Engineered cell for activating NK-like cell, and preparation method and application of engineered cell
An engineering and cellular technology, applied in the field of genetic engineering, to achieve the effects of improving proliferation, enhancing tumor killing ability, and significant tumor cytotoxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Construction of Example 1 NK-like cells
[0048] (1) Design sgRNA (SEQ ID NO:6:gaccatgaactgctcacttg) according to the Bcl11b gene, and add restriction enzyme sites EcoRI and SalI at the 5' end and 3' end of the sequence after artificial synthesis;
[0049] The synthesized sequence fragment was digested with EcoRI and SalI, then connected to the PX458-gBCL11b vector containing the T7 promoter, and the recombinant plasmid was successfully constructed by sequencing.
[0050] (2) will 1×10 7 T cells were resuspended in 3 mL of T cell medium, seeded in one well of a 6-well plate, and activated by adding a combination of 100 ng / mL anti-human CD3 antibody and 100 ng / mL anti-human CD28 antibody;
[0051] After 3 days, remove the suspended cells for counting, centrifuge at 300×g for 10 min, resuspend the cell pellet in 10 mL of Opti-MEM, centrifuge at 300×g for 10 min, resuspend the cell pellet in 100 μL of Opti-MEM, add CRISPR at a concentration of 40 μM / Cas9 plasmid, after ...
Embodiment 2
[0053] Example 2 Construction of engineered cells
[0054] (1) Artificially synthesize T2A-linked nucleic acid molecules containing MICA-encoding genes, IL-12 and CD28 transmembrane regions, CD64 and CD86-encoding genes, and add Pme1 and Spe1 restriction sites and their protective bases at both ends ;
[0055] The nucleic acid molecules were double-digested with restriction endonucleases Pme1 and Spe1, incubated in a water bath at 37°C for 30 minutes, and recovered by 1.5% agarose gel electrophoresis to obtain the digested products containing sticky ends;
[0056] The digested product was ligated into the linearized pWPXLd-eGFP plasmid (containing cohesive ends) digested by Pme1 and Spe1, and the ligation reaction was carried out with the participation of T4 DNA polymerase (Invitrogent) to obtain a lentiviral vector.
[0057] (2) Use 293T cells to prepare recombinant lentivirus. When 293T cells are spread on the bottom of a 100mm culture dish to reach 80-90%, carry out lentiv...
Embodiment 3
[0065] Example 3 In vitro expansion of NK-like cells
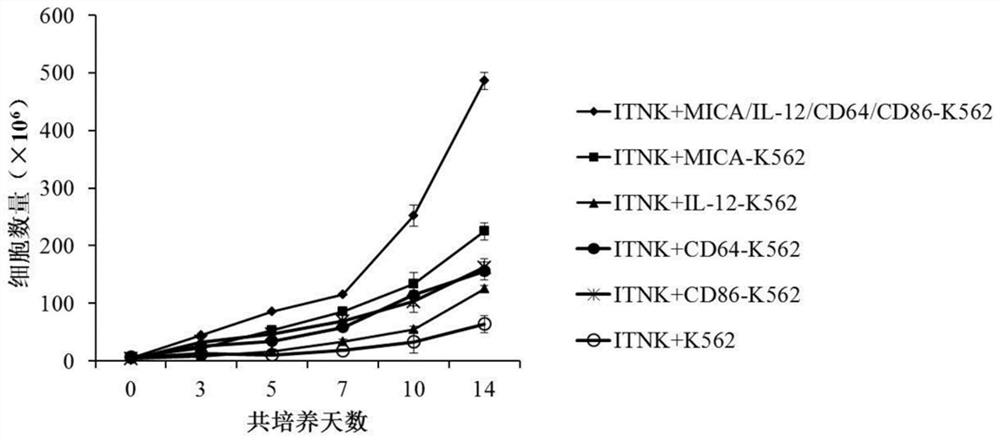
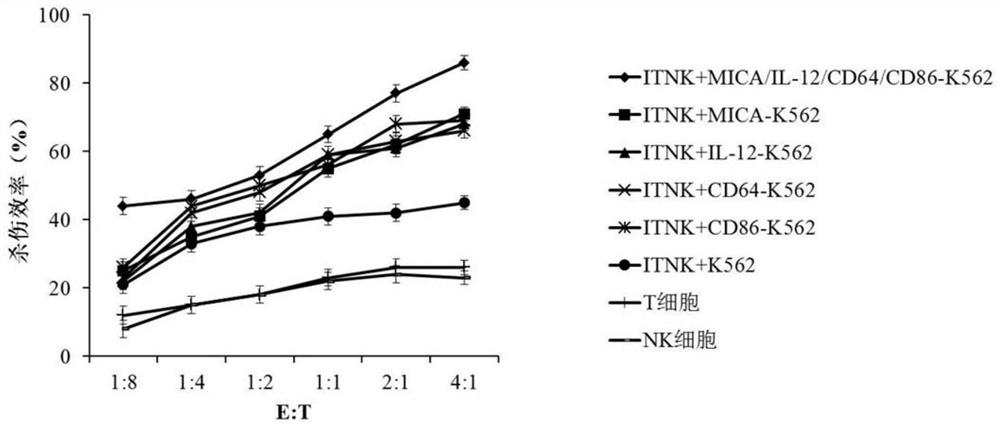
[0066] In this embodiment, NK-like cells were cultured in RPMI-1640 medium containing 10% fetal bovine serum, and the cells were counted after 24 hours of culture, and the cell concentration was adjusted to 2×10 6 cells / mL, an equal proportion of engineered cells were added to the culture medium on day 0, followed by a culture medium containing 2×10 6 Each / mL engineered cells, 10% fetal bovine serum and 50 U / mL IL-2 in RPMI-1640 medium continued to culture for 14 days at 37°C, 5% CO 2 Co-cultivation in the incubator under 500Gy irradiation conditions.
[0067] Such as figure 1 As shown, after NK-like cells were co-cultured with MICA-IL-12-CD64-CD86 engineered cells, the number of cells increased significantly. After 2 weeks of culture, the number of NK-like cells increased by about 100 times, which was significantly higher than that of the control group.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com