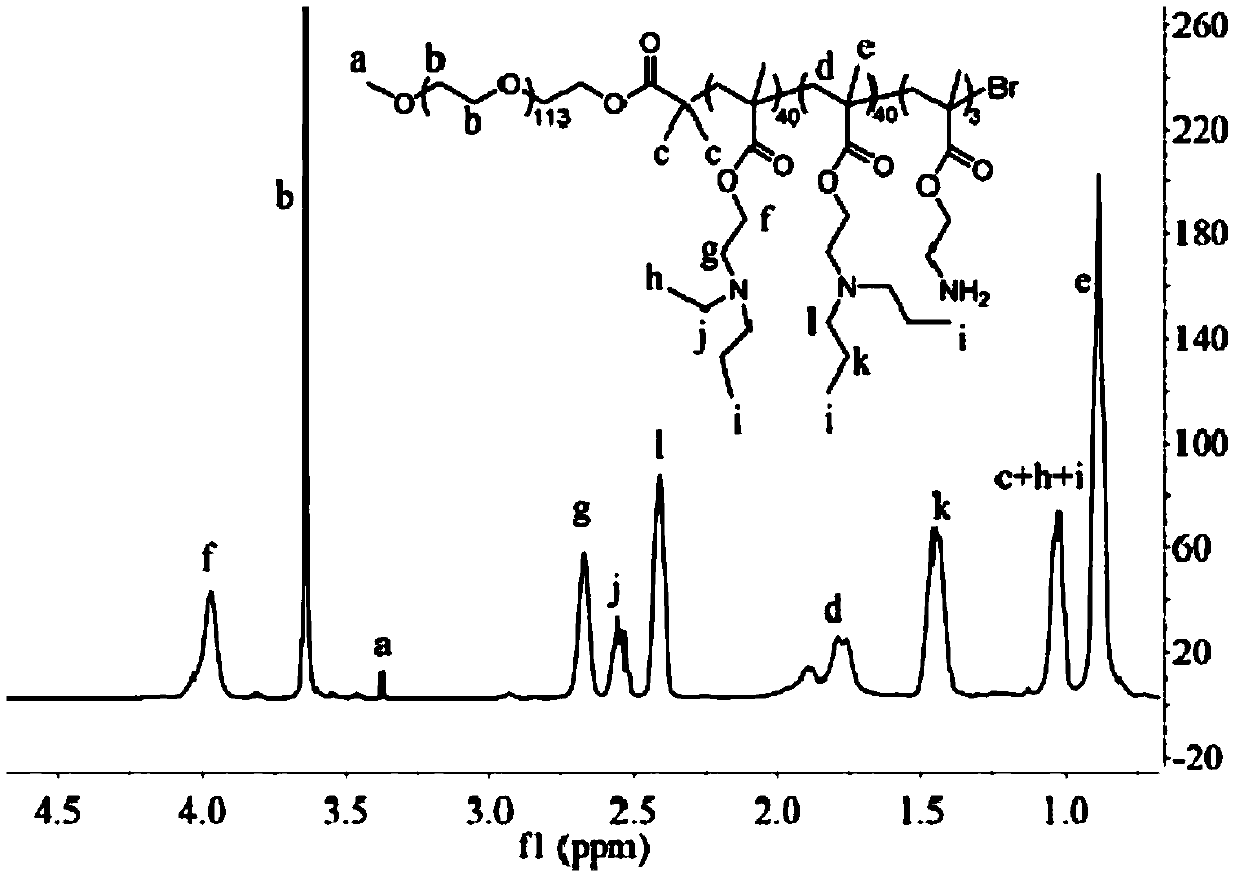
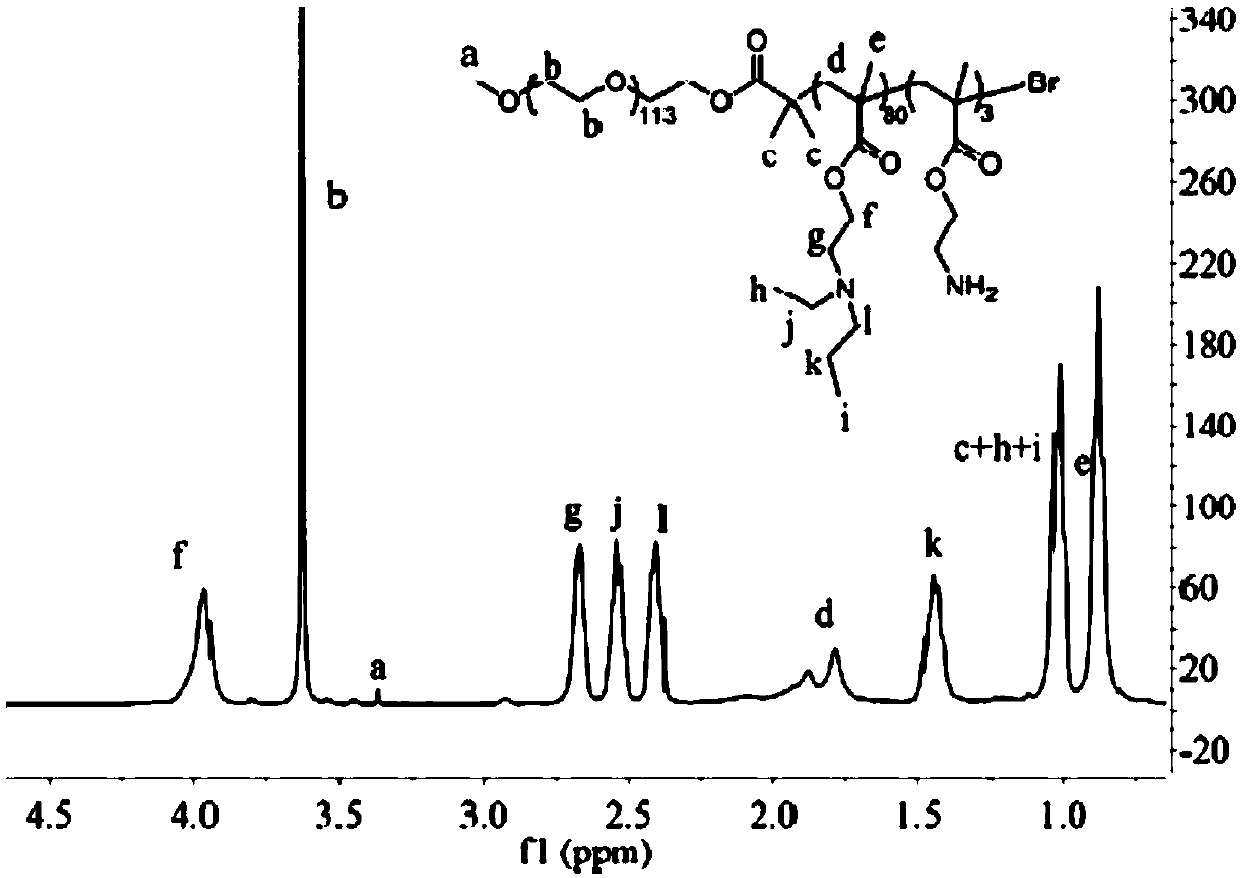
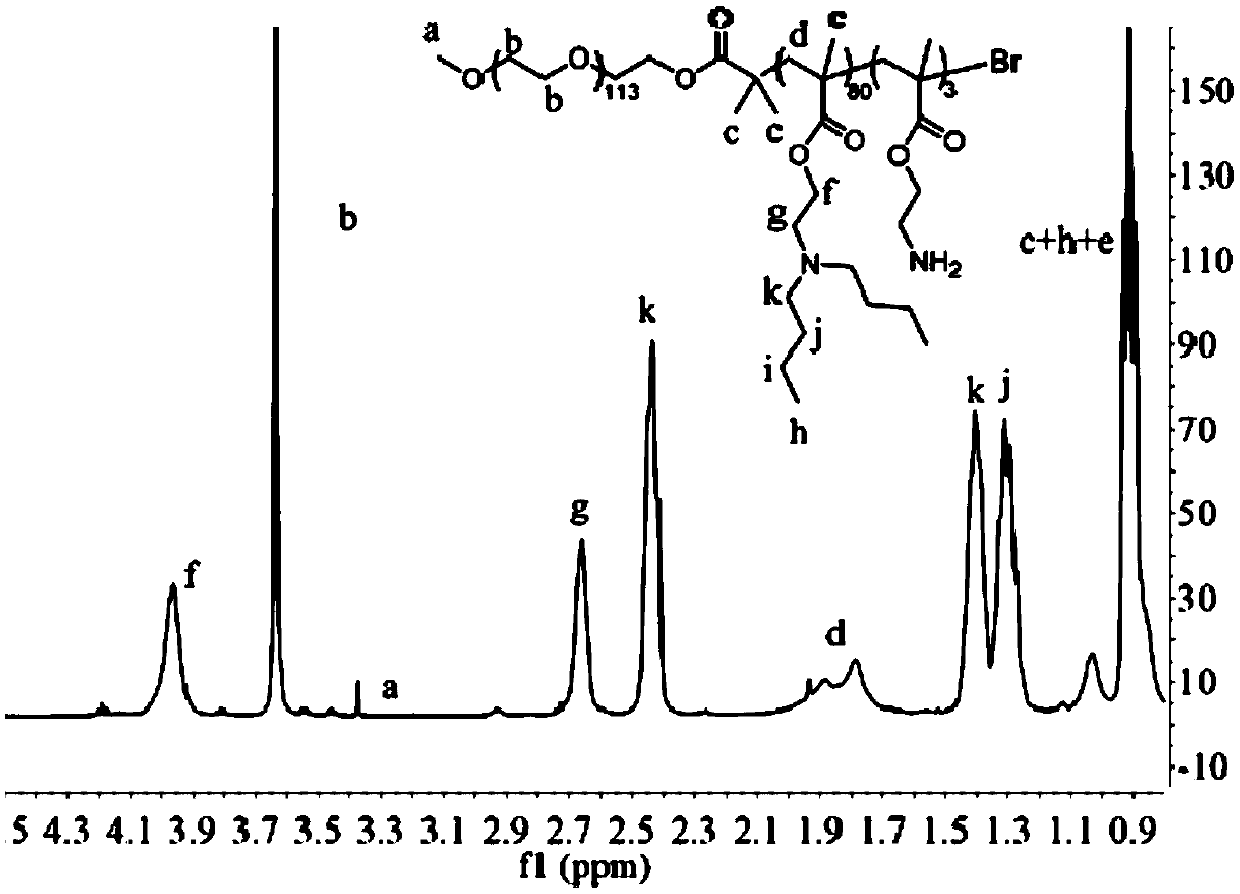
PH-sensitive conjugate, micelle, and preparation method and application thereof
A conjugate and sensitive technology, applied in drug combination, sensory disease, drug delivery, etc., can solve the problems of poor response to disease signals and low activation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0122] As the preparation method of the pH-sensitive conjugated micelles of the present invention, common formulation preparation methods in the field can be used, such as thin-film ultrasonic method, reverse evaporation method, ethanol injection method, desolventization method, etc., preferably desolventization method.
[0123] For example, the preparation method of the pH-sensitive conjugate micelles of the present invention comprises the following steps: 1) adding the pH-sensitive conjugates respectively linked with various marker molecules to an organic solvent to dissolve completely; 2) under the condition of ultrasonic probe, Quickly add the solution obtained in step 1 into ultrapure water and perform ultrasonication; 3) Ultrafiltration or dialysis with ultrapure water to remove the organic solvent; 4) Concentrate the micelles and discard the precipitated insoluble part to obtain the micelles.
[0124] In particular, the present invention provides micelles comprising conj...
Embodiment
[0147] The present invention is illustrated and explained through the following specific synthesis examples and examples, but the present invention is not limited by these specific examples.
[0148] In this document, unless otherwise stated, each symbol stands for the following meanings:
[0149] PEG 5k -OH:
[0150] PEG 5k -Br:
[0151] EPA: The monomer structure is
[0152] DPA: The monomer structure is
[0153] iDPA: The monomer structure is
[0154] DBA: The monomer structure is
[0155] AMA: The monomer structure is for ligation of marker molecules
[0156] QSY21-NHS: A succinimide-functionalized fluorescence quencher
[0157]
[0158] BHQ3-NHS: a succinimide-functionalized fluorescence quencher
[0159]
[0160] ICG-NHS: a succinimide-functionalized photothermal probe
[0161]
[0162] MTT: Cell Viability Assay Reagent 3-(4,5-Dimethylthiazole-2)-2,5-Diphenyltetrazolium
Synthetic example 1
[0163] Synthesis Example 1: Synthesis of Polyethylene Glycol Macroinitiator
[0164] Precisely weigh PEG 5k -OH (20g, 4mmol) was dissolved in a round-bottomed flask with 250mL of dichloromethane, and then 5 equivalents of triethylamine and 4-dimethylaminopyridine were added to the reaction solution. Stir and sonicate to dissolve. Add 5 times equivalent of 2-bromoisobutyryl bromide at low temperature, and react at room temperature for 24 hours. After the reaction was completed, the reaction solution was concentrated to about 50 mL with a rotary evaporator. The reaction solution was transferred to a separatory funnel, washed 3 times with 10% sodium bicarbonate solution, and then washed 3 times with saturated sodium chloride solution. The organic phase was added dropwise to glacial ether, and a precipitate was precipitated. The solid was collected by suction filtration and dried in vacuo. Repeat the ether precipitation operation 3 times to obtain 16.3g of macromolecular init...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com