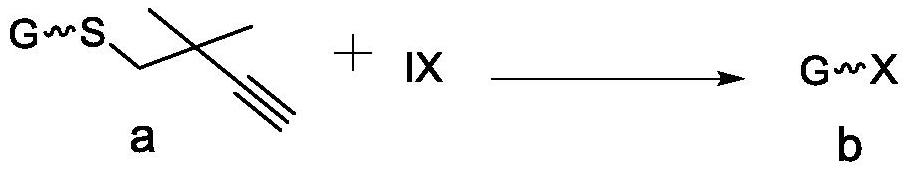A kind of method for preparing halogenated sugar under mild conditions
A technology of halogenated sugar and conditions, which is applied in chemical instruments and methods, preparation of sugar derivatives, sugar derivatives, etc., to achieve the effects of wide application range, mild reaction conditions and thorough reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020]
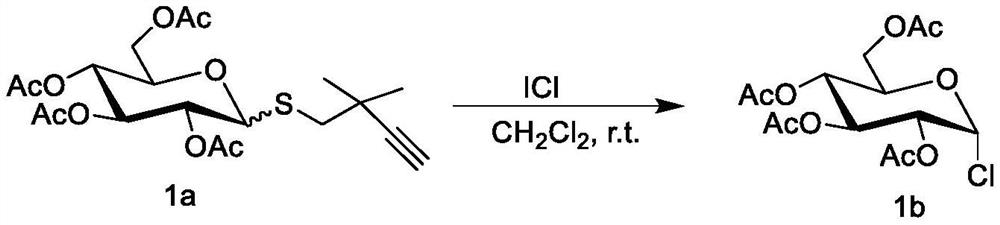
[0021] 32.80 mg (0.074 mmol) of the peracetylated glucosinolate donor represented by formula 1a was azeotroped three times with toluene, and added Molecular sieve, add 0.7 mL of dichloromethane under argon atmosphere, then add 90 μL of 1 mol / L iodine chloride solution in dichloromethane, stir at room temperature for 2 h, after the reaction is completed, filter, remove the organic solvent by vacuum distillation, the residual The product was purified by column chromatography (eluent is a mixture of petroleum ether and ethyl acetate in a volume ratio of 3:1) to obtain 23.0 mg of chloroperacetylated glucose represented by formula 1b, with a yield of 85%. The structural characterization data of the obtained product are: 1 H NMR (400MHz, CDCl 3 )δ6.37(d,J=4.0Hz,1H),5.52(dd,J=3.2,1.2Hz,1H),5.42(dd,J=10.8,3.2Hz,1H),5.25(dd,J=10.8 ,4.0Hz,1H),4.54-4.49(m,1H), 13 C NMR (400MHz, CDCl 3 )δ170.41,170.22,170.01,169.87,91.26,69.44,67.94,67.30,67.18,61.11,20.81,20.78,20.72,20....
Embodiment 2
[0023]
[0024] 41.71 mg (0.094 mmol) of the peracetylated glucosinolate donor represented by formula 1a was azeotroped three times with toluene, and added Molecular sieve, add 0.9 mL of dichloromethane under argon atmosphere, then add 110 μL of 1 mol / L iodine bromide solution in dichloromethane, stir at room temperature for 1 h, after the reaction, filter, remove the organic solvent by vacuum distillation, the residual The product was purified by column chromatography (the eluent was a mixture of petroleum ether and ethyl acetate in a volume ratio of 3:1) to obtain 33.5 mg of bromoperacetylated glucose represented by formula 2b with a yield of 78%. The structural characterization data of the obtained product are: 1 H NMR (400MHz, CDCl 3 )δ6.61(d,J=4.0Hz,1H),5.55(t,J=9.6Hz,1H), 5.16(t,J=9.6Hz,1H),4.83(dd,J=10.0,4.0Hz, 1H), 4.35-4.26(m, 2H), 4.12(d, J=10.8 Hz, 1H), 2.10(s, 3H), 2.09(s, 3H), 2.05(s, 3H), 2.03(s, 3H) ; 13 C NMR (400MHz, CDCl 3 )δ 170.57, 169.91, 169.85, ...
Embodiment 3
[0026]
[0027] 39.25 mg (0.088 mmol) of the peracetylated glucosinolate donor represented by formula 1a was azeotroped three times with toluene, and added Molecular sieves, 0.9 mL of dichloromethane was added under an argon atmosphere, and then 27.9 mg (0.11 mmol) of iodine was added, and the reaction was stirred at room temperature for 2 h. After the reaction was completed, it was filtered, and the organic solvent was removed by distillation under reduced pressure. The residue was passed through the column layer. Analysis and purification (the eluent is a mixture of petroleum ether and ethyl acetate in a volume ratio of 3:1) to obtain 37.1 mg of iodo peracetylated glucose represented by formula 3b, with a yield of 92%. The structural characterization data of the obtained product are: 1 H NMR (400MHz, CDCl 3 )δ6.99(d,J=4.4Hz,1H),5.46(t,J=9.6Hz,1H), 5.21-5.15(m,1H),4.34(dd,J=12.8,4.0Hz,1H), 4.20(dd, J=10.0, 4.4Hz, 1H), 4.11(dd, J=12.4, 2.0Hz, 1H), 4.05(ddd, J=10.4, 3.6, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com