Liposome co-loaded with alkannin and anthracycline chemotherapeutic drugs as well as preparation method and application of liposome
An anthracycline, shikonin technology, applied in the application of anti-tumor immunotherapy, in the field of liposome preparation, can solve problems such as low bioavailability, poor water solubility, toxic and side effects, and achieve high drug encapsulation efficiency , the effect of reducing leakage, reducing the use of
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] A preparation method comprising shikonin and mitoxantrone hydrochloride co-loaded liposomes, which mainly includes the following steps:
[0044] (1) Dissolve hydrogenated soybean lecithin (HSPC), cholesterol (Chol), and PEGylated phospholipid (DSPE-mPEG2000) (mass ratio 95.5:5.2:0.5) in chloroform, evaporate the organic solvent under reduced pressure, and form a dry lipid membrane;
[0045] (2) Add 200mM copper gluconate solution (adjust the pH to 7.4 with triethanolamine), hydrate at 55-65°C for 30 minutes, and pass through a polycarbonate filter membrane to form a liposome membrane with copper gluconate inside and outside The nano-sized liposome of the solution, utilizes the agarose gel chromatographic column to exchange the liposome external water phase to be HEPES buffer salt;
[0046] (3) Add shikonin DMSO solution to the liposome prepared in step (2), incubate at 55-65° C. for 30 min, then add mitoxantrone hydrochloride aqueous solution, continue to incubate for ...
Embodiment 2
[0049] Screening the Synergistic Ratio of Mitoxantrone Hydrochloride and Shikonin in Co-loaded Preparations
[0050] 1. Screen synergy ratio by cytotoxicity test
[0051] Cells B16F10 (melanoma cells) in the logarithmic growth phase were spread in a 96-well plate with 3000 cells per well, adhered to the wall for 12 hours, and different molar ratios were prepared according to the method in Example 1 (mitoxantrone: shikonin=10 :1,5:1,2:1,1:1,1:2,1:5,1:10,1:20) shikonin and mitoxantrone were co-loaded liposomes with different molar ratios (Mitoxantrone:Shikonin=10:1,5:1,2:1,1:1,1:2,1:5,1:10,1:20) liposome, shikonin and mitoxantrone hydrochloride aqueous physical mixture, were diluted with culture medium to the required concentration (co-loaded liposome concentration gradient: 10 μg / ml, 5 μg / ml, 2.5 μg / ml, 1.25 μg / ml, 0.625 μg / ml , 0.312μg / ml, 0.156μg / ml, 0.078μg / ml, 0.039μg / ml; physical mixture concentration gradient: 2.5μg / ml, 1.25μg / ml, 0.625μg / ml, 0.312μg / ml, 0.156μg / ml , 0...
Embodiment 3
[0059] Screening of the ratio of ICD effect induced by mitoxantrone hydrochloride and shikonin co-loaded liposomes synergistically
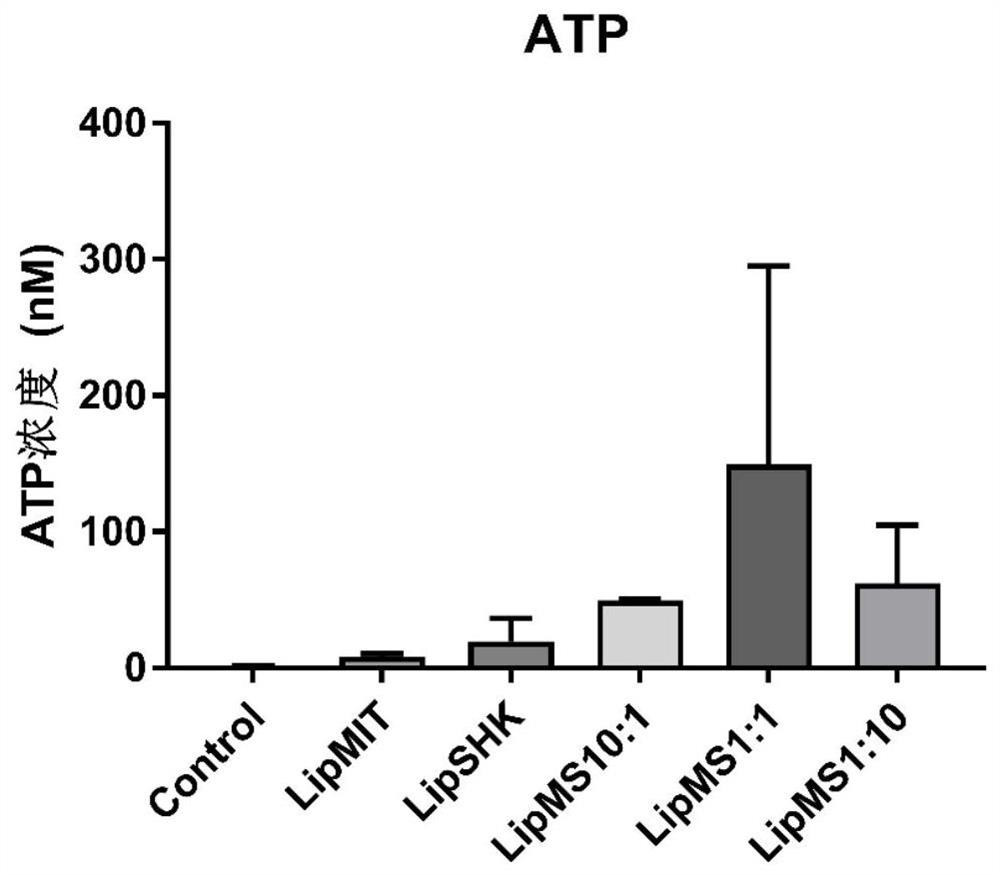
[0060] Cells B16F10 (melanoma cells) in the logarithmic growth phase were spread in a 24-well plate with 100,000 cells per well, adhered to the wall for 12 hours, and different molar ratios were prepared according to the method in Example 1 (mitoxantrone: shikonin=10 :1,1:1,1:10) of shikonin and mitoxantrone co-loaded liposomes, preparation of shikonin single-loaded liposomes, preparation of mitoxantrone single-loaded liposomes, using medium Dilute the single-loaded and co-loaded liposome solutions until the liposomes contain a total drug amount of 5 μg / ml, incubate in the incubator for 24 hours, and use the ATP kit to measure the ATP concentration released by the cells in the medium. Experimental results such as image 3 .
[0061] The results showed that different molar ratios of shikonin and mitoxantrone co-loaded liposomes could synergistic...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com