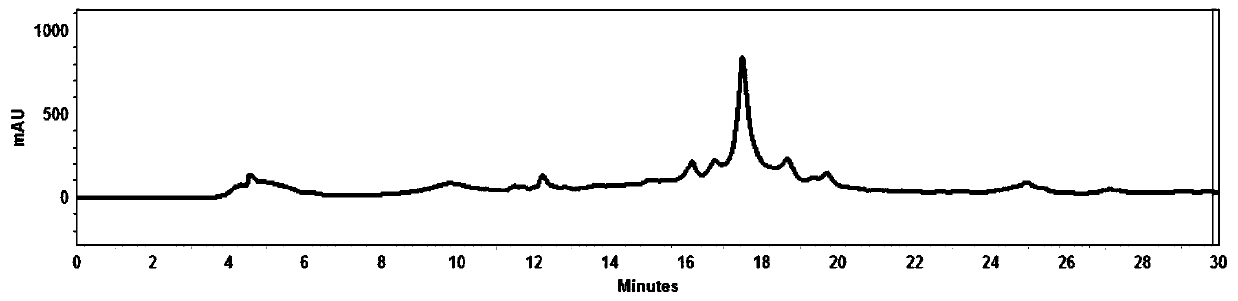
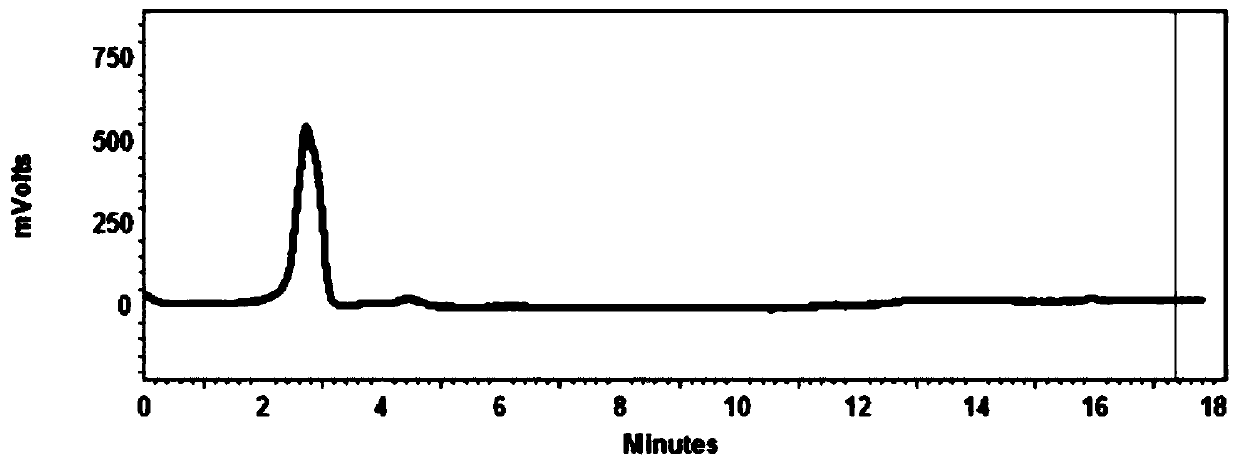
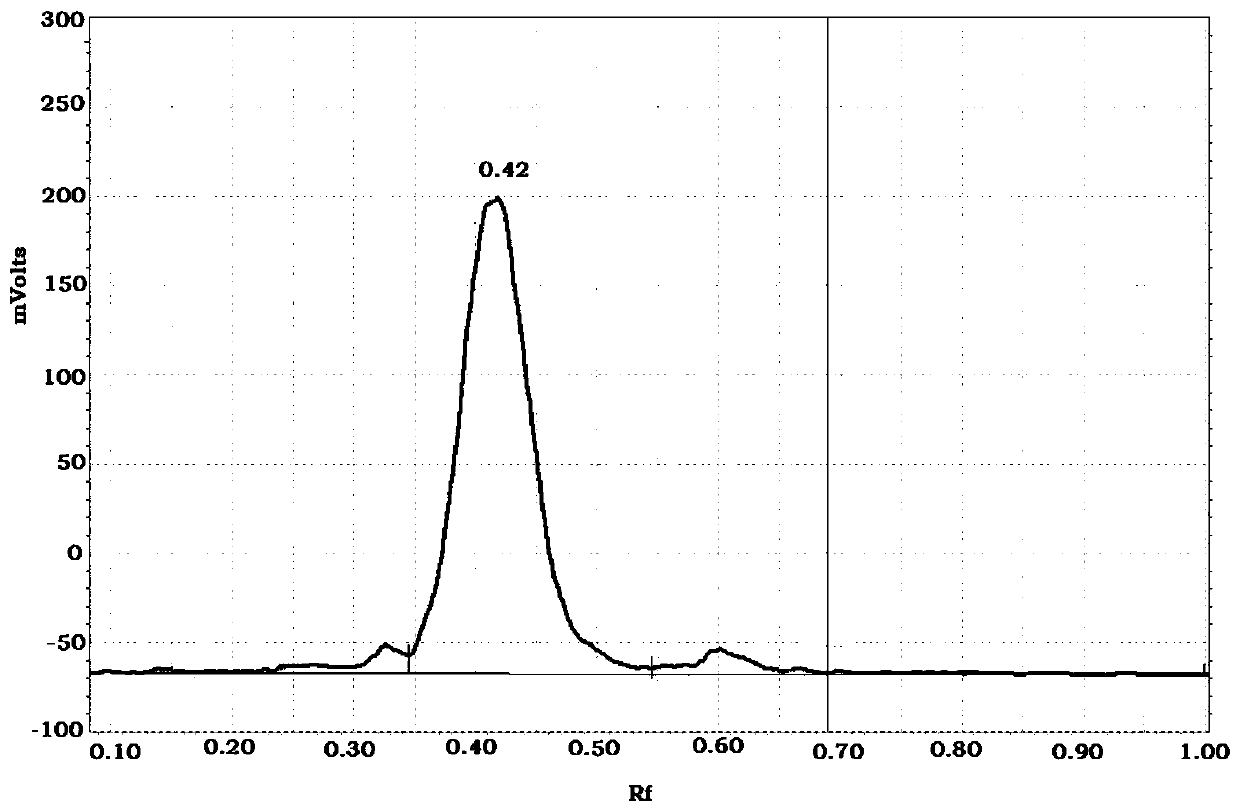
18F-labeled fluoropropionyl ornithine as well as preparation method and application thereof
A technology for fluoropropionylation of ornithine and ornithine, which is applied in the preparation of carboxylic acid amides, chemical instruments and methods, and the preparation of organic compounds, etc., can solve the problems of false negatives and achieve good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] The present invention provides the described above-mentioned technical solution 18 The preparation method of F-labeled fluoropropionylated ornithine comprises the following steps:
[0032] (1) put N 5 -(2-Bromopropionyl)-N 2 -Boc-Ornithine tert-butyl ester, solvent and [ 18 F]F - The source is mixed, and the nucleophilic reaction is carried out to obtain N 5 -(2- 18 F-fluoropropionyl)-N 2 -Boc-tert-butyl ornithine; The [ 18 F]F - source contains [ 18 F]F - and catalysts;
[0033] (2) the N 5 -(2- 18 F-fluoropropionyl)-N 2 -Boc-ornithine tert-butyl ester is mixed with hydrochloric acid for hydrolysis reaction to obtain 18 F-labeled fluoropropionylated ornithine.
[0034] In the present invention, unless otherwise specified, the raw materials and reagents used are commercially available products well known to those skilled in the art.
[0035] The present invention will N 5 -(2-Bromopropionyl)-N 2 -Boc-Ornithine tert-butyl ester, solvent and [ 18 F]F -...
Embodiment 1
[0058] Preparation of N 5 -(2-[ 18 F]-fluoropropionyl) ornithine, wherein the reaction formulas of acylation reaction, nucleophilic reaction and hydrolysis reaction are shown in formula a, formula b and formula c respectively:
[0059]
[0060] Will N 2 -Boc-L-ornithine tert-butyl ester (1g, 3.473mmol), triethylamine (6.946mmol) and anhydrous dichloromethane (20mL) were mixed, stirred evenly under the condition of ice-water bath (0°C), and slowly Add 2-bromopropionyl bromide (0.25mL, 2.315mmol) dropwise to the reaction system, stir thoroughly for 30min, then return to room temperature (23°C), continue stirring for 20h for acylation reaction, TLC detects that the reaction is complete, then filter, and add to the obtained Add 50mL water to the filtrate, use ethyl acetate (3×50mL) to extract, combine the organic phase, anhydrous Na 2 SO 4 After drying, the solvent was evaporated to obtain 0.51 g of yellow powdery solid, namely N 5 -(2-Bromopropionyl)-N 2 -Boc-ornithine t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com