Multienzyme complex as well as construction method and application thereof
A technique for building methods, complexes, and applications in chemical instruments and methods, microorganism-based methods, biochemical equipment and methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Embodiment 1: Construction of engineering bacteria of CvFAP-linker-SpyTag and TLL-linker-SpyCatcher
[0056] All construction processes are based on the basic principle of polymerase chain reaction (PCR).
[0057]In order to construct pET28a-CvFAP-linker-SpyTag (the selected linker is a flexible peptide segment (EAAAK) 2 That is, EAAAK EAAAK), using the seamless cloning method, because the SpyTag gene fragment is very short (only 39bp), so this gene fragment was designed in the primer by insertion mutation, using pET28a-CvFAP as the template, and CvFAP-linker -SpyTag-F / R is used as a primer, and the plasmid pET28a-CvFAP-linker-SpyTag is obtained by PCR amplification. After the PCR product is purified, it is transformed, and after the positive plasmid is obtained, it is sequenced and verified. The accurately sequenced plasmid is stored for future use.
[0058] In order to construct pET28a-TLL-linker-SpyCatcher (here the linker is (GGGGS) 2, That is, GGGGSGGGGS), becaus...
Embodiment 2
[0065] Example 2: Fusion protein expression, purification and characterization
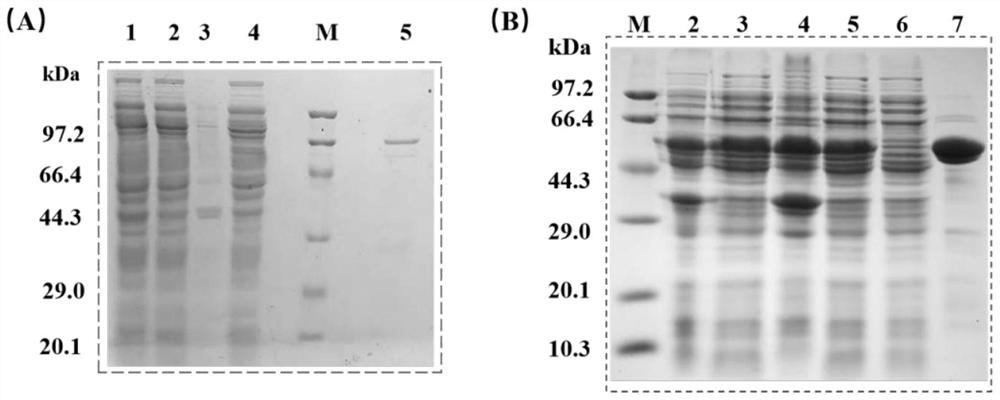
[0066] (1) Transformation and expression of recombinant plasmids: the obtained fusion protein recombinant plasmids pET28a-CvFAP-linker-SpyTag and pET28a-TLL-linker-SpyCatcher were introduced into E.coli BL21(DE3) by heat shock transformation method, and the positive clones obtained After amplified culture, IPTG (final concentrations of 0.5 mM and 0.1 mM, respectively) was added to induce expression, and cultured at 17° C. and 20° C. for 20 h, respectively. After the fermentation process is completed, the TLL-linker-SpyCatcher crude enzyme is purified by affinity chromatography to obtain an enzyme protein with a purity higher than 95%. After the eluted protein is collected, it is concentrated and subpackaged and quickly frozen with liquid nitrogen.- Store at 20°C for later use; in addition, because the catalytic activity of the crude CvFAP enzyme is higher than that of the pure enzyme, the CvFAP-li...
Embodiment 3
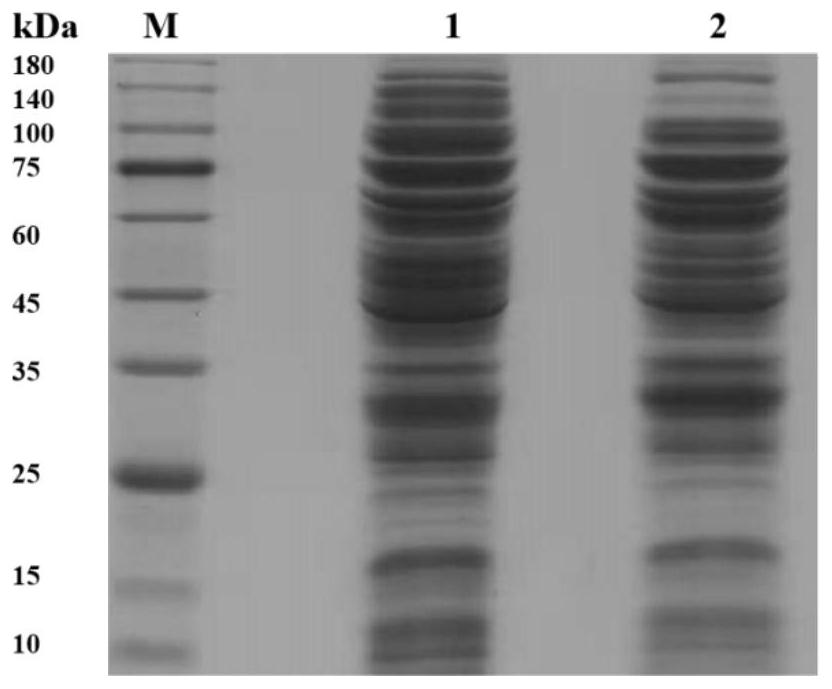
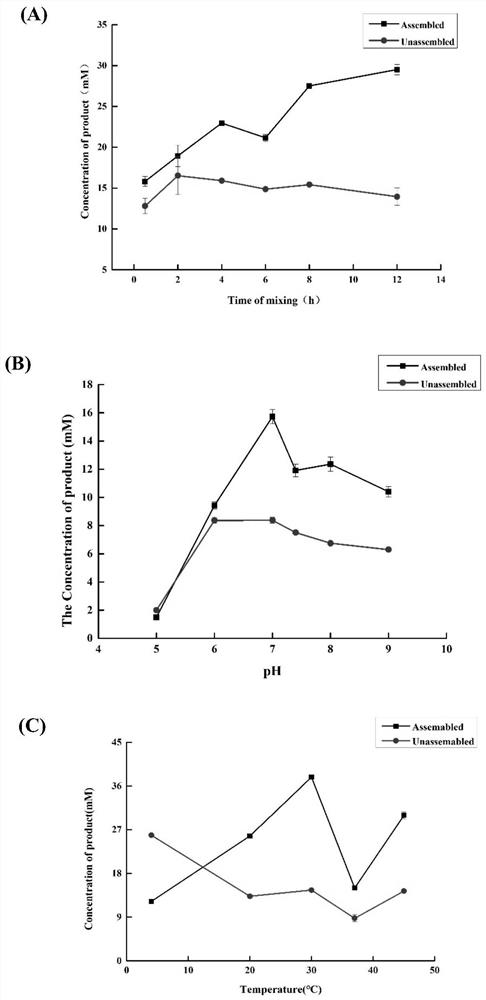
[0069] Example 3: Construction of multi-enzyme complex in vitro and optimization of assembly conditions
[0070] (1) Construction of TLL-CvFAP multi-enzyme complex in vitro: mix CvFAP-linker-SpyTag crude enzyme and TLL-linker-SpyCatcher pure enzyme extracellularly in 20mM, pH 7.4 PBS at a molar ratio of 1:1 of the target protein, each The final concentration of the enzyme was 20 μM / L, and stood at 30°C for 12 hours; after the assembly, samples were taken for SDS-PAGE protein electrophoresis, and 30 μL soybean oil was used as the substrate (the density of soybean oil was 0.91 g / mL, and its average molecular weight was 900g / moL), 30°C, 1000rpm, under blue light irradiation, take part of the assembly mixture to catalyze the conversion of soybean oil; after the reaction, fully extract with 1mL ethyl acetate, centrifuge at 10000rpm, room temperature for 4min, take a clear sample of the upper layer for high-efficiency gas chromatography analysis , to detect the formation of the prod...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com