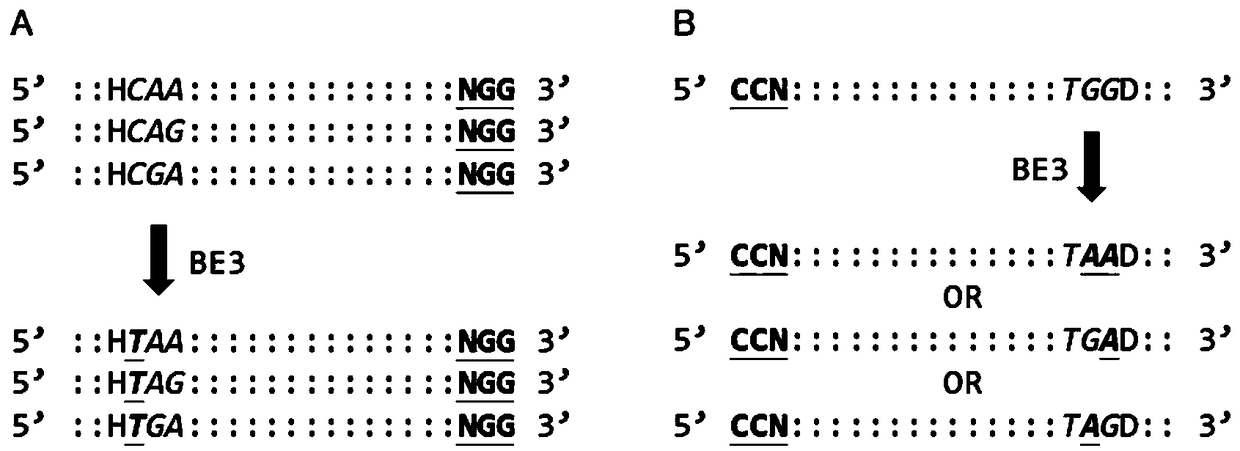
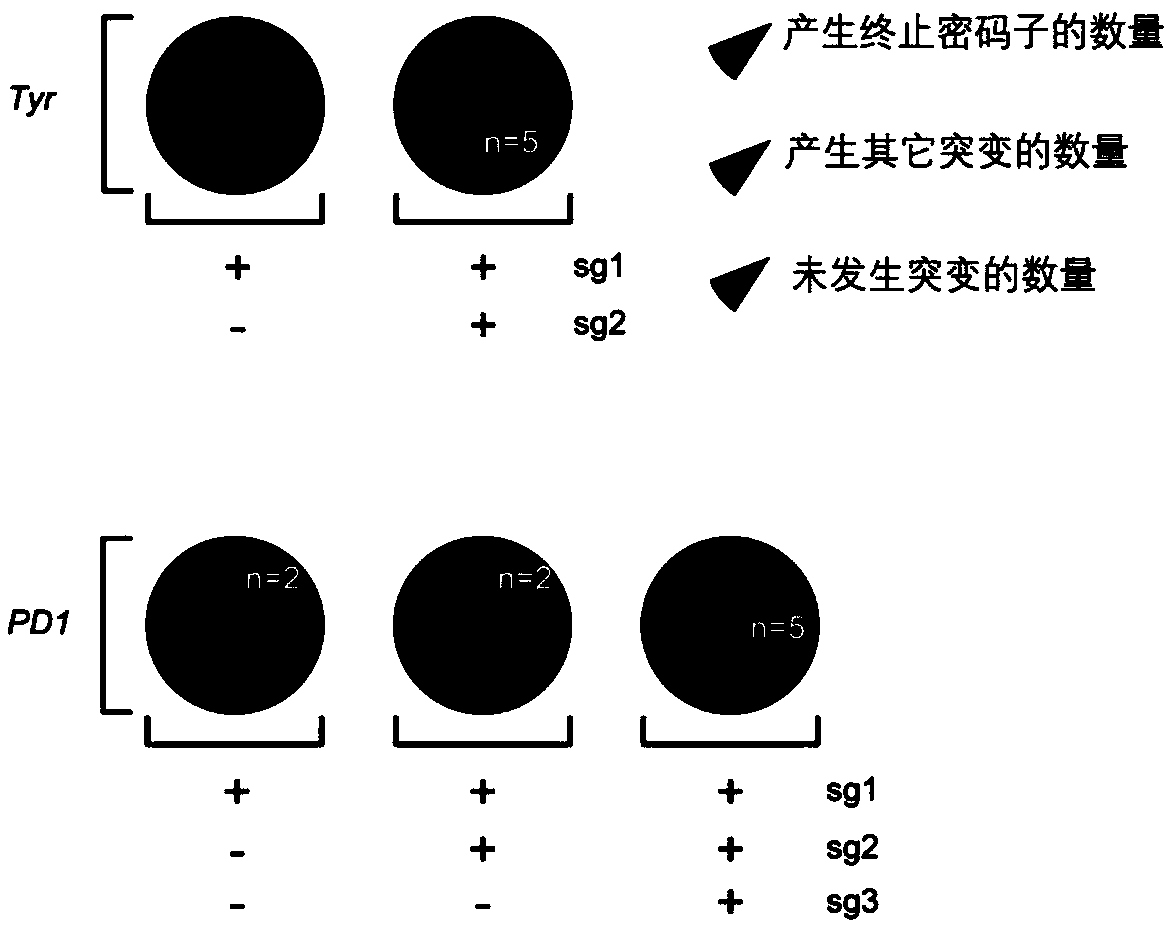
Effective knockout of same gene achieved by co-injection of multiple sgRNAs
A gene knockout and gene technology, applied in genetic engineering, using microinjection method, recombinant DNA technology, etc., can solve the problems of gene acquisition, frame shift mutation, imprecise gene editing, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] BE3-mediated base editing was performed on cell lines, and stop codons were introduced to achieve gene knockout. Carry out gene knockout of cell lines (by electroporation or liposome transfection) according to routine operation, taking liposome transfection as an example.
[0038] (1) Taking N2a cells as an example, the present invention carries out culture and transfection of eukaryotic cells: N2a cells are inoculated and cultured in DMEM high-sugar culture solution (HyClone, SH30022.01B) supplemented with 10% FBS, which contains penicillin ( 100U / ml) and streptomycin (100μg / ml).
[0039] (2) Divide into 6-well plates before transfection, and perform transfection when the density reaches 70%-80%.
[0040] (3) Transfection Take liposome transfection as an example. Follow Lipofectamine TM 2000 Transfection Reagent (Invitrogen, 11668-019) operating manual, taking SpCas9nickase as an example, mix 2 μg BE3 plasmid and 1 μg pGL3-U6-EGFP-sgRNA plasmid, co-transfect into ea...
Embodiment 2
[0079] BE3-mediated base editing was performed on mouse embryos, and a stop codon was introduced to achieve gene knockout.
[0080] (1) In vitro transcription: BE3 plasmid was linearized with AgeI enzyme (NEB, R3552L), and transcribed in vitro with T7ULTRA (Ambion, AM1345). BE3 mRNA was purified using RNeasy Mini Kit (QIAGEN, 74104). The sgRNA nucleotides were annealed into the pUC57 sgRNA expression vector with T7 promoter (Addgene, 51132). The sgRNAs were then amplified and transcribed using the MEGAshorttranscript T7 kit (Ambion, AM1345). sgRNA was purified with MEGAclear kit (Ambion, AM1908) and recovered by ethanol precipitation.
[0081] (2) Microinjection: Female C57BL / 6J mice treated with superovulation were mated with male C57BL / 6J mice, and fertilized eggs were collected from the oviduct on day 0.5. Fertilized eggs were injected with BE3mRNA (50ng / μl) and specific sgRNA (25ng / μl). The injections are as follows:
[0082] 1. Pcdc1
[0083] Combination 1: BE3mRNA+...
Embodiment 3
[0113] Construction of BE3-mediated knockout mice.
[0114] Mouse embryo collection, microinjection, embryo culture and embryo transfer were performed according to routine operations.
[0115] (1) Microinjection: Fertilized eggs were injected with BE3 mRNA and specific sgRNA (Experiment 1: PD1-sg1+2+3, Tyr-sg1+2), or BE3 mRNA and specific sgRNA (Experiment 2: PD1-sg1+2 +3, Tyr-sg1+2+3). Routine embryo transfer;
[0116] (2) Genotype analysis:
[0117] A. Genomic DNA was extracted by pruning the tail of conventional mice: after cleavage and digestion with 100 μg / ml proteinase K in the lysate (10 μM Tris-HCl, 0.4M NaCl, 2 μM EDTA, 1% SDS), dissolve to 50 μl after phenol-chloroform extraction deionized water.
[0118] B. Use a pair of primers N-For and N-Rev to carry out PCR amplification, and use AxyPrep PCR cleanup kit (AXYGEN, AP-PCR-250G) to purify and obtain PCR recovered products. The PCR reaction system is:
[0119] 300-400ng genomic DNA
[0120] 25μl 2X Buffer
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com