Method for catalytically synthesizing long-chain alkyl aromatic hydrocarbons
A technology for long-chain alkyl aromatic hydrocarbons and a synthesis method, which is applied in the field of catalytic synthesis of long-chain alkyl aromatic hydrocarbons, can solve the problems of difficulty in evaluating the activity stability of solid acid catalysts, difficulty in large-scale continuous production, poor activity stability, and the like, and achieves The effect of good catalyst activity stability, high product linearity and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] The preparation of embodiment 1 Sr-Al-SBA-15 molecular sieve catalyst
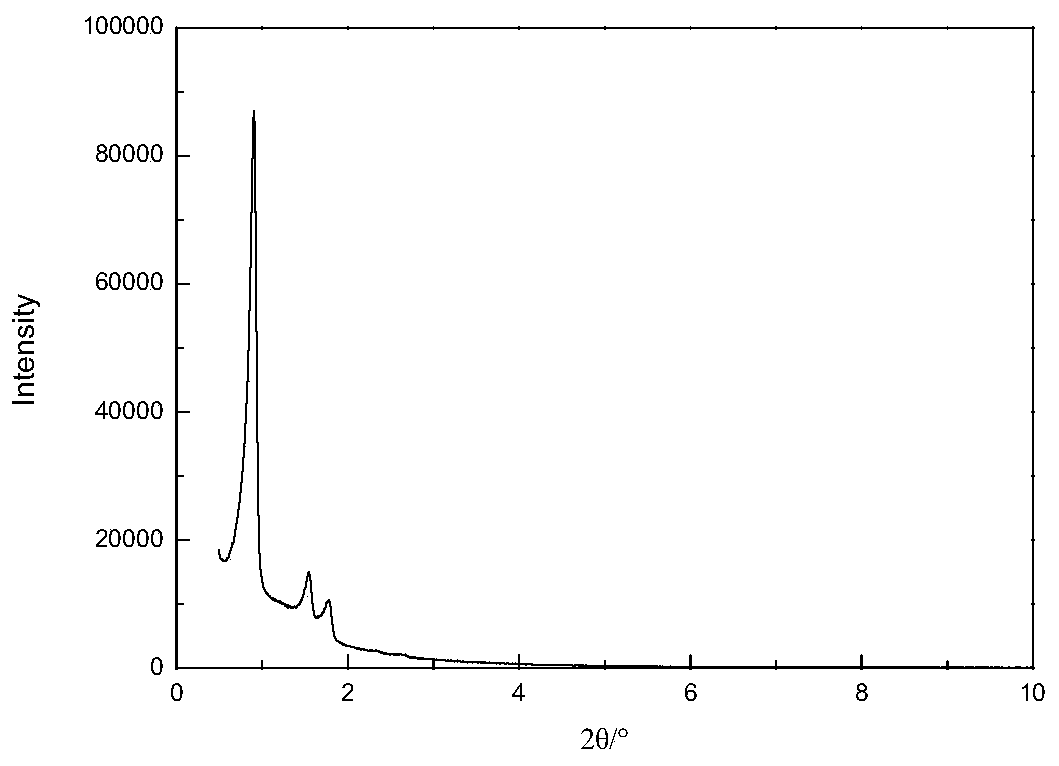
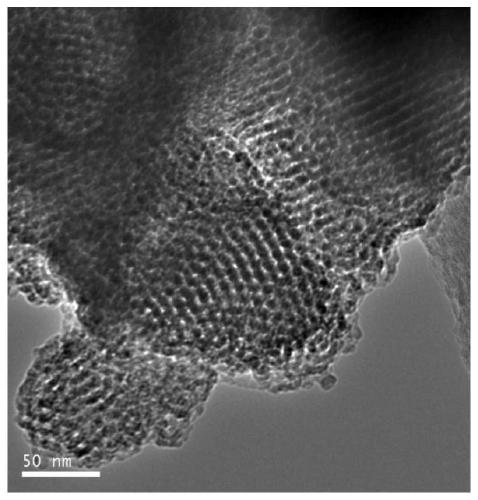
[0068] (1) According to the molar ratio P123: Al 2 o 3 : SiO 2 :H 3 PO 4 :SrO:H 2 O is calculated as 1:3.0:63.0:310:1.0:10000, weigh 20g of triblock polymer P123, mix with calculated amount of distilled water and phosphoric acid, stir and mix at 40°C for 1h, add calculated amount of monohydrate oxidation Aluminum and strontium nitrate, continue to stir and mix for 1 hour; then, slowly add the calculated amount of tetraethyl orthosilicate under stirring conditions, continue stirring at 40°C for 5 hours; crystallize at 95°C for 48 hours, then filter and wash , dried, and finally in a muffle furnace at a heating rate of 2°C / min from 25°C to 550°C, and roasted at a constant temperature for 5 hours to remove the template agent to obtain Sr-Al-SBA-15 molecular sieve powder, whose Al 2 o 3 with SiO 2 The molar ratio is 0.0476, SrO and SiO 2 The molar ratio was 0.0159. X’Pert PRO X-ray diffractome...
Embodiment 2
[0070] The preparation of embodiment 2 Mg-Al-SBA-15 molecular sieve catalyst
[0071] (1) According to the molar ratio P123: Al 2 o 3 : SiO 2 :H 2 SO 4 :MgO:H 2O is calculated as 1:4.0:60.0:290:2.5:9000, weigh 20g of triblock polymer P123, mix with calculated amount of distilled water and sulfuric acid, stir and mix at 42°C for 1h, add calculated amount of monohydrate oxidation Aluminum and magnesium acetate, continue to stir and mix for 1 hour; then, slowly add the calculated amount of tetraethyl orthosilicate under stirring conditions, and continue to stir for 5 hours at 42°C; crystallize at 95°C for 48 hours, then filter and wash , drying, and finally in a muffle furnace at a heating rate of 1 °C / min from 20 °C to 550 °C, and constant temperature roasting for 5 hours to remove the template agent to obtain Mg-Al-SBA-15 molecular sieve powder, its Al 2 o 3 with SiO 2 The molar ratio is 0.0667, MgO and SiO 2 The molar ratio was 0.0417. Characterized by X-ray diffract...
Embodiment 3
[0073] Embodiment 3 Preparation of Ba-Al-SBA-15 molecular sieve catalyst
[0074] (1) According to the molar ratio P123: Al 2 o 3 : SiO 2 :HCl:BaO:H 2 O is calculated as 1:10.0:62.0:300:3.0:11000, weigh 20g of triblock polymer P123, mix with calculated amount of distilled water and hydrochloric acid, stir and mix at 40°C for 1h, add calculated amount of monohydrate oxidation Aluminum and barium nitrate, continue to stir and mix for 1 hour; then, slowly add the calculated amount of tetraethyl orthosilicate under stirring conditions, continue stirring at 40°C for 6 hours; crystallize at 100°C for 48 hours, then filter and wash , dried, and finally in a muffle furnace at a heating rate of 2°C / min from 20°C to 550°C, and roasted at a constant temperature for 5 hours to remove the template agent to obtain Ba-Al-SBA-15 molecular sieve powder, its Al 2 o 3 with SiO 2 The molar ratio is 0.1613, BaO and SiO 2 The molar ratio was 0.0484. Characterized by X-ray diffraction and tr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com