A method for the continuous solvent-free reaction synthesis of hexazinone
A cycloazinone and solvent-free technology is applied in the field of continuous solvent-free reaction synthesis of cycloazinone, can solve the problems of increasing the cost of synthesizing cycloazinone, harm to the environment and human body, solvent loss, etc., and achieves reduction of solvent addition, The effect of saving production cost and quick response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
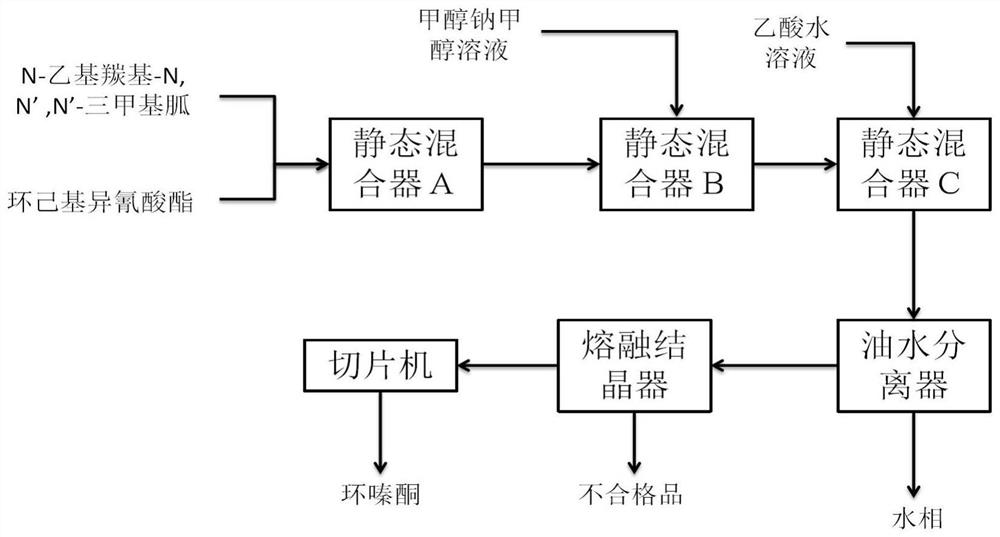
[0046] A method for the continuous solvent-free reaction synthesis of hexazinone, the specific steps are as follows,
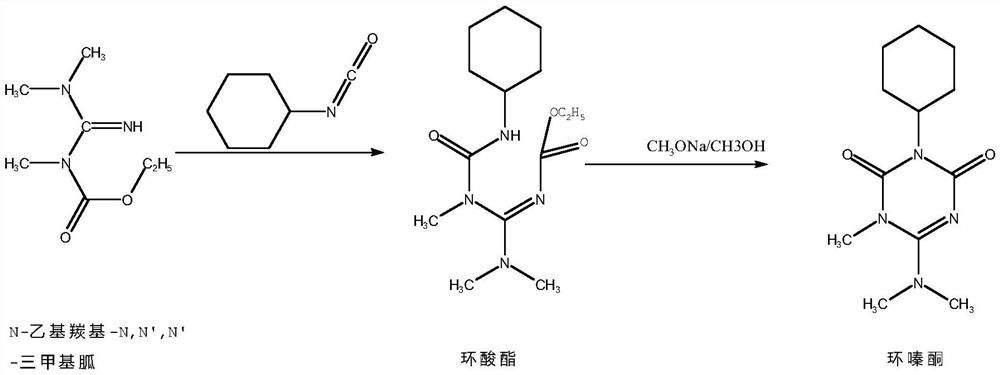
[0047] (1) Preparation of cyclic ester compound
[0048] N-ethylcarbonyl-N, N', N'-trimethylguanidine with a mass fraction of 98% is pumped into the straight static mixer A at 176.7g / min with an electromagnetic metering pump, and cyclohexyl isocyanate is used The electromagnetic metering pump is pumped into the straight static mixer A at 126.5g / min for reaction, the residence time is 20s, and the temperature of the heat transfer oil in the straight static mixer A is 95°C to obtain the cyclic ester compound;
[0049] (2) Synthesis of Hexazinone
[0050] The cyclic ester compound obtained by the reaction is added to the straight static mixer B, and the methanol solution with a mass fraction of 28% sodium methoxide is pumped into the straight static mixer B at 1 g / min with an electromagnetic metering pump for reaction , the residence time is 20s, the temperature ...
Embodiment 2
[0057] A method for the continuous solvent-free reaction synthesis of hexazinone, the specific steps are as follows,
[0058] (1) Preparation of cyclic ester compound
[0059] N-ethylcarbonyl-N, N', N'-trimethylguanidine with a mass fraction of 98% is pumped into the straight static mixer A with an electromagnetic metering pump at 1767g / min, and cyclohexyl isocyanate is The metering pump is pumped into the straight static mixer A at 633g / min for reaction. The temperature of the heat transfer oil in the straight static mixer A is 95° C., and the residence time is 20s to obtain the cyclic ester compound;
[0060] (2) Synthesis of Hexazinone
[0061] The cyclic ester compound obtained by the reaction is added to the straight static mixer B, and the methanol solution with a mass fraction of 28% sodium methoxide is pumped into the straight static mixer B with an electromagnetic metering pump at 6.5g / min. Reaction, residence time is 20s, the heat transfer oil temperature of straig...
Embodiment 3
[0068] A method for the continuous solvent-free reaction synthesis of hexazinone, the specific steps are as follows,
[0069] (1) Preparation of cyclic ester compound
[0070] N-ethylcarbonyl-N, N', N'-trimethylguanidine with a mass fraction of 98% is pumped into the straight static mixer A with an electromagnetic metering pump at 3534g / min, and cyclohexyl isocyanate is The metering pump is pumped into the straight static mixer A at 2530g / min for reaction, the temperature of the heat transfer oil in the straight static mixer A is 100°C, and the residence time is 30s to obtain the cyclic ester compound;
[0071] (2) Synthesis of Hexazinone
[0072] The cyclic ester compound obtained by the reaction is added to the straight static mixer B, and the methanol solution with a mass fraction of 28% sodium methoxide is pumped into the straight static mixer B at 10 g / min with an electromagnetic metering pump for reaction , the residence time is 30s, the temperature of the heat transfe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com