Diltiazem hydrochloride controlled-release pill and preparation method thereof
A technology of diltiazem hydrochloride and controlled-release pills, which is applied in the directions of pill delivery, pharmaceutical formulations, and medical preparations with inactive ingredients, etc., can solve problems such as poor controlled-release properties and difficulty in maintaining the health of those taking the medicine.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
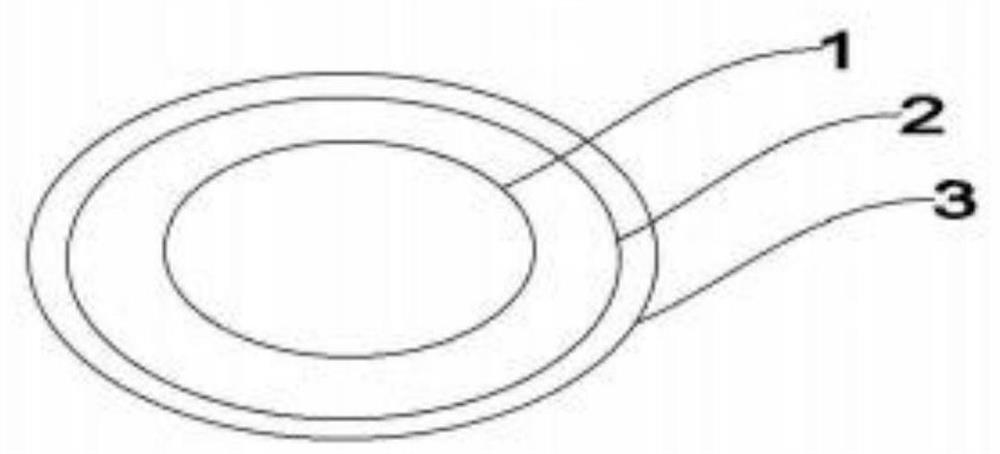
[0036] For ease of understanding, see figure 1, the application provides an embodiment of a diltiazem hydrochloride controlled-release pill, comprising: a pill core 1, a controlled-release layer 2 and an immediate-release layer 3 arranged sequentially from the inside to the outside, and in the prescription, by parts by weight, Pill core 1 includes: 15-80 parts of diltiazem hydrochloride, 10-85 parts of filling excipients, 3-15 parts of lubricant, 2-12 parts of binder, 2-14 parts of swelling agent and 4-15 parts of osmotic pressure active substances; Based on the total weight of the pill core 1, the controlled release layer 2 includes: 8%-20% controlled release film material, 4%-12% porogen and 2%-8% plasticizer; Gross weight, immediate-release layer 3 comprises: 12%-30% diltiazem hydrochloride, 5%-15% immediate-release film material and 1%-4% adhesive; Diltiazem hydrochloride content in immediate-release layer 3 is higher than pill The content of diltiazem hydrochloride in co...
Embodiment 2
[0040] As a further improvement to Example 1, the present application provides an example of a diltiazem hydrochloride controlled-release pill. Optionally, the filling excipient is one or more of lactose, calcium carbonate and sodium bicarbonate. The lubricant is one or more of magnesium stearate, stearic acid, zinc stearate or polyethylene glycol; the controlled-release layer 2 and / or the immediate-release layer 3 include the lubricant. The binder is crospovidone, polyvinylpyrrolidone, methylcellulose or absolute alcohol. The swelling agent is polyoxyethylene or hydroxypropylmethylcellulose. The osmotic pressure active substance is one or more of sodium chloride, potassium chloride, mannitol, lactose and sorbitol. The controlled-release membrane material and the immediate-release membrane material are Eudragit RS / RL, Eudragit NE 30D, hypromellose, ethyl cellulose or cellulose acetate. The plasticizer is one or more of phthalates, triethyl citrate and glycerol. The porogen ...
Embodiment 3
[0043] The application also provides a preparation method of diltiazem hydrochloride controlled-release pills, comprising the following steps:
[0044] Step 1, material preparation: According to the prescription, prepare pill core 1 material, controlled-release layer 2 material, and quick-release layer 3 material, wherein, diltiazem hydrochloride is crushed with a pulverizer and passed through a 80-90 mesh sieve, and the osmotic pressure active substance is passed through a 75-85 mesh sieve. Mesh sieve;
[0045] Step 2. Mixing to make a pill core 1: After uniformly mixing filling materials, binders, swelling agents, sieved diltiazem hydrochloride and osmotic pressure active substances, the drug-containing granules are made into drug-containing granules through a granulator, and the drug-containing granules are mixed and added After the lubricant, the pill core 1 is formed by pressing the tablet machine, wherein the pill core 1 includes: 15-80 parts of diltiazem hydrochloride, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com