Positive electrode material for improving interface stability of sulfide electrolyte and application
A technology of sulfide electrolyte and positive electrode material, which is applied in the field of materials, can solve the problems of unstudied material optimization and modification of the performance of all-solid-state sulfide batteries, and achieve the goal of reducing electrochemical side reactions at the interface, optimizing electrochemical performance, and increasing capacity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
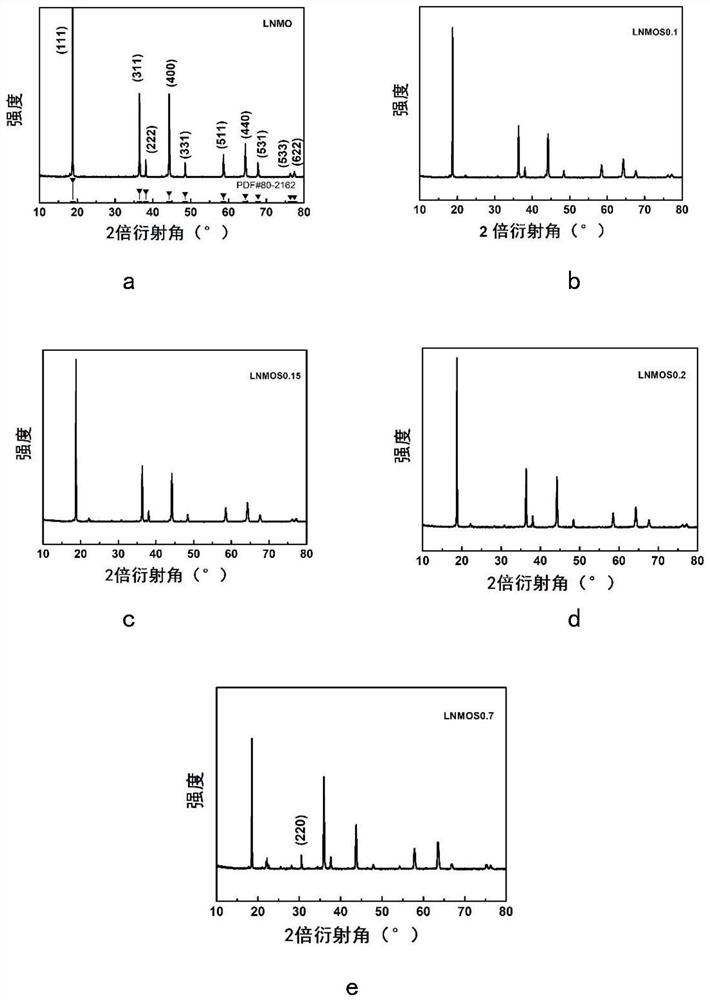
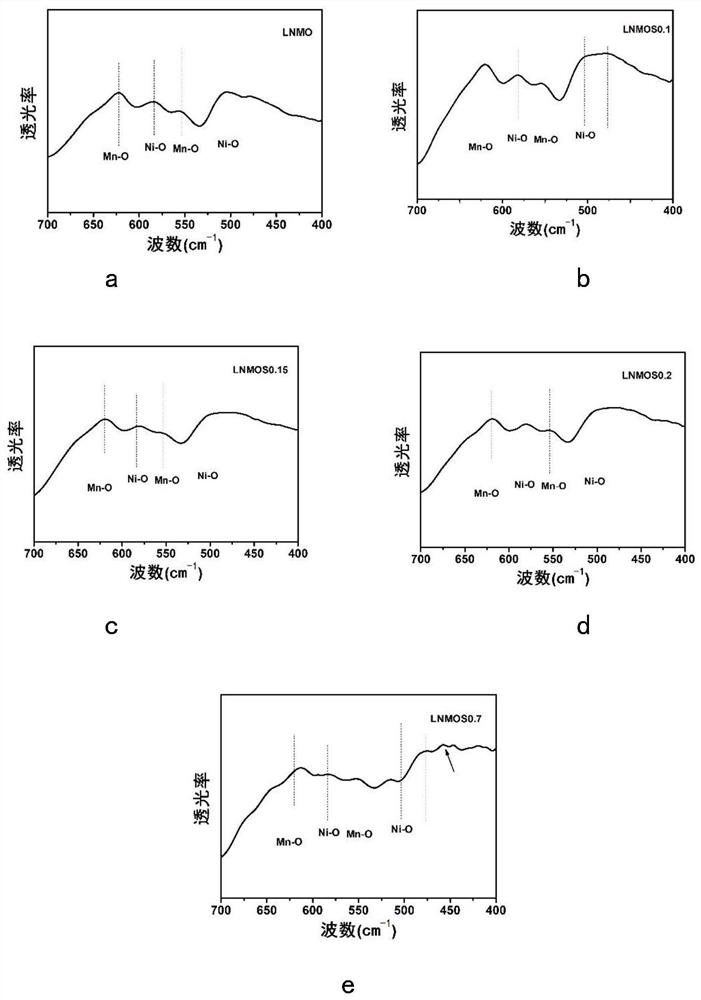
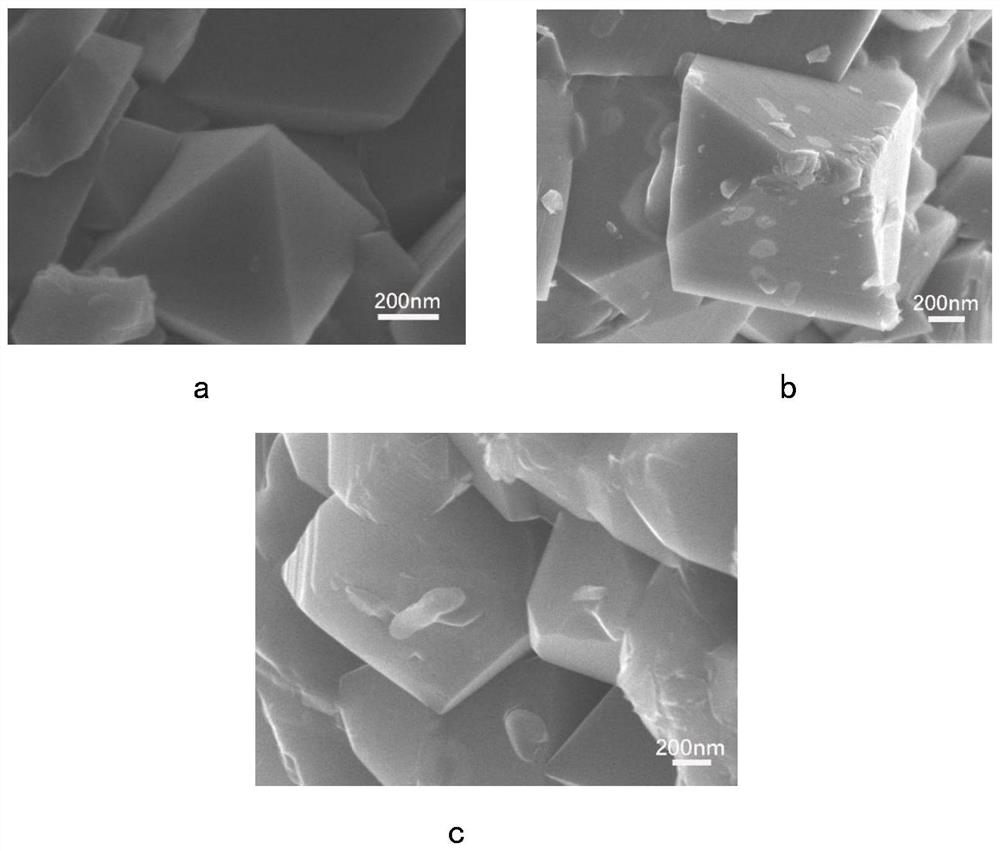
[0056] This embodiment is used to compare raw material LiNi 0.5 mn 1.5 o 4 , LiNi protected by the present invention 0.5 mn 1.5 S x o 4-x (x=0.1,0.15,0.2), and excessive doping of S element LiNi beyond the scope of the present invention 0.5 mn 1.5 S x o 4-x (x=0.7) characteristics of the positive electrode material.
[0057] First illustrate the test equipment and test conditions of the present embodiment:
[0058]X-ray diffraction measurements were performed on a Bruker AXSD8 laser with copper Kα radiation of λ=1.54178 in the range of 10°≤2θ≤80°. Scanning electron microscopy tests were performed using a Hitachi S4800 scanning electron microscope, and elemental surface scans of the test surfaces were performed using energy dispersive X-ray spectroscopy (EDX). Fourier Transform Infrared Spectroscopy (FTIR) testing was performed using a Nicolette 8700 infrared spectrometer. The functional groups of all samples were tested using Fourier transform infrared spectroscopi...
Embodiment 2
[0078] This embodiment provides the positive electrode material LiNi with cation doping on the basis of the LNMOS0.15 of the above-mentioned embodiment 1. 0.415 mn 1.245 Cu 0.05 Mg 0.02 Y 0.01 B 0.01 Fe 0.1 S 0.15 o 3.85 .
[0079] This embodiment selects nickel-manganese precursor Ni 0.25 mn 0.75 (OH) 2 , the lithium source is Li 2 CO 3 , the sulfur source is sulfur powder.
[0080] Put the nickel-manganese precursor, an excess of 5% lithium source, sulfur powder, copper oxide, magnesium oxide, iron oxide, boric acid, and yttrium oxide in a mortar and grind for 2 hours according to the required molar ratio, so that the lithium source powder and the sulfur source The powder is uniformly mixed with the nickel-manganese precursor to obtain a mixed material. During batch preparation, the material can also be placed in a ball mill tank with a rotating speed of 230-400 rpm, a mixing time of 2-6 hours, and a material filling efficiency of 55% in the equipment to obtain...
Embodiment 3
[0089] This embodiment provides the positive electrode material LiNi with cation doping on the basis of the LNMOS0.15 of the above-mentioned embodiment 1. 0.45 mn 1.35 Al 0.06 Fe 0.1 Ti 0.03 Nb 0.01 S 0.15 o 3.85 .
[0090] This embodiment selects nickel-manganese precursor Ni 0.25 mn 0.75 (OH) 2 , the lithium source is Li 2 CO 3 , the sulfur source is sulfur powder.
[0091] Put the nickel-manganese precursor, an excess of 5% lithium source, sulfur powder, niobium pentoxide, iron oxide, titanium oxide, boric acid, and aluminum oxide in a mortar and grind for 2 hours according to the required molar ratio to make the lithium source powder, The sulfur source powder is uniformly mixed with the nickel-manganese precursor to obtain a mixed material. During batch preparation, the material can also be placed in a ball mill tank with a rotating speed of 230-400 rpm, a mixing time of 2-6 hours, and a material filling efficiency of 55% in the equipment to obtain a mixed mat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Reversible discharge capacity | aaaaa | aaaaa |
| First cycle discharge capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com