C2-symmetry-removing diphenylamine-type chiral bisoxazoline ligands as well as synthetic method and application thereof
A diphenylamine-type and bisoxazoline technology, which is applied in the field of de-C2-symmetrical diphenylamine-type chiral bisoxazoline ligands and its synthesis and application, can solve the problem of single electronic effect, and achieve a simple synthesis method , improve enantioselectivity, mild condition effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041]
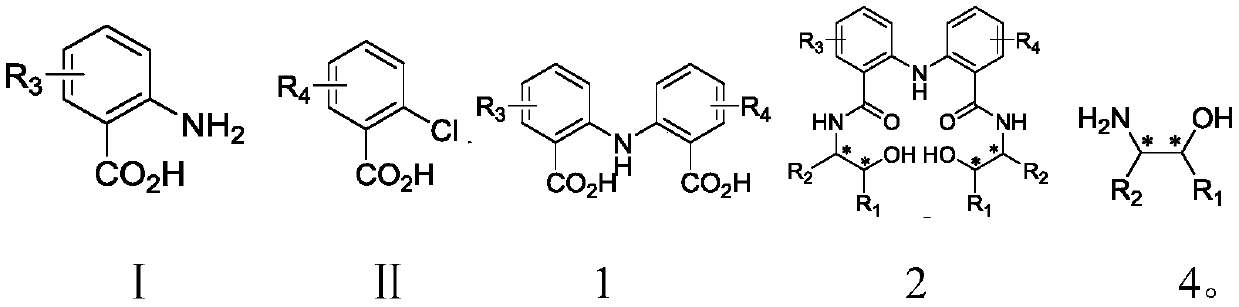
[0042] 5.48g anthranilic acid, 7.44g 2-chloro-5-methoxybenzoic acid, 5.52g K 2 CO 3 , 0.378g copper powder and 1.14g CuI were added to the round bottom flask and mixed, then DMF was added, and placed at reflux temperature to react for 3 hours. After cooling, filter, add dilute hydrochloric acid to the filtrate until the solution is weakly acidic, stir and filter with suction to obtain the solid product 2-((2-carboxyphenyl)amino)-5-methoxybenzoic acid.
[0043] 1.35g (R)-(-)-2-phenylglycinol, 2.20g 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride, 1.55g 1-hydroxy Benzotriazole was dissolved in THF, and 1.44 g of 2-((2-carboxyphenyl)amino)-5-methoxybenzoic acid was added. React at room temperature for 12 hours. Quench the reaction with ammonium chloride solution, remove the solvent under reduced pressure, add ethyl acetate to dissolve, wash with water, and extract three times. The organic phase is dried with sodium sulfate, the solvent is removed, and i...
Embodiment 2
[0047]
[0048] 5.48g anthranilic acid, 6.98g 2-chloro-5-fluorobenzoic acid, 5.52g K 2 CO 3 , 0.378g copper powder and 1.14g CuI were added to the round bottom flask and mixed, then DMF was added, and placed at reflux temperature to react for 3 hours. After cooling, filter, add dilute hydrochloric acid to the filtrate until the solution is weakly acidic, stir and filter with suction to obtain the solid product 2-((2-carboxyphenyl)amino)-5-fluorobenzoic acid.
[0049] 1.35g (R)-(-)-2-phenylglycinol, 2.20g 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride, 1.55g 1-hydroxy Benzotriazole was dissolved in THF, and 1.10 g of 2-((2-carboxyphenyl)amino)-5-fluorobenzoic acid was added. React at room temperature for 12 hours. Quench the reaction with ammonium chloride solution, remove the solvent under reduced pressure, add ethyl acetate to dissolve, wash with water, and extract three times. The organic phase is dried with sodium sulfate, the solvent is removed, and it i...
Embodiment 3
[0053]
[0054] 5.48g anthranilic acid, 9.42g 2-chloro-5-bromobenzoic acid, 5.52g K 2 CO 3 , 0.378g copper powder and 1.14g CuI were added to the round bottom flask and mixed, then DMF was added, and placed at reflux temperature to react for 3 hours. After cooling, filter, add dilute hydrochloric acid to the filtrate until the solution is weakly acidic, stir and filter with suction to obtain the solid product 2-((2-carboxyphenyl)amino)-5-bromobenzoic acid.
[0055] 1.68g 2-((2-carboxyphenyl)amino)-5-bromobenzoic acid and 10mL SOCl 2 Add it to a round-bottomed flask, heat to reflux for 4 hours, and remove excess SOCl by rotary evaporation 2 , Add the diacid chloride obtained from the precipitation into dichloromethane. Under the condition of ice bath, the dichloromethane solution of diacid chloride was added dropwise to the dichloromethane solution containing 1.35g (R)-(-)-2-phenylglycinol and 3.8mL triethylamine. After the dropwise addition was completed, it was stirred...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com