A gene therapy carrier and its use
A gene therapy and vector technology, applied in gene therapy, genetic engineering, plant genetic improvement, etc., can solve problems such as inability to cure diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
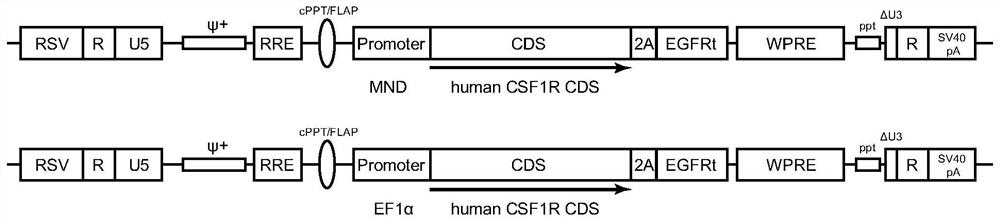
[0106] Example 1: Construction of CSF1R lentiviral vector
[0107] In this example, the vectors pMND-CSF1R-LV and pEF1α-CSF1R-LV were respectively constructed as follows: a third-generation lentiviral vector containing a chimeric 5′LTR; the myeloproliferative sarcoma virus enhancer negative control region was deleted and dl587rev primer binding site substituted (MND) promoter or short elongation factor 1α (EF1α) promoter; polynucleotide encoding CSF1R polypeptide; and self-inactivating (SIN) 3′LTR (see figure 1 ). Specifically, the pMND-CSF1R-LV vector mainly includes: chimeric 5'LTR, Psi (ψ) packaging signal, human immunodeficiency virus (HIV) rev response element (RRE), cPPT / FLAP, myeloproliferative sarcoma virus enhancer Negative control region deleted and dl587rev primer binding site replacement (MND) promoter, polynucleotide encoding CSF1R polypeptide, truncated epidermal growth factor receptor (EGFRt) tag, woodchuck hepatitis virus post-transcriptional regulatory elemen...
Embodiment 2
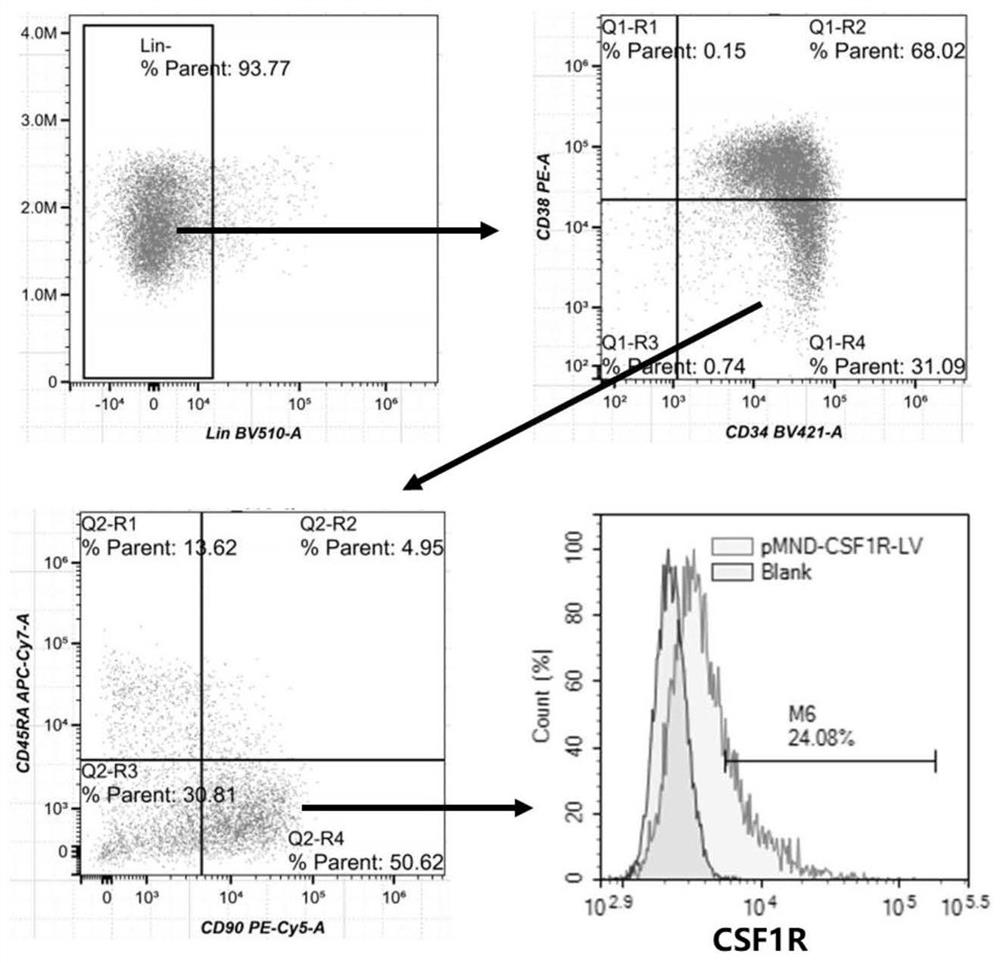
[0138] Example 2: Transduction of human CD34 with a lentiviral vector encoding CSF1R + hematopoietic stem cells
[0139] (1) Treat the cell plate with recombinant human fibronectin (Retronectin)
[0140] The recombinant human fibronectin solution was diluted to 60 μg / ml with buffer. Add the recombinant human fibronectin dilution to the cell culture plate (0.2 mL in a 48-well plate). Place at room temperature for 4 hours, or overnight at 4°C in the dark. Aspirate the recombinant human fibronectin solution, and use an appropriate amount of 2% BSA (bovine serum albumin) to block with DPBS (Duchenne's phosphate buffered saline, without calcium and magnesium). Place at room temperature for 30min. Aspirate the BSA blocking solution and wash the cell plate twice with DPBS. Add appropriate amount of DPBS for later use.
[0141] (2) HSCs pre-stimulation
[0142] The isolated hematopoietic stem cells were cultured in cell plates pretreated with recombinant human fibronectin, cell...
Embodiment 3
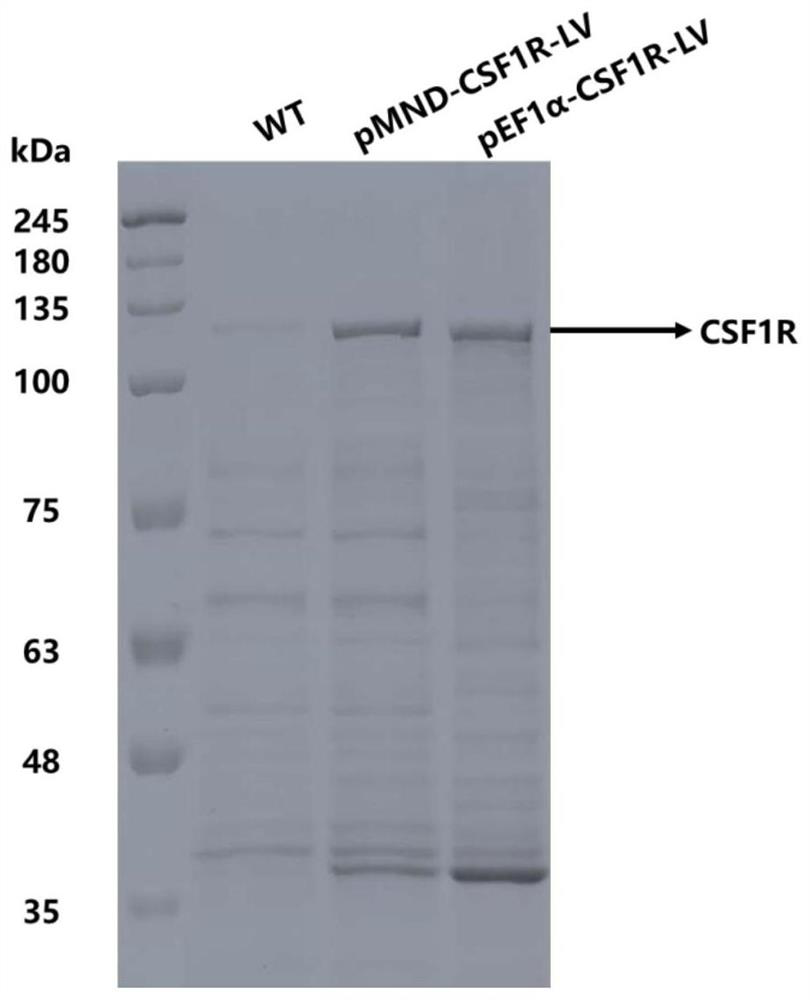
[0145] Example 3: Detection of protein expression in cells transduced by a lentiviral vector encoding CSF1R (Coomassie Brilliant Blue staining detection)
[0146] 1. Three days after infecting hematopoietic stem cells with lentivirus encoding CSF1R, the cells were collected and the expression of related proteins was detected.
[0147] 2. Prepare a separating gel with a concentration of 8% and a volume of 15ml
[0148] h 2 O: 6.9ml
[0149] 30% gel stock solution: 4.0ml
[0150] 1.5mol / L Tris-HCl (pH8.8): 3.8ml
[0151] 10% SDS (sodium dodecyl sulfate): 0.15ml
[0152] 10% ammonium persulfate: 0.15ml
[0153] TEMED (tetramethylethylenediamine): 0.01ml
[0154] 3. Once TEMED is added, immediately after mixing, carefully inject the separating gel into the prepared gap between the glass plates, leaving enough space for the layering glue. Use a capillary to gently add a few milliliters of n-butanol to its top layer to prevent the inhibitory effect of oxygen in the air on gel...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com