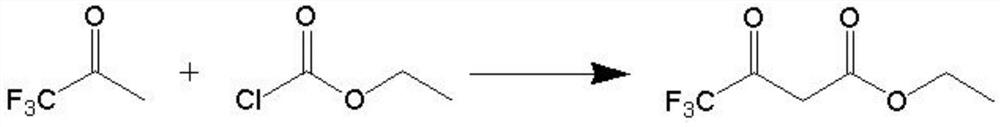
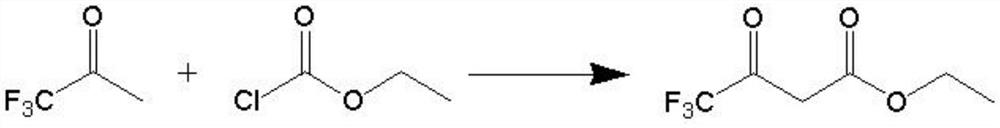
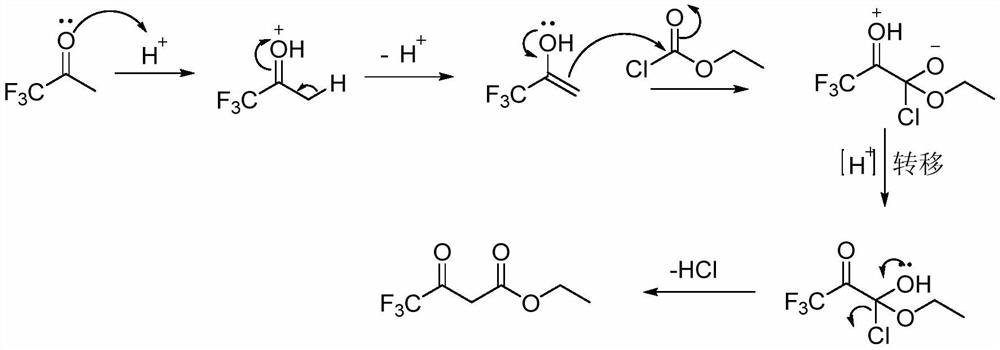
A kind of synthetic method of 4,4,4-trifluoroethyl acetoacetate
A technology of ethyl trifluoroacetoacetate and a synthesis method, which is applied in the field of synthesis of ethyl 4,4,4-trifluoroacetoacetate, can solve the problems of side reactions and many hidden dangers in production, and achieves the effect of improving mass transfer and heat transfer. , High environmental benefits, avoid the accumulation of by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] (1) Weigh 114.3 g (1.0 mol) of trifluoroacetone and 228.6 g of toluene in a mixing bottle and stir them evenly to serve as solution A; 110.2 g (1.0 mol) of ethyl chloroformate (content 98.5%) serve as solution B;
[0032] (2) the temperature of the pipeline reactor is set to 50°C, the pressure is 0.5MPa, the flow rate of the pump A is set to 8ml / min, the flow rate of the pump B is set to 2ml / min, and the exhaust gas exhaust valve and the hydrogen chloride gas inlet valve are opened after reaching the set temperature, Introduce hydrogen chloride gas; open pump A to enter the mixed solution of trifluoroacetone and toluene, open pump B to enter ethyl chloroformate;
[0033] (3) 0.49% residual ethyl chloroformate of the reaction solution at the outlet of the reactor was detected by sampling after 20 min, and the reaction was qualified;
[0034] (4) after the reaction finishes, the reaction solution recovers toluene and a small amount of residual raw materials through a norm...
Embodiment 2
[0036] (1) 125.7 g (1.1 mol) of trifluoroacetone and 377.1 g of cyclohexane were weighed and stirred in a mixing bottle to serve as solution A; 110.2 g of ethyl chloroformate (content 98.5%) (1.0 mol) was used as solution B;
[0037] (2) The temperature of the pipeline reactor is set to 70°C, the pressure is 0.6MPa, the flow rate of the pump A is set to 9.5ml / min, and the flow rate of the pump B is set to 1.5ml / min. After reaching the set temperature, open the exhaust exhaust valve and the hydrogen chloride gas inlet. Valve, feed hydrogen chloride gas; open pump A to enter the mixed solution of trifluoroacetone and cyclohexane, open pump B to enter ethyl chloroformate;
[0038] (3) 0.45% residual ethyl chloroformate of the reaction solution at the outlet of the reactor was detected by sampling after 20 minutes, and the reaction was qualified;
[0039] (4) after the reaction finishes, the reaction solution recovers cyclohexane and a small amount of residual raw materials throug...
Embodiment 3
[0041] (1) Weigh 125.7 g (1.1 mol) of trifluoroacetone and 377.1 g of cyclohexane in a mixing bottle and stir them evenly to serve as solution A; 110.2 g (1.0 mol) of ethyl chloroformate (content 98.5%) serve as solution B;
[0042] (2) The temperature of the pipeline reactor is set to 80°C, the pressure is 0.7MPa, the flow rate of pump A is set to 9.5ml / min, and the flow rate of pump B is set to 1.5ml / min. After reaching the set temperature, open the exhaust gas exhaust valve and the hydrogen chloride gas inlet. Valve, feed hydrogen chloride gas; open pump A to enter the mixed solution of trifluoroacetone and cyclohexane, open pump B to enter ethyl chloroformate;
[0043] (3) 0.2% residual 0.2% of ethyl chloroformate in the reaction solution at the outlet of the reactor is detected by sampling after 20 minutes, and the reaction is qualified;
[0044] (4) after the reaction finishes, the reaction solution recovers cyclohexane and a small amount of residual raw materials throug...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com