Oxygen carrier for chemical-looping combustion and cracking and preparation method and application thereof
A technology of chemical chain combustion and oxygen carrier, applied in chemical instruments and methods, combustion methods, carbon preparation/purification, etc. Ease of industrial production, improved stability, environmentally friendly effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
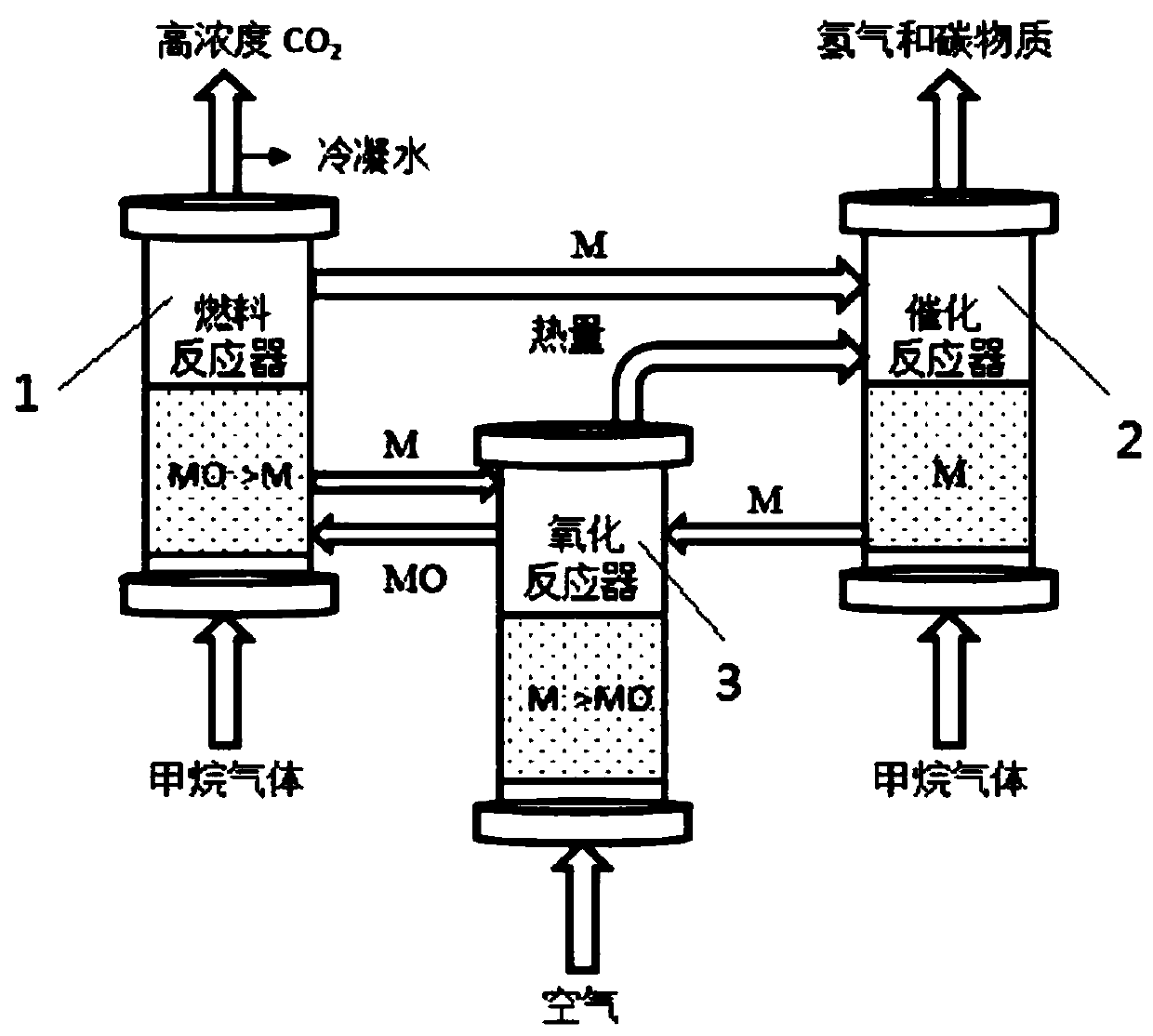
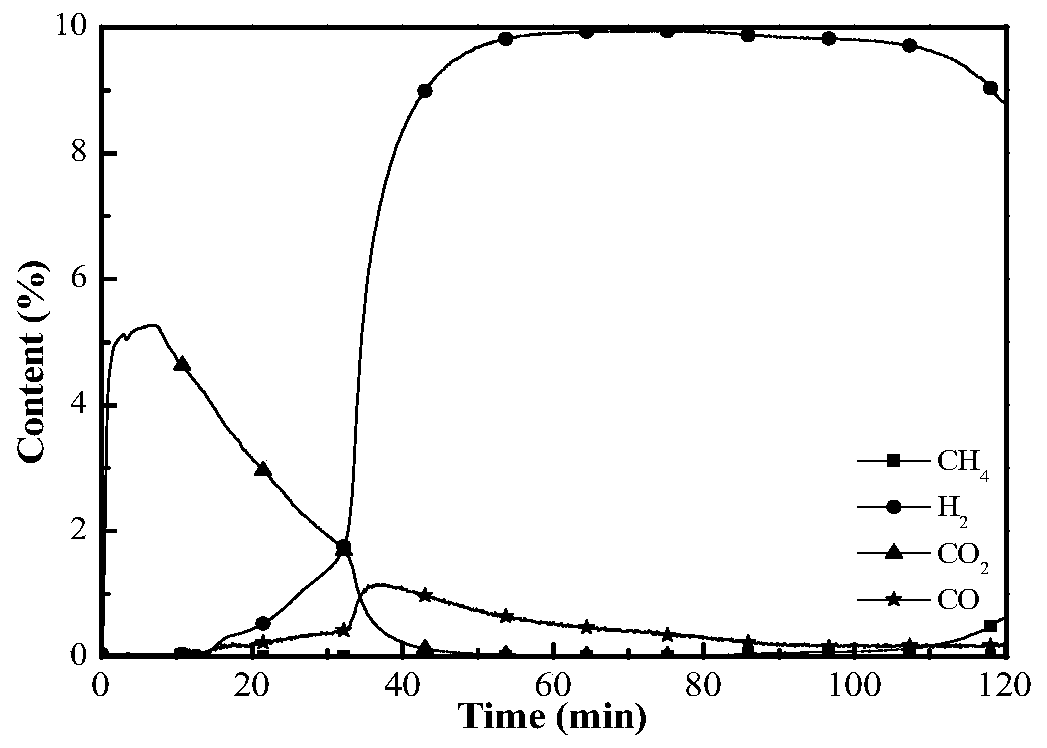
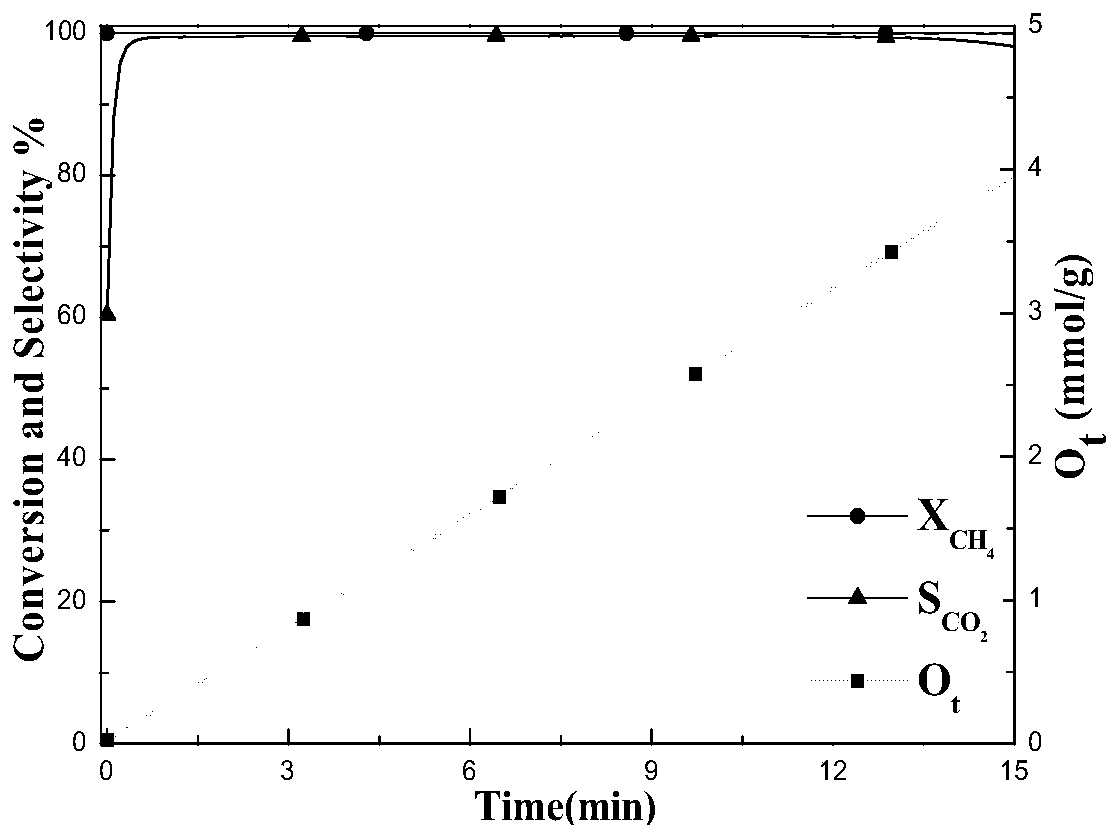
Image
Examples
preparation example Construction
[0037] The preparation method of the above-mentioned oxygen carrier is one of preparation methods such as co-precipitation method, sol-gel method, combustion method and impregnation method.
[0038] Specifically, the oxygen carrier is prepared by the co-precipitation method: measure a certain amount of deionized water, heat it to 60°C, add the precursors of La / Ba, Fe, Al, and Ni in a certain proportion and order to prepare a uniformly mixed solution . Add the homogeneously mixed precursor solution to the saturated ammonium carbonate solution (saturated ammonium carbonate is excessive, 1.5 times the amount of substances required for precipitation of lanthanum / barium, iron, aluminum, nickel), add ammonia water to adjust the pH to 8-11 , stirred evenly at 60°C for 6h, filtered the precipitate and dried it, then calcined at 400-500°C for 2-5h, then calcined at 600-1000°C for 2-5h, and naturally cooled to room temperature to obtain NiO-AFe x Al 12-x o 19 (A = La, Ba) carrier.
...
Embodiment 1
[0049] NiO-BaFe 2 al 10 o 19 Composite oxygen carrier, the molar ratio of nickel to iron is 5:1.
[0050] Measure a certain amount of deionized water, heat it to 60°C, add barium nitrate, iron nitrate, aluminum nitrate and nickel nitrate in a molar ratio of 1:2:10:10, and prepare a uniformly mixed nitrate solution, barium nitrate, nitric acid The concentrations of iron, aluminum nitrate and nickel nitrate are all 1mol / L. Add the uniformly mixed nitrate solution to 1mol / L ammonium carbonate solution (excess saturated ammonium carbonate, which is 1.5 times the content required for the precipitation of barium, iron, aluminum, nickel), add ammonia water to adjust the pH to 10, at 60°C Stir evenly for 6 hours, filter the precipitate, and dry the precipitate, firstly calcining at a heating rate of 2°C / min from room temperature to 500°C for 4 hours, then heating at a rate of 2°C / min to 700°C for 4 hours, and then cooling down to room temperature naturally , to get NiO-BaFe 2 al 1...
Embodiment 2
[0052] Measure a certain amount of deionized water, heat it to 60°C, add lanthanum nitrate, ferric chloride, aluminum chloride and nickel chloride in a molar ratio of 1:4:8:8, and prepare a well-mixed salt solution. Lanthanum nitrate The concentrations of , ferric chloride, aluminum chloride and nickel chloride are all 1mol / L. Add the well-mixed salt solution to 0.9mol / L ammonium carbonate solution (saturated ammonium carbonate is excessive, which is 1.5 times the content required for the precipitation of lanthanum, iron, aluminum, and nickel), add ammonia water to adjust the pH to 8, at 60°C Stir evenly for 6 hours, filter the precipitate, and dry the precipitate, firstly calcining at a heating rate of 2°C / min from room temperature to 400°C for 5 hours, then heating at a heating rate of 2°C / min to 800°C for 2 hours, and then cooling down to room temperature naturally , to obtain oxygen carriers.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com