Target drug loaded micelle suitable for loading of hydrophobic chemical drugs
A drug-loaded micelle, hydrophobic technology, used in drug combinations, anti-tumor drugs, pharmaceutical formulations, etc., can solve problems such as high cost, complex reaction process, and off-target targeting groups
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
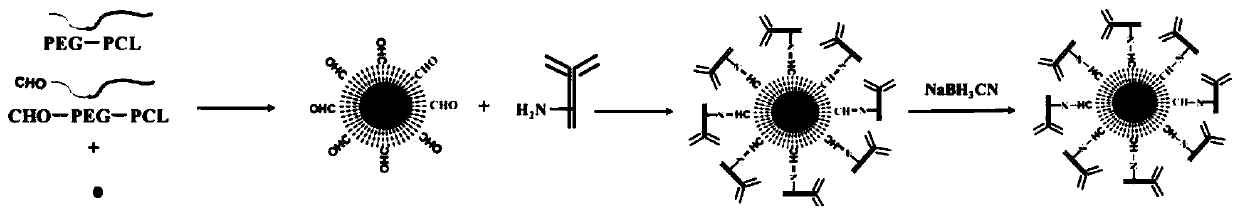
[0123] Embodiment 1 Preparation method of non-targeting nanocrystal micelles (that is, non-targeting drug-loaded micelles)
[0124] The drug loading is the percentage of the mass of paclitaxel to the mass of the polymer carrier material.
[0125] The molecular weight of PCL in the PCL-PEG-CHO used in this example is 5000Da; the molecular weight of PEG is 2000Da. The molecular weight of PCL in the PCL-PEG used in this example is 2000Da; the molecular weight of PEG is 2000Da.
[0126] Specifically, the preparation method of non-targeted drug-loaded micelles comprises the following steps:
[0127]Mix the PCL-PEG-CHO and the PCL-PEG according to the mass ratio of 2:1, then add paclitaxel with 5% of the total mass of PCL-PEG-CHO and PCL-PEG, and co-dissolve in 1ml chloroform and shake until completely dissolved and mix evenly; then add 10ml of deionized water and sonicate in a water bath with 100% power until a white uniform emulsion is formed. Remove the chloroform by rotary ev...
Embodiment 2
[0131] Embodiment 2, the preparation method of targeted nanocrystalline micelles (that is, targeted drug-loaded micelles)
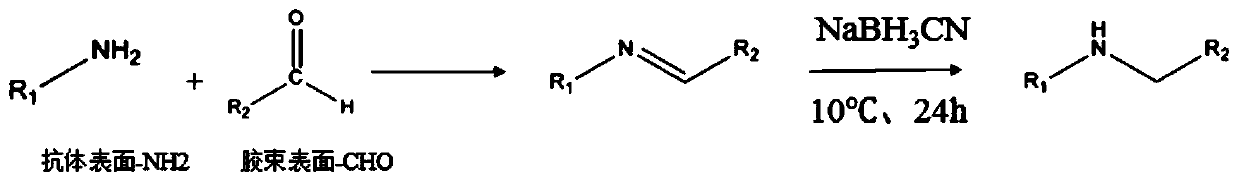
[0132] Targeted drug-loaded micelles using NaCNBH 3 Preparation by reduction method. That is, in phosphate buffered saline solution, the aldehyde groups on the surface of the non-targeted drug-loaded micelles obtained in Example 1 and the primary amino groups on the surface of Herceptin (molar ratio -CHO / -NH2=15:1) pass through The schiff reaction forms a C=N bond, followed by NaCNBH 3 A stable C-N bond is formed under the action, and the reaction is carried out at 2-10°C and 350rpm for 24h, and the obtained targeted drug-loaded micelles are stored at 4°C for future use.
[0133] For details of the reaction diagram, see figure 2 .
Embodiment 3
[0134] Embodiment 3, performance determination of nanocrystalline micelles (comprising blank micelles, non-targeted drug-loaded micelles, targeted drug-loaded micelles)
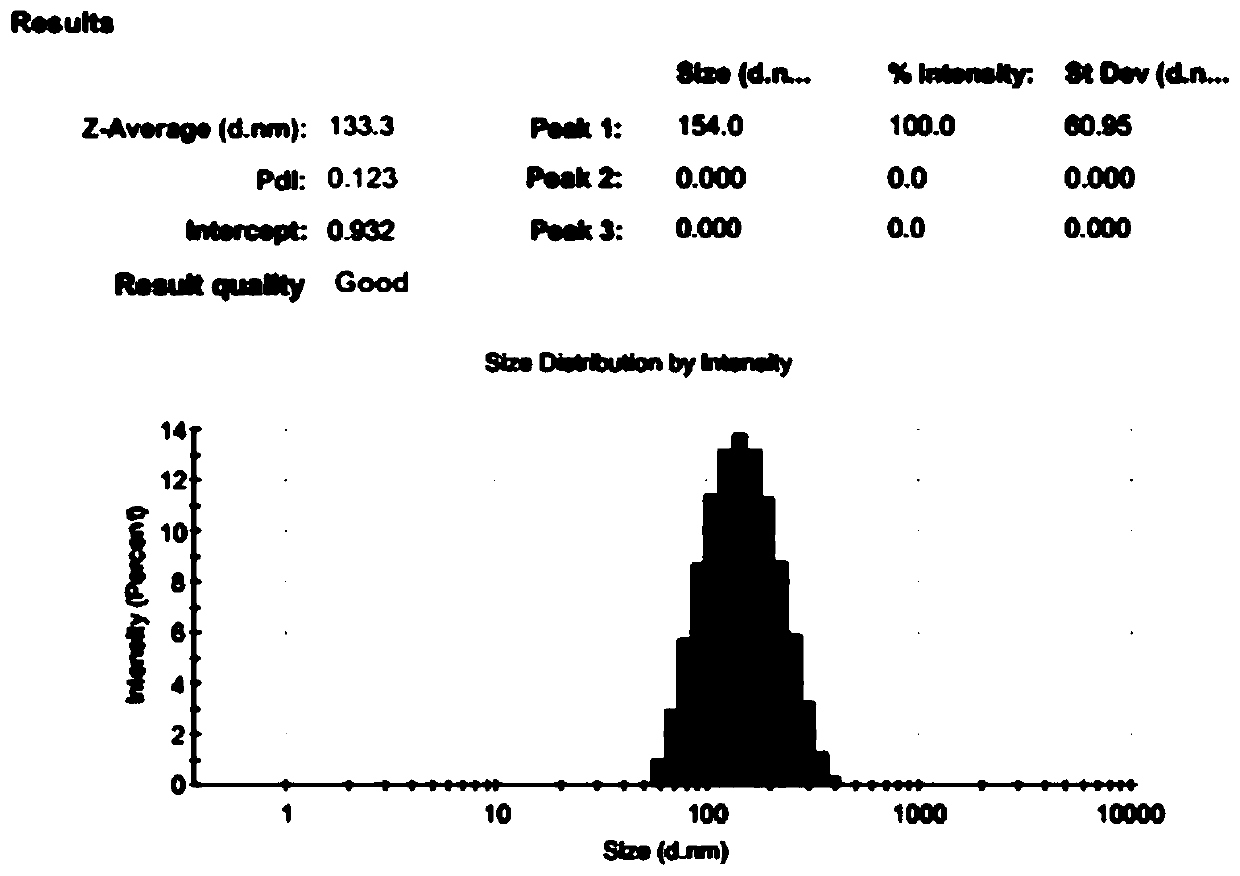
[0135] 1. Determination of particle size
[0136] The particle size and polydispersity of the blank micelles obtained in Example 1, the non-targeted drug-loaded micelles obtained in Example 1, and the targeted drug-loaded micelles obtained in Example 2 were respectively measured at room temperature using a laser scattering particle size analyzer. coefficient.
[0137] The particle diameter of the blank micelle that embodiment 1 obtains, polydispersity index (PDI) see Figure 3A . For the particle size and polydispersity index (PDI) of the non-targeted drug-loaded micelles obtained in Example 1, see Figure 3B . For the particle size and polydispersity index (PDI) of the targeted drug-loaded micelles obtained in Example 2, see Figure 3C .
[0138] From Figure 3A , Figure 3B and Figure 3C , it ca...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com