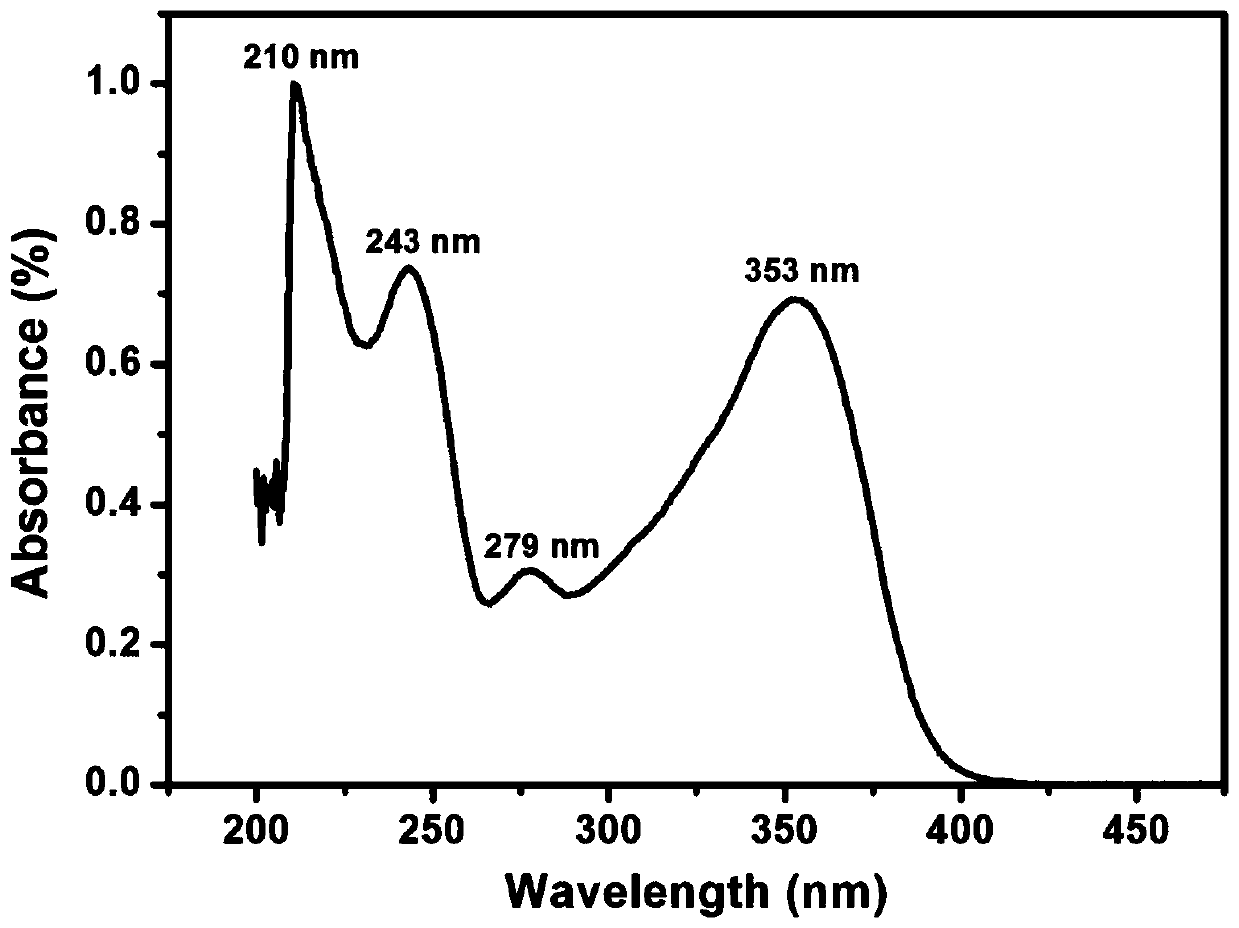
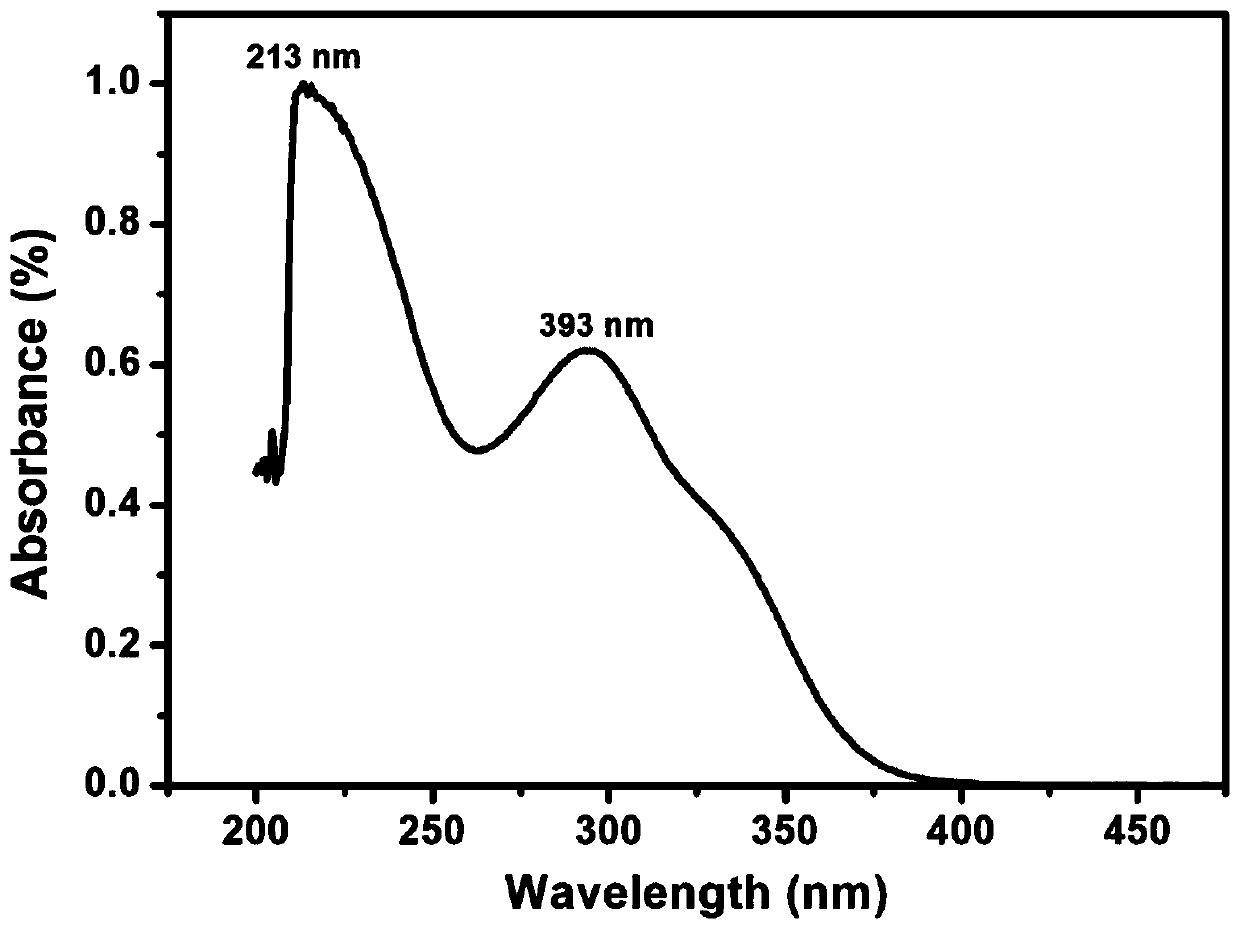
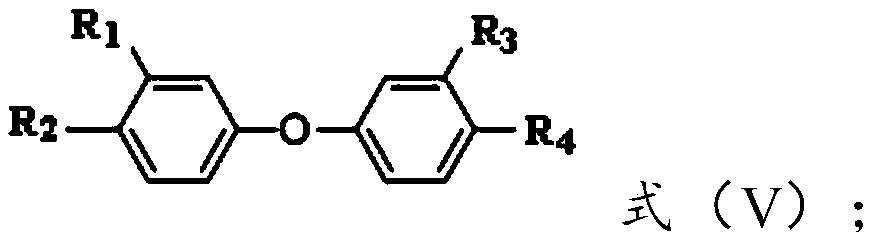
Aromatic diamine monomer and preparation method thereof
A diamine monomer and aromatic technology, applied in the field of aromatic diamine monomer and its preparation, can solve the problems of large specific surface area of nanoparticles, poor dispersion of nanoparticles, poor heat resistance, etc., and achieve process controllability High, simple route, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0049] The present invention also provides a method for preparing the aromatic diamine monomer described in the above technical solution, comprising the following steps:
[0050] a) carry out etherification reaction with methoxyphenol and the substituted nitrobenzene of structure shown in formula (I) in the presence of basic catalyst, obtain the compound with structure shown in formula (II); Described methoxyphenol is 3-methoxyphenol or 4-methoxyphenol;
[0051]
[0052] In formula (I), X is fluorine, chlorine, bromine, iodine, methanesulfonyloxy, trifluoromethanesulfonyloxy or p-toluenesulfonyloxy, R 7 and R 8 are independently selected from hydrogen or nitro, and R 7 with R 8 different;
[0053]
[0054] In formula (II), R 9 and R 10 are independently selected from hydrogen or methoxy, and R 9 with R 10 different;
[0055] b) Carrying out a Friedel-Crafts acylation reaction between a compound having a structure shown in formula (II) and a nitrobenzoyl halide i...
Embodiment 1
[0135] (1) Add 69.52g (0.56mol) of 3-methoxyphenol, 88.23g (0.56mol) of p-nitrochlorobenzene, 85.14g (0.616mol) of potassium carbonate and 200g of dimethyl sulfoxide into the reactor in sequence , heated to 160°C and reacted for 6h; cooled to 60°C, added to 2000mL water, precipitated crude product, filtered out, dissolved in dichloromethane after washing, dried over anhydrous magnesium sulfate, concentrated solvent, obtained crude product again, and re- Crystallization obtained 114.23 g of the refined product of compound (II-1) represented by formula (II); the yield was 83.2%.
[0136] Utilize nuclear magnetic resonance to characterize the structural compound (II-1) shown in the formula (II) that obtains, the proton nuclear magnetic resonance spectrum result that obtains is: 1 H NMR (400MHz, DMSO) δ = 8.280–8.205 (m, 2H), 7.389 (t, J = 8.2Hz, 1H), 7.170–7.095 (m, 2H), 6.875 (dd, J = 8.3Hz, 2.2Hz , 1H), 6.776(t, J=2.2Hz, 1H), 6.731(dd, J=8.0Hz, 2.0Hz, 1H), 3.765(s, 3H).
[01...
Embodiment 2
[0144] (1) Refer to step (1) of Example 1 to obtain the refined product of compound (II-1) shown in formula (II).
[0145] (2) 18.56g (0.1mol) 3-nitrobenzoyl chloride, 14.67g (0.11mol) aluminum trichloride, 250g 1,2-dichloroethane and 22.07g (0.09mol) formula (II) Compounds (II-1) showing the structure were sequentially added into the reactor, stirred and reacted at 20°C for 30 h; then slowly added to ice-hydrochloric acid for treatment, separated, dried over anhydrous magnesium sulfate, and concentrated solvent to obtain a crude product, which was subjected to Recrystallization obtained 21.02 g of refined product of compound (III-3) represented by formula (III); the yield was 59.2%.
[0146] Utilize nuclear magnetic resonance to characterize the structural compound (III-3) shown in the formula (III) that obtains, the proton nuclear magnetic resonance spectrum result that obtains is: 1 HNMR (400MHz, DMSO) δ=8.490(d, J=8.0Hz, 1H), 8.425(s, 1H), 8.312(d, J=9.1Hz, 2H), 8.129(d, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Tensile strength | aaaaa | aaaaa |
| Tensile strength | aaaaa | aaaaa |
| Tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com