Platelet anti-human globulin experimental immune zone distribution detection method
A technology for detecting the distribution of anti-human globulin, applied in the field of immunology and medical testing, can solve the problems of complicated experimental procedures, easy to be activated and aggregated, and long operation time, so as to improve accuracy and sensitivity, avoid loss of antigenicity, The effect of high detection accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Example 1: Preparation of quantum dot immunolabeled anti-human globulin
[0064] (1) Preparation of anti-human globulin
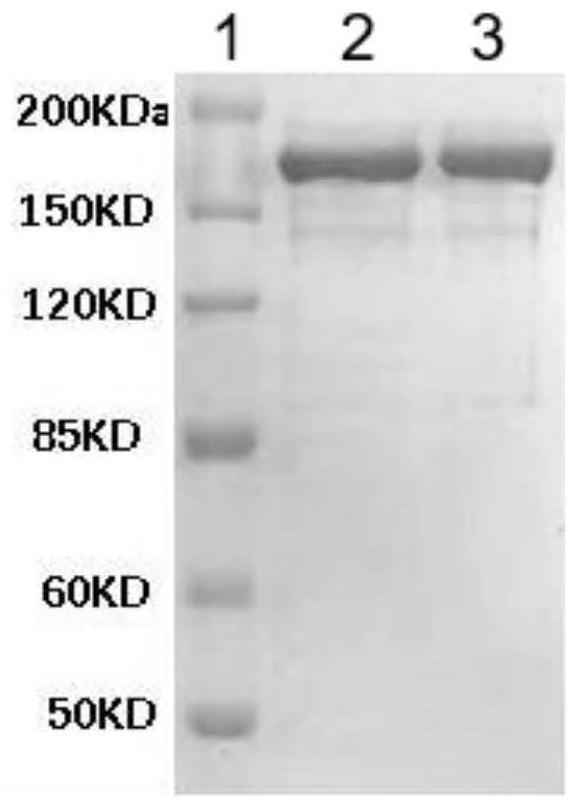
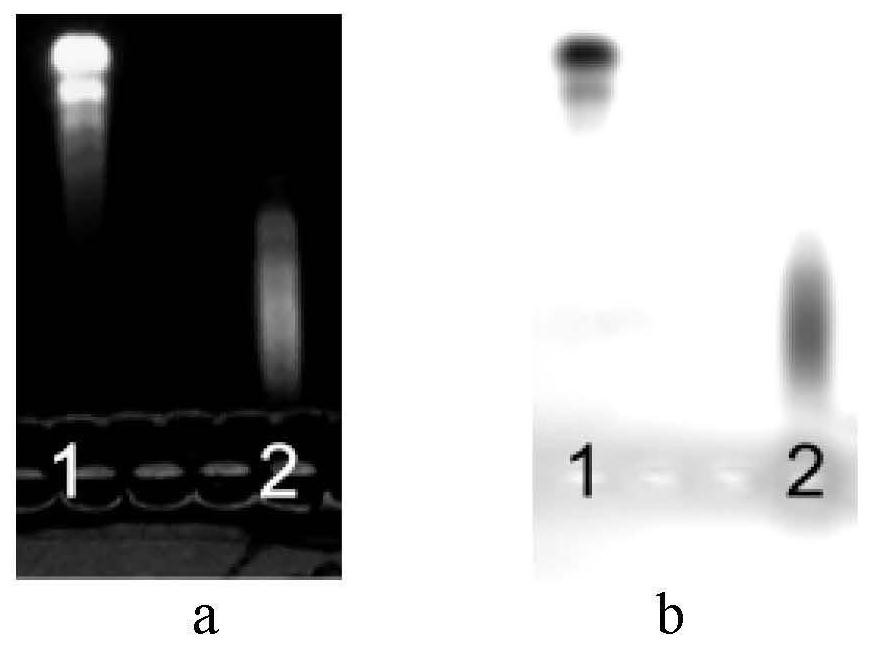
[0065] Extract gamma globulin from human plasma, emulsify with adjuvant, immunize sheep (1 mg / rat) or rabbit (0.5 mg / rat), etc., immunize at multiple points each time, and draw venous blood after 5 times of immunization. The whole blood was centrifuged at 2000rpm for 30min to separate the plasma, and the protein was precipitated with 50% ammonium sulfate, and purified with DEAE-52 ion exchange chromatography column, the purified protein was collected, and the protein concentration was determined by SDS-PAGE electrophoresis purity test and test tube anti-globulin After the titer is detected by the protein experiment, it is ready for use. Test results such as figure 2 Shown, wherein, 1, Marker; 2, 0.05M NaCl solution eluent; 3, 0.1M NaCl solution eluent. The test results showed that the purity of the antihuman globulin in the eluate of 0.05M NaCl s...
Embodiment 2
[0071] Example 2: Platelet Antibody Detection
[0072] (1) Preparation of platelet suspension
[0073] Three portions of fresh O-type EDTA anticoagulated whole blood were collected and centrifuged at 900 rpm for 10 minutes to obtain platelet-rich plasma, which was diluted 2-fold with PBS solution containing 1% BSA to form a platelet suspension. If platelets are collected by machine, the platelet suspension is diluted 10 times with PBS solution containing 1% BSA.
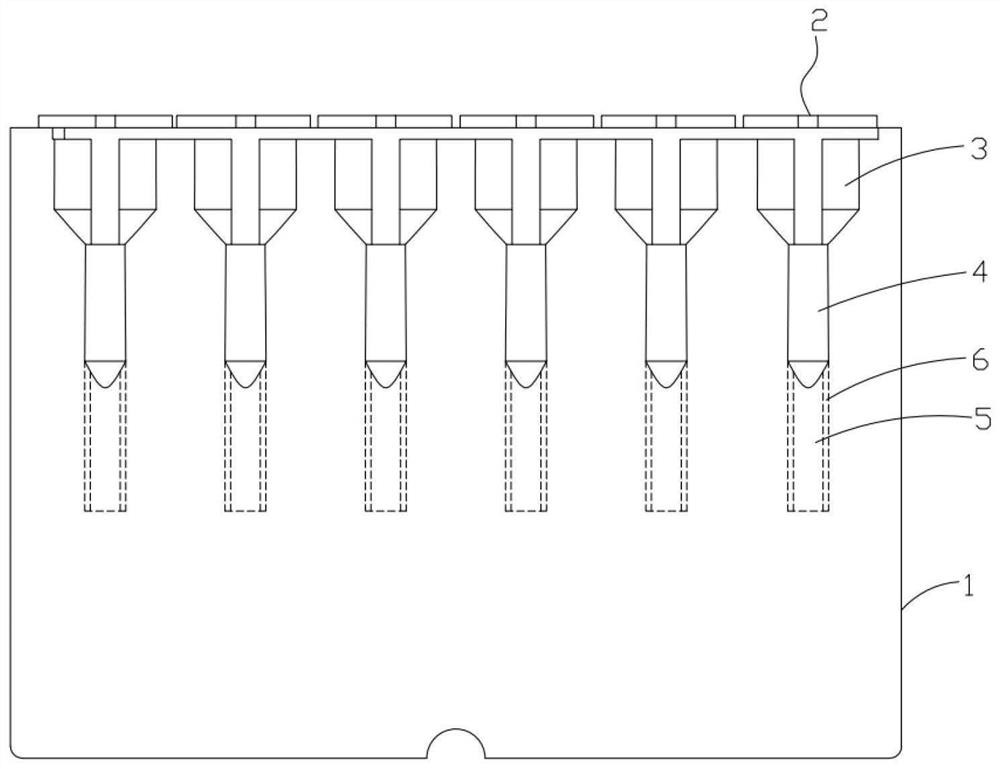
[0074] (2) Preparation of zone distribution detection card
[0075] Add 100 μL of immune complex separation enhancement medium into the reaction chamber of the zone distribution detection card. The immune complex separation enhancement medium has a specific gravity of 1.05 to 1.10, an osmotic pressure of 280 to 350 mmol / L, and contains 0.1 to 1 mg / mL of secondary antibody. The solution was centrifuged at 1000rpm for 1min before use.
[0076] (3) Detection steps
[0077] Add 50 μL platelet suspension, 50 μL sample...
Embodiment 3
[0081] Embodiment 3: platelet cross-matching type
[0082] (1) Preparation of platelet suspension
[0083] Collect EDTA anticoagulated whole blood from blood donors with the same ABO blood type as the patient, centrifuge at 900 rpm for 10 minutes, and take the upper layer of platelet-rich plasma (PRP); dilute it with PBS solution containing 1% BSA twice to form a platelet suspension. If platelets are collected by machine, the platelet suspension is diluted 10 times with PBS solution containing 1% BSA.
[0084] (2) Preparation of test card
[0085]Add 100 μL of immune complex separation enhancement medium to the reaction chamber of the zone distribution detection card. The immune complex separation enhancement medium has a specific gravity of 1.05-1.10, an osmotic pressure of 280-350 mmol / L, and contains 0.1-1 mg / mL secondary antibody solution , centrifuged at 1000rpm for 1min and then used for later use.
[0086] (3) Cross-matching step
[0087] Add 50 μL donor platelet su...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Osmotic pressure | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com