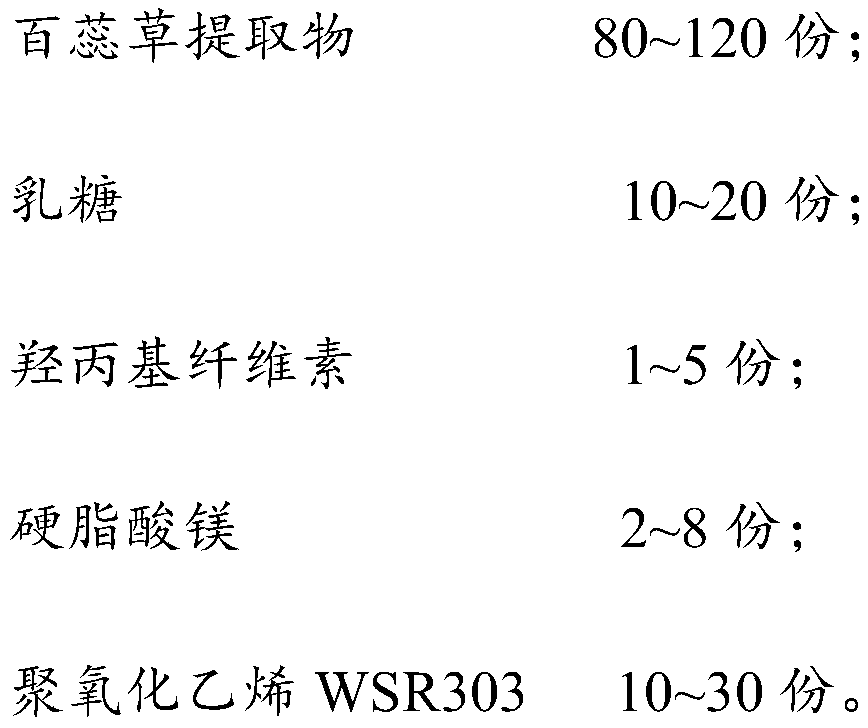
Thesium chinense tablet and preparation method and application thereof
A technology of Bairui tablet and Bairui grass, applied in the field of Bairui tablet and its preparation, can solve the problems of low in vitro dissolution rate, low bioavailability, large difference in tablet weight and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0023] Experimental Example 1 Prescription Screening
[0024] 1. Preparation of test samples:
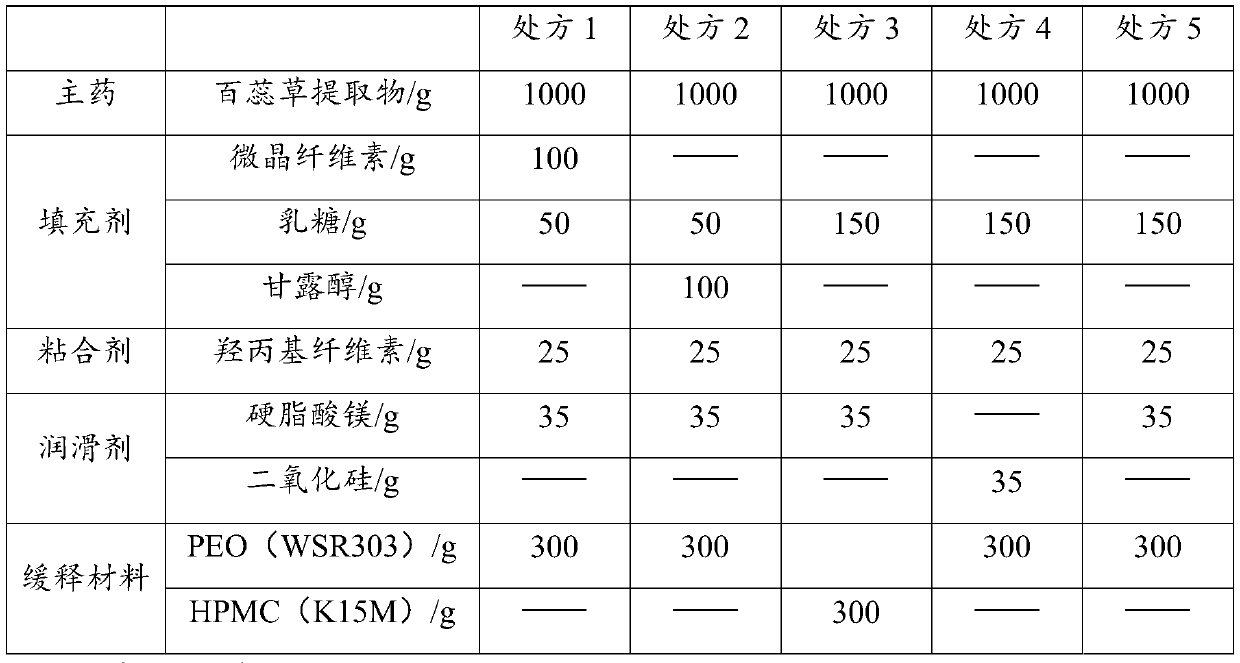
[0025] The main drug of this product is thyme extract, and its active ingredient is flavonoid. For the convenience of patients, the tablet weight is planned to be controlled at 400mg / tablet. The prescription screening experiments are shown in Table 1. The preparation process is as follows: (1) pass the above-mentioned auxiliary materials and main ingredients through 80-mesh and 100-mesh sieves respectively, and set aside; (2) take the adhesive and add an appropriate amount of water, and use a dispersing homogenizer to mix evenly to obtain a mass percentage of 7% (3) the thyme extract (prepared according to the method of the following example 1), filler and lubricant are mixed uniformly by the equal addition method to obtain the mixed material, and the above-mentioned adhesive is added Mixture solution, granulation, drying, and granulation to obtain Bairui granules. (4) Take the s...
experiment example 2
[0038] Experimental Example 2 Stability Investigation
[0039] The tablets prepared by Prescription 1 and Prescription 5 were used as test substances and tested as follows.
[0040] (1) Illumination stability: place the test substance under 25°C and 4500 Lux light conditions to measure the drug stability for 5 days and 10 days, respectively, take samples at the same time on the 5th and 10th day of the experiment period, and use the above-mentioned HPLC method to determine And calculate the 12h cumulative dissolution rate of flavonoids, the results are shown in Table 3, the results show that the tablets prepared by prescription 1 and prescription 5 all have good light stability.
[0041] (2) Stability at high temperature: place the test substance at 60°C to measure the drug stability for 5 days and 10 days respectively, take samples at the same time on the 5th day and the 10th day during the experiment period, use the above HPLC method to measure and calculate the 12h stability...
Embodiment 1
[0046] Composition: 800g of thyme extract, 200g of lactose, 20g of hydroxypropyl cellulose, 50g of magnesium stearate, 100g of PEO (WSR303);
[0047] Preparation method: take Thyme officinalis and add 75% ethanol aqueous solution by volume, extract 3 times, extract for 3 hours each time, combine the extracts, concentrate under reduced pressure to a relative density of 1.15, and then vacuum-dry to obtain Thyme officinalis extract . Pass the above auxiliary materials and main ingredients through 80-mesh and 100-mesh sieves respectively, and set aside; take hydroxypropyl cellulose, add appropriate amount of water, and use a dispersing homogenizer to mix evenly to prepare a binder solution with a mass percentage of 7%, set aside; The thyme extract, lactose and magnesium stearate are uniformly mixed according to an equal delivery method to obtain a mixed material, the above-mentioned binder solution is added, granulated, dried, and sized to obtain the thyme granule. Take PEO (WSR3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com