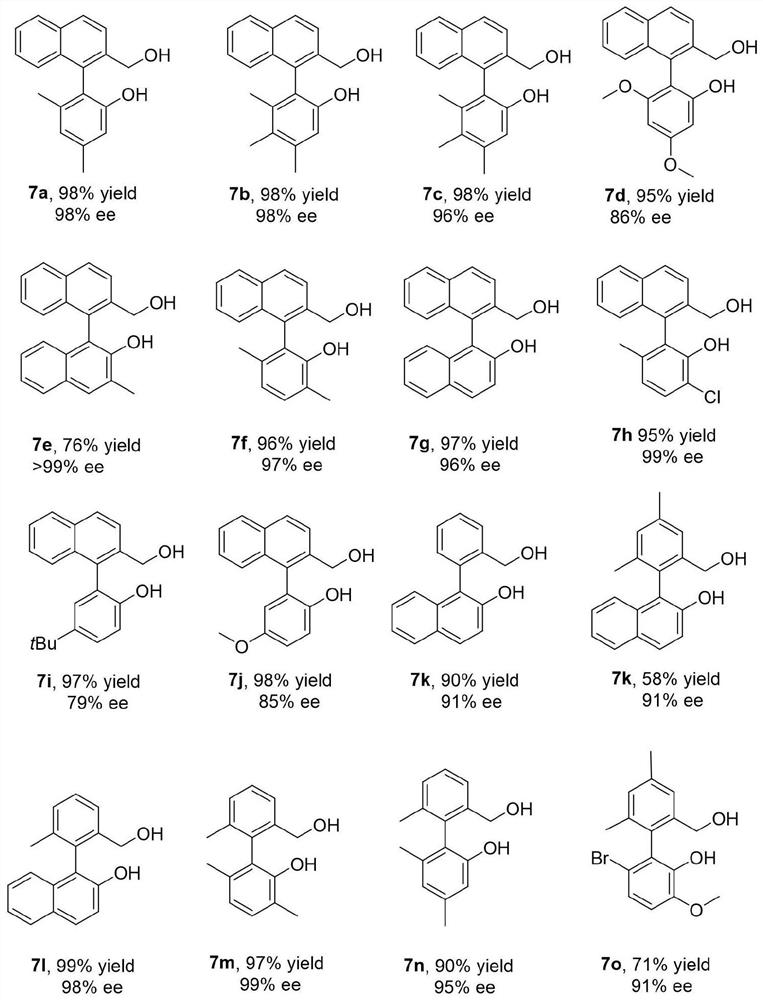
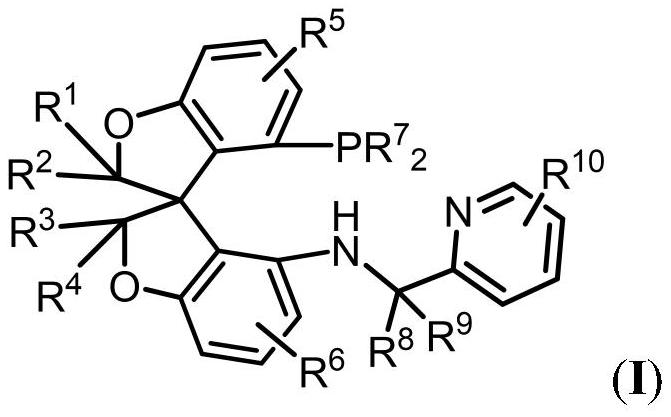
Synthesis and application of a kind of oxaspiro ring pnn type ligand
A synthesis route and reaction technology, which is applied in the field of synthesis of novel oxaspirocyclic bisphosphine ligands, can solve the problems of limited substrate range and loss of activity of the catalytic system, and achieve high enantioselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
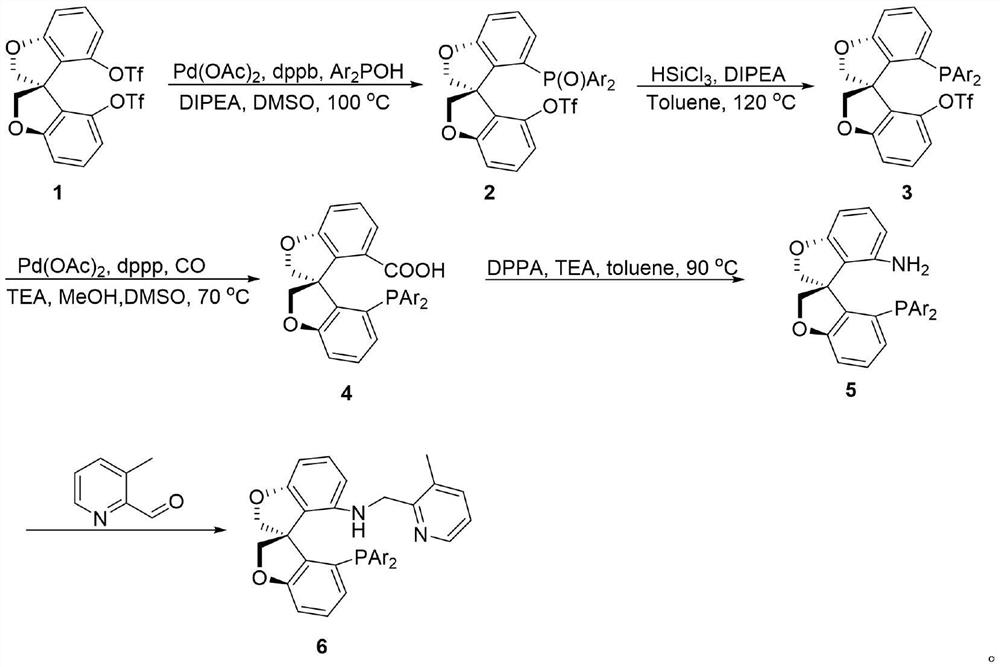
[0029] (S)-4'-(Di-3,5-di-tert-butylphenylphosphinooxy)-2H,2'H-3,3'-spirobis[benzofuran]-4-trifluoromethane Synthesis of sulfonate 2a:
[0030]
[0031] N 2 Under atmosphere, add 1 (5.2g, 10mmol), dppb (213mg, 0.05mmol), Ar 2 POH (6.39g, 15mmol), Pd(PAc) 2 (112mg, 0.05mmol) and DIPEA (6.5mL, 40mmol), and finally 50mL of anhydrous and oxygen-free DMSO was added. Reaction at 100°C for 6h. After being cooled to room temperature, water was added to quench the reaction, and the reaction system was extracted with ethyl acetate. The organic phase was dried with anhydrous sodium sulfate and the solvent was removed under reduced pressure. After simple column chromatography, the product 2a (7.43 g, yield = 93%).
[0032] white solid. 1 H NMR (500MHz, CDCl 3 )δ1.18(s,9H,CH 3 ),1.19(s,9H,CH 3 ),1.20(s,9H,CH 3 ),1.21(s,9H,CH 3 ),4.54-4.56(m,1H,CH 2 ),4.62-4.64(m,1H,CH 2 ),4.80-4.82(m,2H,CH 2 ),6.63-6.68(m,2H,Ar),6.82-6.88(m,6H,Ar),6.90-6.94(m,2H,Ar),7.15-7.21(m,1H,Ar),7.26-...
Embodiment 2
[0034] (S)-4'-(Di-3,5-di-tert-butylphenylphosphino)-2H,2'H-3,3'-spirobis[benzofuran]-4-trifluoromethanesulfonic acid Synthesis of ester 3a:
[0035]
[0036]In a sealed 100 mL tube, 2a (3.98 g, 5 mmol), DIPEA (6.6 mL, 40 mmol), 20 mL of toluene and trichlorosilane (2.0 mL, 20 mmol) were added. The reaction was stirred overnight at 120°C. The reaction system was quenched with excess saturated sodium bicarbonate solution, 100 mL of ethyl acetate was added, filtered through diatomaceous earth, and the organic phase was dried over anhydrous sodium sulfate. The solvent was removed under reduced pressure, followed by column chromatography to obtain white solid 3a (3.51 g, yield = 90%).
[0037] white solid. 1 H NMR (500MHz, CDCl 3 )δ1.17(s,9H,CH 3 ),1.19(s,9H,CH 3 ),1.20(s,9H,CH 3 ),1.21(s,9H,CH 3 ),4.52-4.55(m,1H,CH 2 ),4.60-4.63(m,1H,CH 2 ),4.77-4.82(m,2H,CH 2 ),6.61-6.69(m,2H,Ar),6.80-6.94(m,6H,Ar),7.14-7.22(m,2H,Ar),7.28-7.34(m,2H,Ar). 13 C{1H}NMR (126MHz, CDCl 3...
Embodiment 3
[0039] Preparation of (S)-4-bis-3,5-di-tert-butylphenylphosphoryl-2H,2'H-3,3'-spirodibenzotetrahydrofuran-4-carboxylic acid 4a:
[0040]
[0041] Into a 250 mL reactor was charged palladium acetate (259 mg, 1.16 mmol) and 1,3-bis(diphenylphosphine)propane (478 mg, 1.16 mmol), 3a (5.51 g, 7.0 mmol). The autoclave was then transferred to a glove box, and dimethyl sulfoxide (60 mL) was added followed by methanol (42 mL) and triethylamine (11.6 mL). The reaction kettle was then removed from the glove box, and the pressure of carbon monoxide was increased to 10 atm. Then the reaction kettle was placed on a 70°C oil bath and stirred overnight. After the reaction was completed, the reaction mixture was transferred to a 500mL separatory funnel and 250mL of ethyl acetate was added, then the organic phase was washed twice with saturated brine, the organic phase was dried, and the solvent was removed by column chromatography to obtain the methyl ester product, which was then subjecte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com