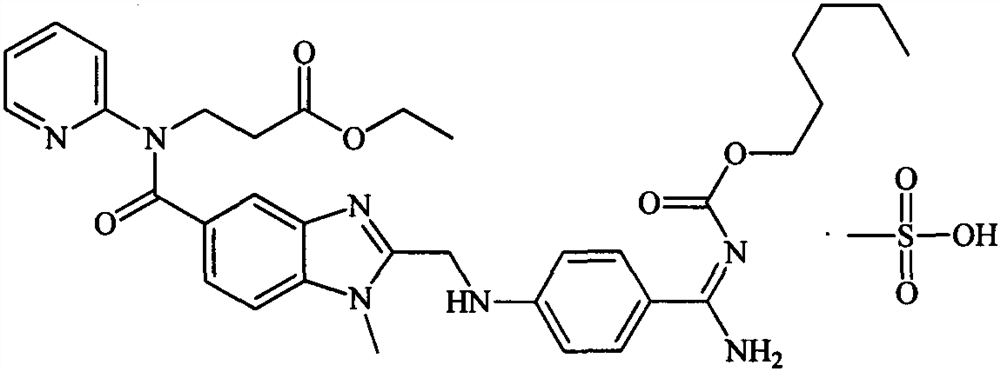
Pharmaceutical composition containing dabigatran etexilate mesylate and preparation method thereof
A technology for dabigatran etexilate mesylate and a composition is applied in the field of dabigatran etexilate mesylate pharmaceutical composition and preparation thereof, and can solve the problem of large variability of bioavailability, excessive residual solvent environment, and active ingredients. Degradation and other problems to achieve the effect of reducing gastric irritation, improving bioavailability in vivo, and reducing variability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
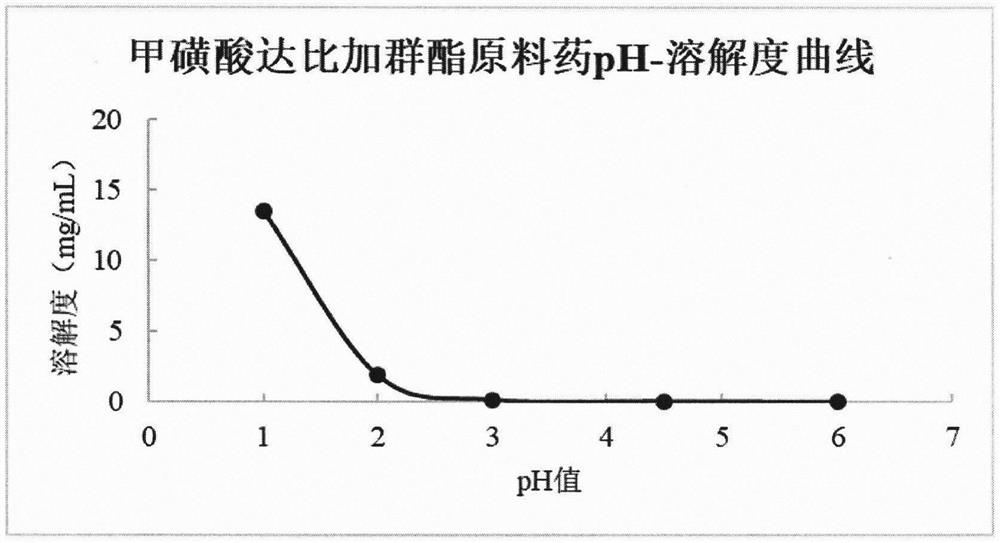
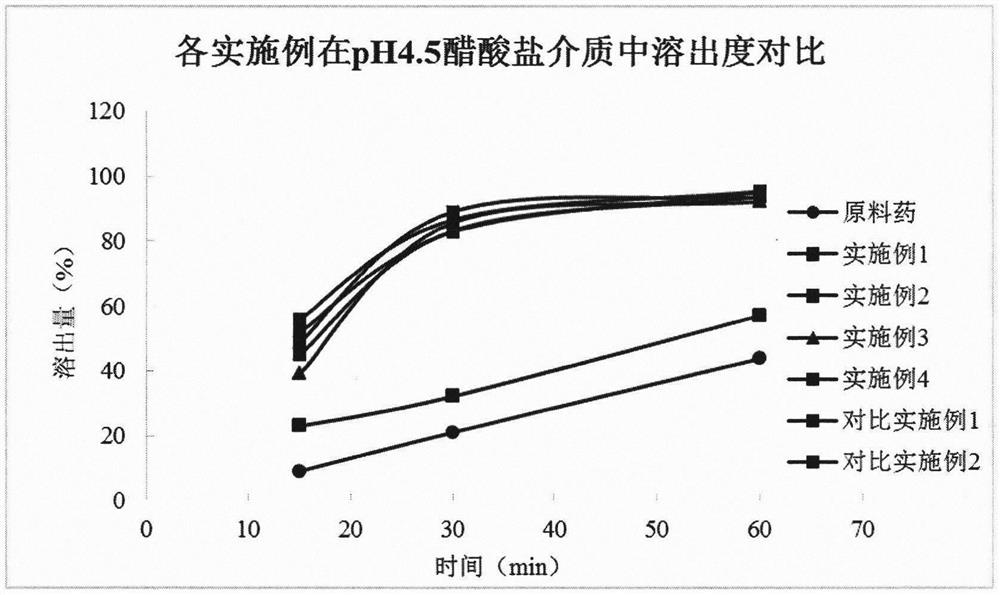
Image
Examples
Embodiment 1
[0025] This embodiment provides the preparation method of the dabigatran etexilate mesylate pharmaceutical composition. The prescription is as follows:
[0026] composition Dosage (mg) Dabigatran etexilate mesylate 126.83 macrogol glyceride laurate 89.40 polyethylene glycol 4000 135.50 microcrystalline cellulose 69.66 Carboxymethyl Starch Sodium 19.50 talcum powder 2.00 Micropowder silica gel 2.00
[0027] Preparation Process:
[0028] Mix polyethylene glycol 4000 and dabigatran etexilate mesylate, melt at 60-70°C, stir and granulate, cool and sizing to obtain Granule 1. Mix granule 1 with macrogolglycerol laurate, melt at 45-55°C, stir and granulate, cool, and granulate to obtain granule 2. Mix granule 2 with microcrystalline cellulose, sodium starch glycolate, talcum powder, and micropowder silica gel evenly, fill capsules, and obtain the product.
Embodiment 2
[0030] This embodiment provides the preparation method of the dabigatran etexilate mesylate pharmaceutical composition. The prescription is as follows:
[0031] composition Dosage (mg) Dabigatran etexilate mesylate 126.83 Ethylene glycol stearate 86.30 polyethylene glycol 6000 123.90 microcrystalline cellulose 42.70 Calcium hydrogen phosphate anhydrous 31.15 Croscarmellose Sodium 21.60 Magnesium stearate 2.60
[0032] Preparation Process:
[0033] Mix polyethylene glycol 6000 and dabigatran etexilate mesylate, melt at 60-70°C, stir and granulate, cool and sizing to obtain Granule 1. Mix granule 1 with ethylene glycol stearate, melt at 50-60°C, stir and granulate, cool, and granulate to obtain granule 2. Then mix the above granule 2 with microcrystalline cellulose, anhydrous calcium hydrogen phosphate, croscarmellose sodium and magnesium stearate, and press into tablets to obtain the product.
Embodiment 3
[0035] This embodiment provides the preparation method of the dabigatran etexilate mesylate pharmaceutical composition. The prescription is as follows:
[0036] composition Dosage (mg) Dabigatran etexilate mesylate 126.83 Propylene Glycol Monolaurate 95.60 polyethylene glycol 4000 142.32 Spray Dried Lactose 61.4 Low-substituted hydroxypropyl cellulose 38.24 Sodium stearate fumarate 5.00
[0037] Preparation Process:
[0038] Mix polyethylene glycol 4000 and dabigatran etexilate mesylate, melt at 60-70°C, stir and granulate, cool, and granulate to obtain Granule 1. Mix granule 1 with propylene glycol monolaurate, melt at 50-60°C, stir and granulate, cool, and granulate to obtain granule 2. Then the above granules 2 are mixed with spray-dried lactose, low-substituted hydroxypropyl cellulose and sodium stearate fumarate, and filled into capsules to obtain the product.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting temperature | aaaaa | aaaaa |
| Melting temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com