Aryl gemfibrozil derivative hypervalent iodine compound and preparation method thereof
A technology for gemfibrozil and derivatives is applied in the field of aryl gemfibrozil derivatives hypervalent iodine compounds and their preparation, and can solve problems that are not conducive to the construction of gemfibrozil drug libraries and gemfibrozil drug molecules. Limited and narrow scope of application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] The preparation of phenyl gemfibrozil methyl ester hypervalent iodine trifluoromethanesulfonate described in this embodiment comprises the following steps:
[0062] (1) In a 100 mL round bottom flask, add 10 mmol of hydroxy(p-toluenesulfonyloxy)iodobenzene into a mixed solution of 30 mL of dichloromethane and 5 mL of 2,2,2-trifluoroethanol, then Add 10 mmol gemfibrozil methyl ester compound, then add 10 mmol trimethylsilyl trifluoromethanesulfonate dropwise, then react at room temperature for 1 h;
[0063] (2) After the reaction was completed, the solvent was distilled off under reduced pressure, and 70 mL of diethyl ether was added to precipitate to obtain hypervalent iodine trifluoromethanesulfonate as a white solid with a yield of 83%.
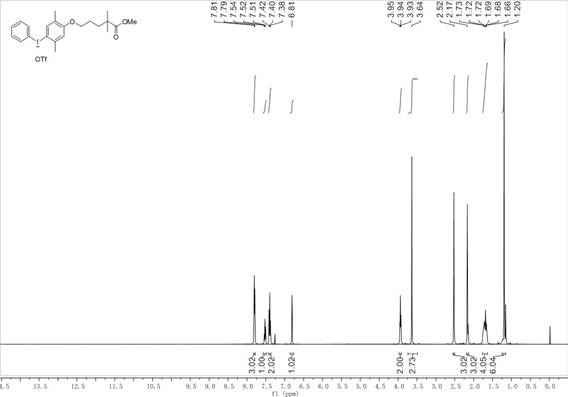
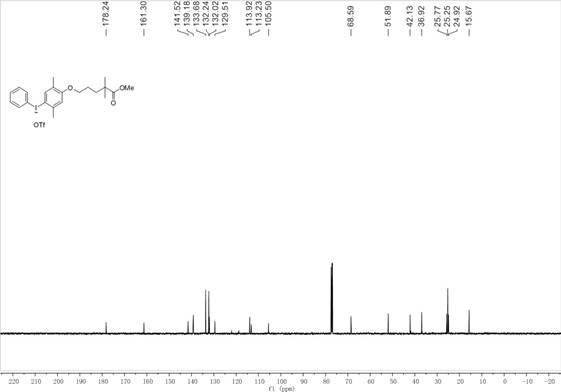
[0064] figure 1 For the hypervalent iodine trifluoromethanesulfonate of phenyl gemfibrozil methyl ester prepared in Example 1 of the present invention 1 H NMR, figure 2 For the hypervalent iodine trifluoromethanesulfonate of phen...
Embodiment 2
[0068] The preparation of 4-methylphenyl gemfibrozil methyl ester hypervalent iodine trifluoromethanesulfonate described in this embodiment comprises the following steps:
[0069] (1) In a 100 mL round bottom flask, dissolve 10 mmol 4-methyliodobenzene in 40 mL methylene chloride, add 10 mmol m-chloroperoxybenzoic acid and then 10 mmol p-toluenesulfonate under stirring condition Acid monohydrate, stirred at room temperature for 1 h, after the reaction was completed, spin the solvent dry, add 70 mL of ether, stir well for 30 minutes to produce a precipitate, filter and dry in vacuum to obtain hydroxyl (p-toluenesulfonyloxy) 4-methyliodobenzene, the yield is 98%;
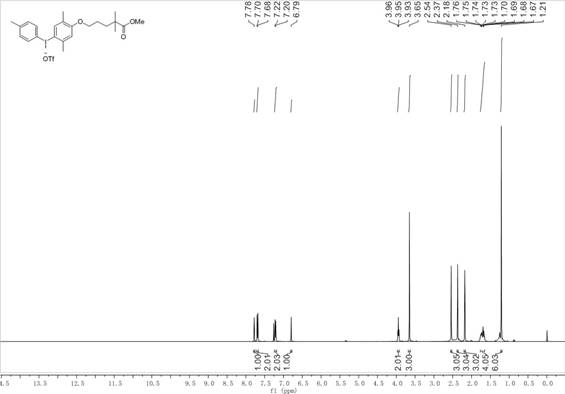
[0070] (2) In a 100 mL round bottom flask, add 8 mmol of hydroxy(p-toluenesulfonyloxy)4-methyliodobenzene in step (1) to 25 mL of dichloromethane and 4 mL of 2,2,2- In the mixed solution of trifluoroethanol, add 8 mmol gemfibrozil methyl ester compound subsequently, then add 8 mmol trimethylsilyl trifluoromethanesulf...
Embodiment 3
[0076] The preparation of hypervalent iodine trifluoromethanesulfonate 2,4,6-trimethylphenyl gemfibrozil methyl ester described in this example comprises the following steps:
[0077] (1) In a 250 mL round bottom flask, dissolve 10 mmol of iodine in 100 mL of dichloromethane, add 20 mmol of mesitylene under stirring, then add 30 mmol of m-chloroperoxybenzoic acid and 20 mmol of p-toluene Sulfonic acid monohydrate was reacted at room temperature for 1 hour. After the reaction was completed, the solvent was spin-dried, and 150 mL of diethyl ether was added, and stirred thoroughly for 30 minutes to produce a precipitate, which was filtered and dried in vacuum, and the obtained hydroxyl group (p-toluenesulfonyl Oxygen)-trimethyliodobenzene, the yield is 97%;
[0078] (2) In a 100 mL round-bottomed flask, add 8 mmol of hydroxy(p-toluenesulfonyloxy)-trimethyliodobenzene obtained in step (1) into 25 mL of dichloromethane and 3 mL of 2,2,2- In the mixed solution of trifluoroethanol, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com