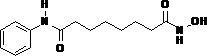
A kind of method utilizing modified mesoporous material to catalyze preparation vorinostat
A technology of vorinostat and mesoporous silica is used in the synthesis of pharmaceutical compounds and the synthesis of suberoylanilide hydroxamic acid, which can solve the problems of harsh reaction temperature and conditions, long reaction time, and many steps.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Preparation of suberic anhydride / coupling agent modified mesoporous silica:
[0050] (1) Add 0.5 mol of suberic anhydride and 0.5 mol of 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride to 100 ml of silane coupling agent KH-550, under ice bath condition Stir the reaction for 5 min, cool to room temperature, filter, and dry in vacuo to obtain solid A;
[0051] (2) Weigh 50 grams of 30 nm mesoporous silica particles and disperse them in 300 ml of 95% methanol, adjust the pH to 9.5 with ammonia water, and stir evenly to obtain a mesoporous silica dispersion;
[0052] (3) Take 2.5 grams of the above solid A and disperse it in 30 ml of ethanol, slowly add it to the above mesoporous silica dispersion, keep warm at 30°C and slowly stir for 6 h, after the reaction, centrifuge and wash with absolute ethanol After solids, disperse them in 120ml methanol-hydrochloric acid mixed solution system (methanol: 30wt% hydrochloric acid volume ratio = 5:1), reflux at 50°C for 5 ...
Embodiment 2
[0054] Preparation of caprylanid:
[0055] Add 0.1 mol (17.4 g) of suberic acid to 350 ml of THF solvent, then add 80 g of the modified mesoporous silica particles prepared above, and stir and pretreat at 40 °C for 2 h to fully modify the suberic acid monolayer Carboxyl group: after pretreatment with suberic acid, add 0.1mol of aniline, raise the temperature to 55°C and continue to stir for 5 hours. After the reaction is completed, filter to remove by-product impurities and silica particles, and wash with THF and absolute ethanol respectively , recovered for later use; vacuum-concentrate the obtained filtrate to one-fifth of the original volume at 60°C, then adjust the pH of the concentrated solution to 10.5 with 1.5 mol / l potassium hydroxide solution, stir at 35°C for 10 min, and Filtrate, add hydrochloric acid solution to the filtrate to adjust the pH to 2, stir for 5 minutes, cool to separate the solid, wash with water, and dry in vacuo to obtain about 23 g of caprylanilic ...
Embodiment 3
[0057] Preparation of Octanoanilic Acid in the Presence of Accelerator
[0058] Add 17.4 g of suberic acid into 300 ml of THF solvent, then add 50 g of the above-mentioned modified mesoporous silica particles, stir at 35°C for 1 h, add 0.1 mol of aniline and 0.05 mol of B- Chlorophthalic borane, heated up to 45°C and stirred for 3.5 h, cooled to room temperature, adjusted the pH value to 11 with 10% sodium hydroxide solution, filtered by suction to remove by-product impurities and silica particles, and used 1 mol / L hydrochloric acid was adjusted to a pH value of 1, stirred for 5 min, cooled and suction filtered, and the filter cake was washed with water and dried to obtain a white solid caprylanilic acid with a purity of 99.3% and a yield of 93.3% as measured by HPLC.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com