Synthetic method of dapoxetine and intermediate thereof
A synthesis method and compound technology, applied in chemical instruments and methods, preparation of organic compounds, preparation of amino hydroxyl compounds, etc., can solve problems such as expensive catalysts, and achieve green and environmentally friendly synthesis processes, low production costs, and cheap and easy-to-obtain raw materials Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0089] Add 200mL of water and 100mL of methanol into a single-mouth 500mL round-bottom flask, add 0.2mol / 32.83g of sodium benzenesulfinate, 0.1mol / 11.72g of tert-butyl carbamate, 0.12mol / 12.72g of benzaldehyde, 70mL of formic acid, Stir well at room temperature 25°C for 3 days to form an emulsion. Use a sand core funnel to filter under reduced pressure, fully stir and wash with 30 mL of distilled water for 3 times, fully stir and wash with 30 mL of diethyl ether for 2 times, fully stir and wash with 30 mL of n-hexane once, and dry naturally to obtain 32.3 g of white powdery solid compound 2 with a yield of 93%. .
[0090] The reaction formula is as follows:
[0091]
Embodiment 2
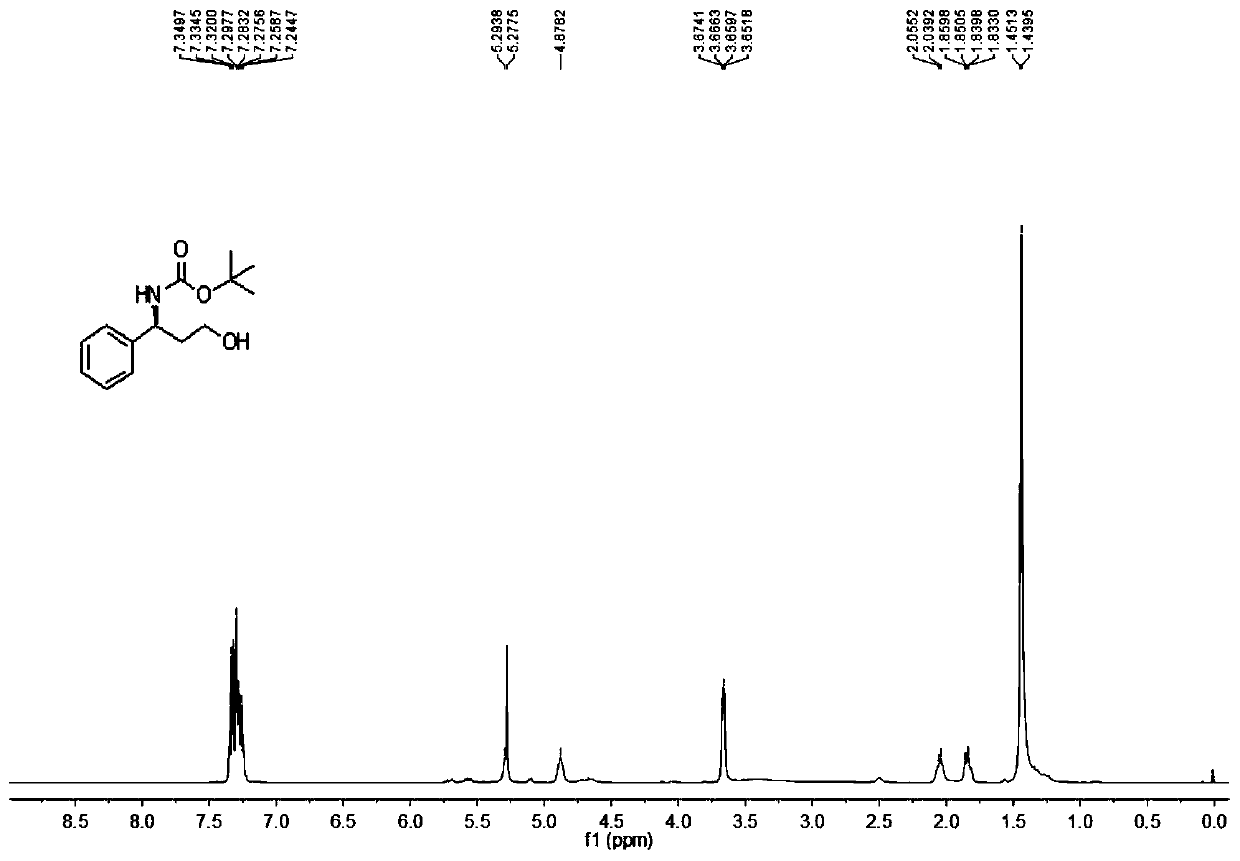
[0093] Add 20mmol / 6.95g of the product of Example 1 into a 500mL round-bottomed flask, stir and dissolve with 160mL of dichloromethane, weigh 62g of potassium carbonate and dissolve it in 160mL of water and stir until it becomes clear, then pour the potassium carbonate aqueous solution into the round-bottomed flask , fully stirred at room temperature for 5 hours, extracted with a 500mL separatory funnel to obtain the organic phase, then extracted twice with 80mL dichloromethane, combined the organic phases, dried with anhydrous sodium sulfate, and obtained 3.78g colorless oil Liquid compound 3, yield 92%.
[0094] The reaction formula is as follows:
[0095]
Embodiment 3
[0097] Preparation of acetaldehyde: Add paraldehyde into the flask, add concentrated sulfuric acid dropwise, distill at normal pressure, control the temperature at 55-60°C, connect the condensing pipe to a low-temperature constant temperature reaction bath, and lower the temperature of the cooling system to -10-0°C , and the receiving bottle was cooled in an ice-water bath. The collected acetaldehyde was stored at low temperature. Concentrated sulfuric acid is used as a catalyst, and the catalyst consumption is 0.5% of the mass of paraldehyde.
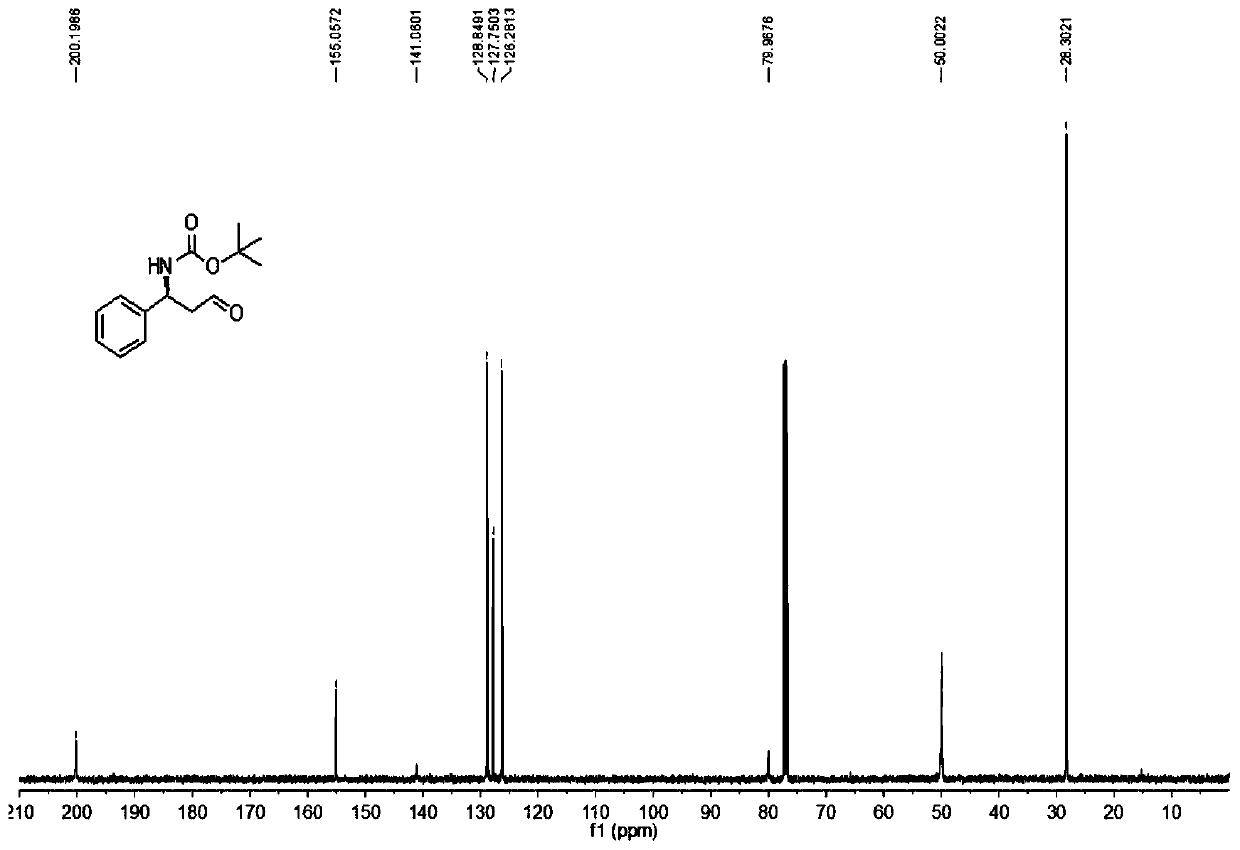
[0098] Add 4mmol / 0.46g of L-proline and 12mmol / 12g of PEG1000 into a 250mL round-bottomed flask, add 30mL of redistilled acetonitrile, stir at room temperature for 15min, and then transfer to 20mmol / 4. In 10g of acetonitrile (50mL) solution, stir at 0°C, then dissolve redistilled acetaldehyde in 70mL of acetonitrile to form a solution and drop it into the reaction flask, after 3.5h, analyze by high-performance liquid chromatography w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com