Preparation method of avibactam intermediate compound
A technology for intermediates and compounds, applied in the field of avibactam intermediate compounds and preparation, can solve the problems of expensive raw materials, cumbersome post-processing, impact on product quality and the like, and achieves simple process operation, high product purity, and easy raw materials. the effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
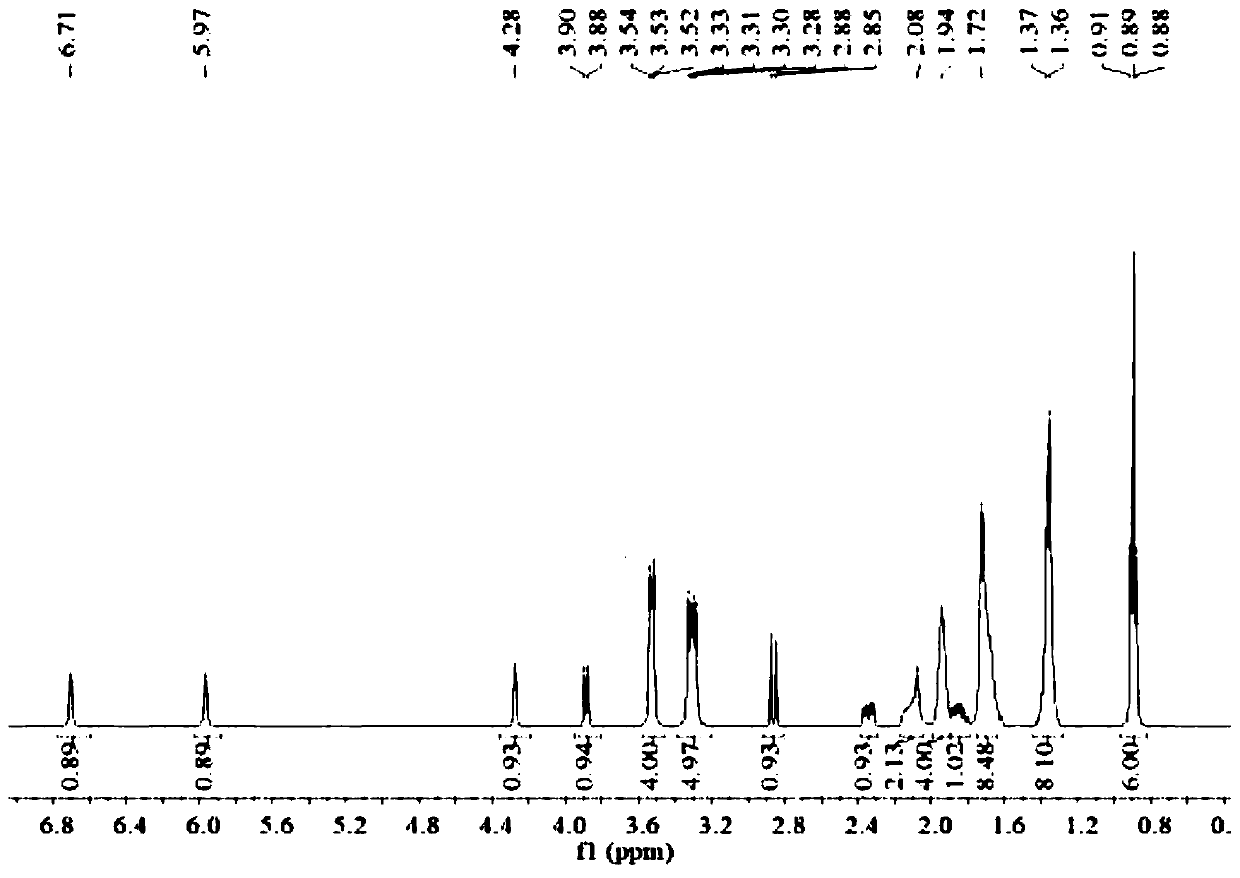
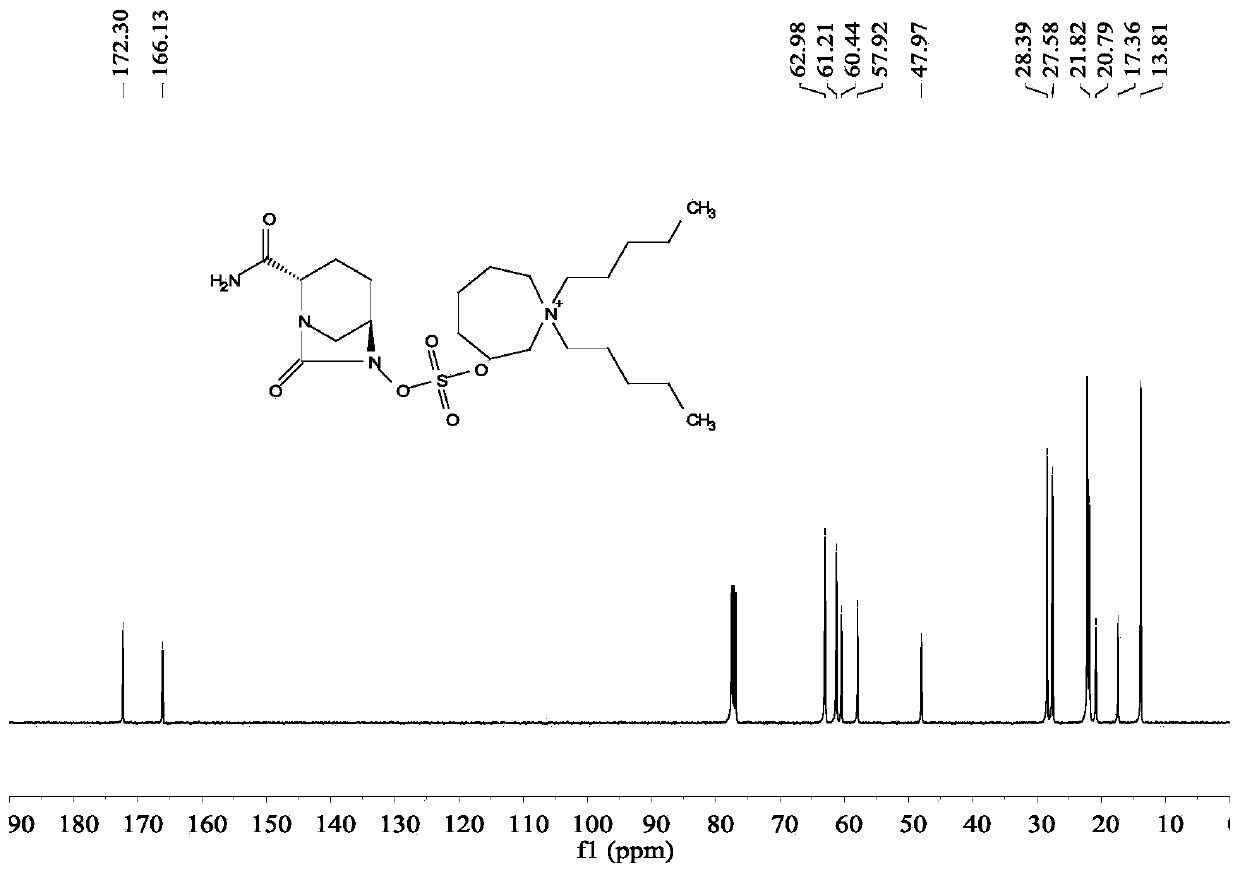
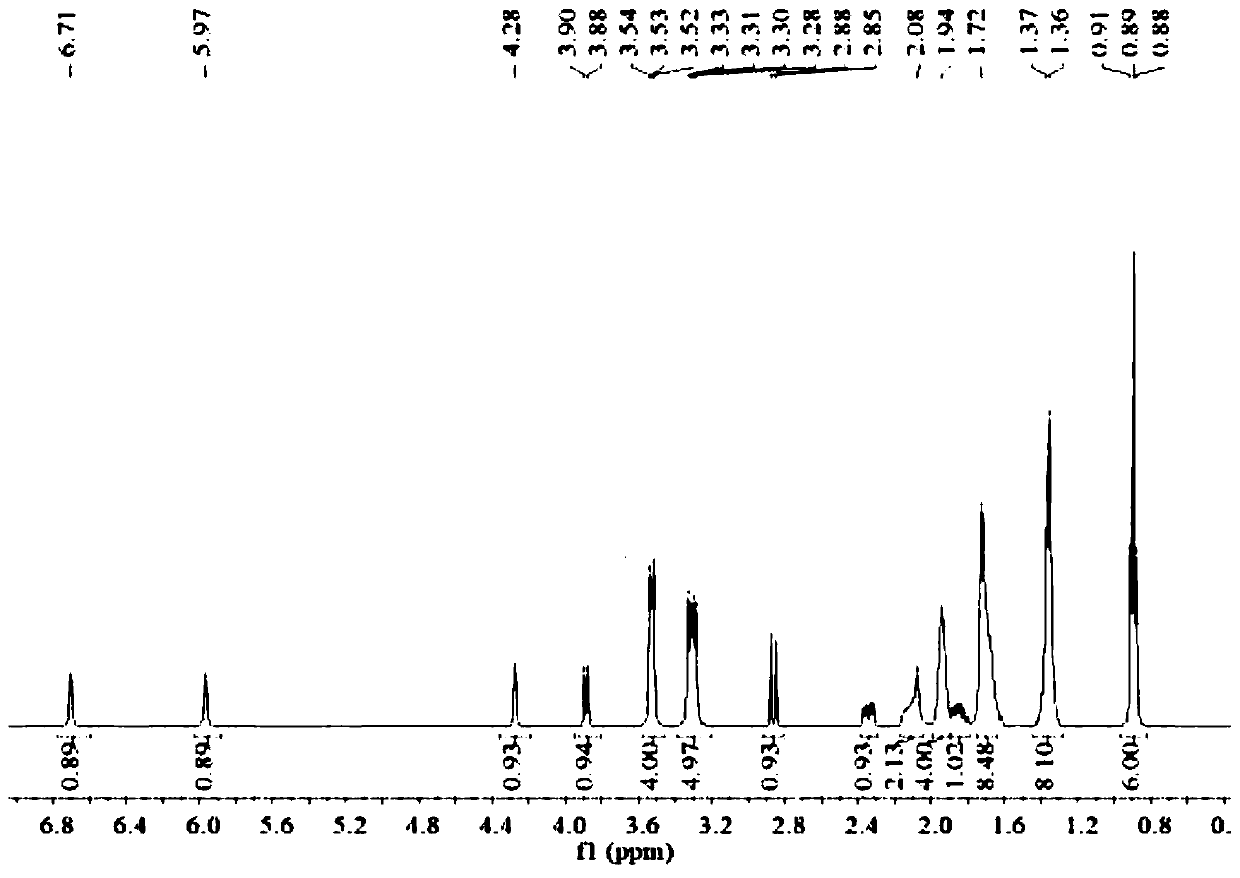
[0025] (2S,5R)-6-(Benzyloxy)-7-oxo-1,6-diazabicyclo[3.2.1]octane-2-carboxamide (5g, 18.2mmol), palladium Carbon (125mg, 10% palladium), sulfur trioxide trimethylamine complex (2.78g, 1.12eq), triethylamine (0.5ml, 0.2eq), isopropanol (25ml) and water (25ml) were sequentially added to 100ml for reaction In the bottle, hydrogen gas was introduced under the condition of stirring, and the reaction was completed at room temperature. Remove palladium carbon by suction filtration and wash the filter cake with water, wash the filtrate once with 25ml ethyl acetate (25ml), then add 1,1-dipentylcyclohexamethylene ammonium bromide (7g, 1.2eq), keep at 40 Under the condition of ℃, react for 2h. Extract with dichloromethane (25ml×2), combine the organic phases and rotary evaporate, then add ethanol and ethyl acetate (3:1) solvent to crystallize at 5°C, filter, wash and dry to obtain 5.8g of avibactam intermediate compound (the step yield was 63%).
[0026] Take by weighing 5g of sulfonic...
Embodiment 2
[0028] (2S,5R)-6-(Benzyloxy)-7-oxo-1,6-diazabicyclo[3.2.1]octane-2-carboxamide (5g,), palladium on carbon ( 125mg, 10% palladium), sulfur trioxide trimethylamine complex (2.78g, 1.12eq), triethylamine (0.5ml, 0.2eq), isopropanol (25ml) and water (25ml) were sequentially added to a 100ml reaction flask , Introduce hydrogen gas under stirring conditions, and react until complete at 30°C. Remove palladium carbon by suction filtration and wash the filter cake with water, wash the filtrate once with 25ml ethyl acetate, then add 80% 1,1-dipentylcyclohexamethylene ammonium bromide (5.6g), keep the reaction at 40°C 2h. Extract with dichloromethane (25ml×2), then add the remaining 20% of 1,1-dipentylcyclohexamethylene ammonium bromide (1.4g) and react at 40°C for 2h, use di Chloromethane extraction (25ml×2), combined organic phase and rotary evaporation, added ethanol and ethyl acetate solvent to crystallize at 5°C, filtered, washed and dried to obtain 5.56g avibactam intermediate ...
Embodiment 3
[0030] (2S,5R)-6-(Benzyloxy)-7-oxo-1,6-diazabicyclo[3.2.1]octane-2-carboxamide (5g,), palladium on carbon ( 125mg, 10% palladium), sulfur trioxide trimethylamine complex (2.78g, 1.12eq), triethylamine (0.5ml, 0.2eq), isopropanol (25ml) and water (25ml) were sequentially added to a 100ml reaction flask , Introduce hydrogen gas under stirring conditions, and react until complete at 30°C. Remove the palladium carbon by suction filtration and wash the filter cake with water, wash the filtrate once with 25ml ethyl acetate, then add 1,1-dibutylcyclohexamethylene ammonium bromide (8.7g, 1.5eq), keep the reaction at 40°C for 2h . Extract with dichloromethane (25ml * 2), combine the organic phase rotary evaporation, add ethanol and ethyl acetate solvent and crystallize under the condition of 5 ℃, then filter, wash and dry to obtain 5.67g avibactam intermediate compound (yield 62%).
[0031] Weigh 5 g of quaternary ammonium salt and dissolve it in ethanol (25 ml), add dropwise the et...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com