Fusion protein, preparation method and application thereof, expression system and vaccine
A fusion protein and protein technology, applied in the biological field, can solve the problems of decreased ability of lymphocyte antibodies, decreased cellular immunity, decreased body resistance to disease, etc., to achieve high expression, enhanced immunogenicity, and stable titer Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0064] A method for preparing the above fusion protein, comprising expressing the gene encoding the fusion protein in a host.
[0065] The method has the advantages of simple preparation method, low cost, powerful vaccine function and the like.
[0066] The host in the present invention can be, but not limited to, an Escherichia coli expression system, a yeast expression system, an insect expression system, a plant expression system or a mammalian expression system. Since the protein expressed by mammalian cells is translated and processed, its structure and biological properties are closer to natural proteins, so the present invention preferably uses a mammalian expression system to express the gene encoding the fusion protein.
[0067] In a preferred embodiment of the present invention, a mammalian expression system is used to express the gene encoding the fusion protein. Since the protein expressed by mammalian cells is translated and processed, its structure and biologica...
Embodiment 1
[0101] Example 1 Expression, identification and purification of fusion protein PA-C3d-Cap-K
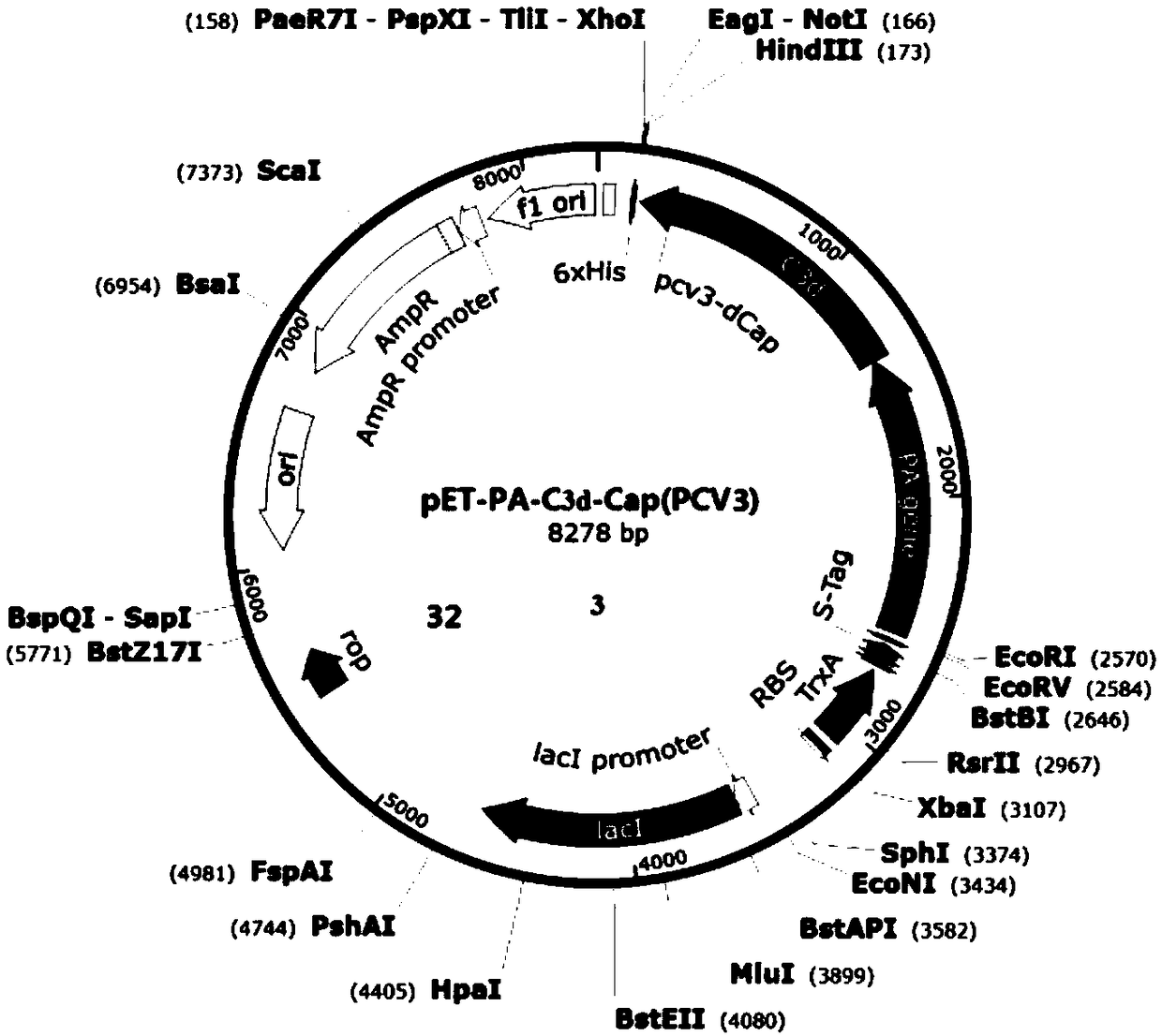
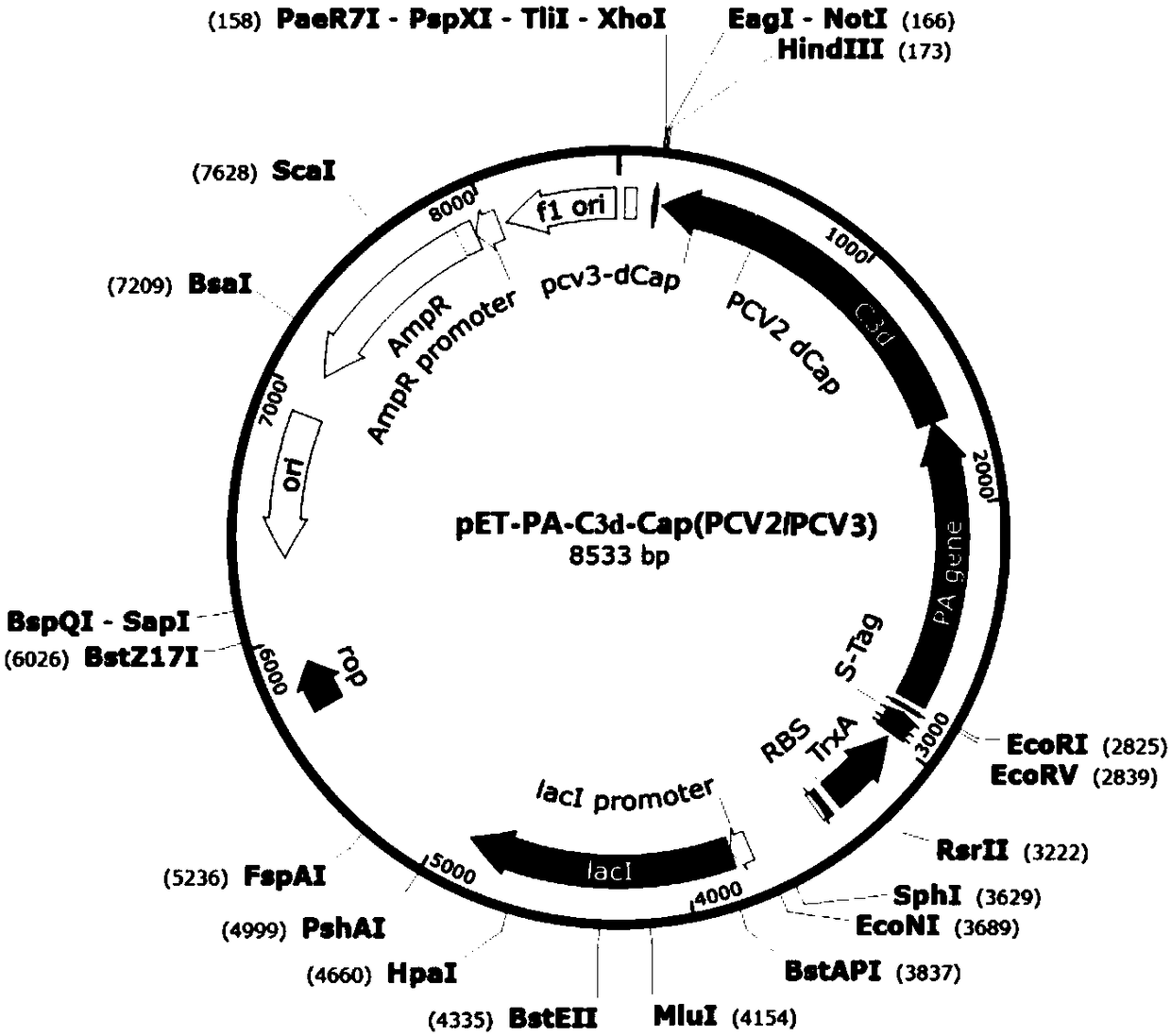
[0102] According to NCBI reports, Pseudomonas aeruginosa PA (Pseudomonas aeruginosa PA, accession number: CP007224.1, see SEQ ID NO.1) gene sequence, porcine complement C3d (see SEQ ID NO.8) gene sequence, PCV2-ADDLPP The Cap protein gene sequence (see SEQ ID NO.2) of the 10069 strain (accession number: EU594437.1) and the Cap protein gene sequence (see SEQ ID NO.2) of the PCV3-US / MN strain (NCBI accession number: KX898030.1) .3) Analyze and finally design Pseudomonas exotoxin A (PEA) domain I and II protein, porcine complement C3d, porcine circovirus Cap protein dominant epitope and carboxyl terminal part containing SEQ ID NO.4 The polypeptide selects flexible amino acids (SEQ ID NO.7) and connects them in series in a certain combination to express a fusion protein with good immunogenicity.
[0103] 1.1 Construction and identification of PEA domains I and II and Cap gene cloning vec...
Embodiment 2
[0127] The preparation of embodiment 2 porcine circovirus vaccine
[0128] Dilute the fusion protein prepared in Example 1 with PBS solution, mix the diluted fusion protein solution with SEPPICISA201R VG adjuvant at a mass fraction of 50%, stir at a speed of 8000r / min for 10min, and add 0.01% (volume ratio) thimerosal solution, so that the final concentration does not exceed 1 / 10,000. After fully oscillating and mixing, according to the requirements of the appendix of the current version of the Chinese Veterinary Pharmacopoeia, after passing the sterility test, viscosity measurement, and stability measurement, place it in 4 ℃ for later use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com