Olopatadine alpha methyl compound, and preparation method and application thereof
A technology for olopatadine and compound, which is applied in the field of olopatadine alpha methyl compound and preparation, and can solve problems such as unfavorable safe production, high safety risk and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1 The method for preparing olopatadine α methyl compound:
[0035]
[0036] Take 20 g of isoket acid (compound 1) and add it to the reaction flask, add 50 ml of isopropanol, stir and cool down to 0-10°C, add 10 g of thionyl chloride dropwise, stir and heat up to reflux after the addition, TLC detects that the reaction is complete After cooling down, thionyl chloride and isopropanol were removed by concentration under reduced pressure, and then 40ml of isopropanol was added for recrystallization to obtain 22g of compound 2.
[0037]
[0038] Take 20g of compound 2, add 200ml of N,N-dimethylformamide, stir and dissolve completely, control the temperature below 10°C, add 8.47g of potassium tert-butoxide, stir and control the temperature below 10°C, add 10.26g of methyl iodide dropwise, and the reaction is complete at room temperature , drop 150ml of purified water to quench the reaction, then use dichloromethane to extract completely, use anhydrous sodium ...
Embodiment 2
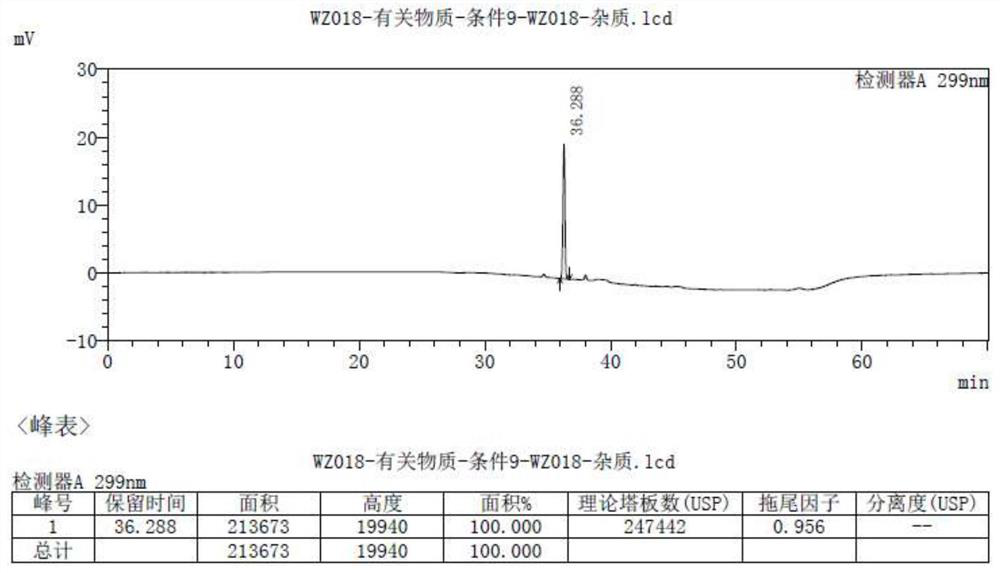
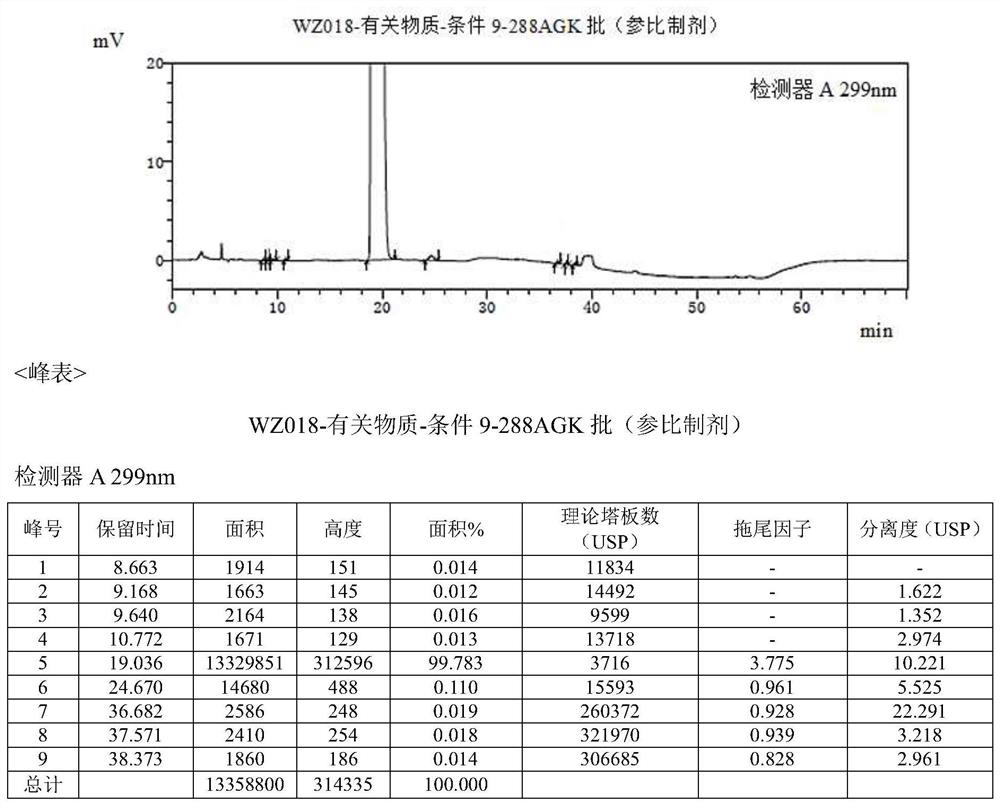
[0043] Example 2 Confirmation of impurities
[0044] Multiple batches of the original drug olopatadine hydrochloride (trade name: Alloca) were tested using the following method: mobile phase A was potassium dihydrogen phosphate solution-acetonitrile (75:25), and mobile phase B was potassium dihydrogen phosphate Solution-Acetonitrile (40:60), AgelaVenusil MP C8 (4.6*250mm) chromatographic column, the detection wavelength is 299nm.
[0045] result figure 2 Shown, detect and separate and find the impurity compound of about 0.019% trace (see figure 2 36.682min place), the same separation environment, fully matched with the compound obtained in Example 1. It can be seen that the impurity is the olopatadine α-methyl compound prepared in Example 1.
Embodiment 3
[0047] Preparation system: prepare with reference to the method in CN1042211676:
[0048]
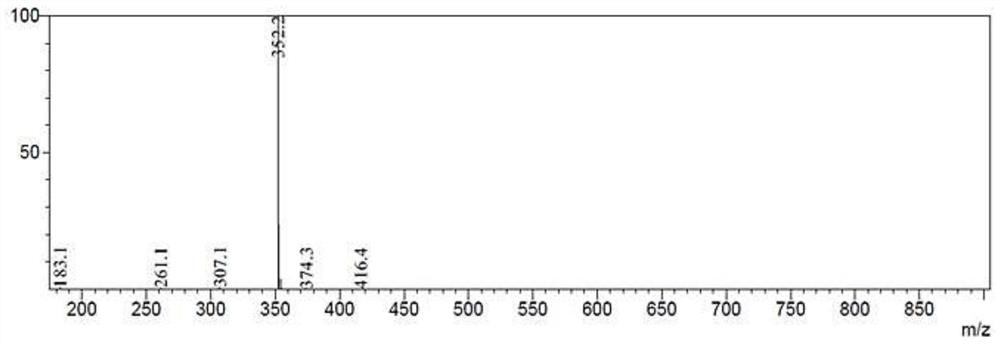
[0049] It is extremely difficult to separate during the separation and preparation of the mother liquor. It exists in a small amount in the mother liquor, and is similar in polarity to other impurities. The peak time in HPLC (high performance liquid chromatography) is relatively close, but it is not easy to prepare and separate. After using more methods for purification and separation (silica gel column chromatography, Sephadex, ODS reverse phase, HPTLC, HPLC, etc.), try to use HPLC for separation, and replace different chromatographic columns (Agela Venusil MP C8 (4.6*250mm), phenomenonex Ultracarb (4.6*150mm, etc.), changing the mobile phase ratio, buffer salt type and ratio, etc., finally found that adding a small amount of trifluoroacetic acid can be effectively separated, and then obtained trace amounts of the Impurities, for structural confirmation.
[0050] Specific process: ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com